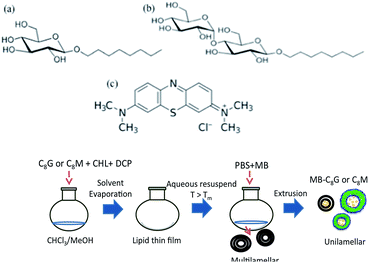
Alkyl mono- and di-glucoside sugar vesicles as potential drug delivery vehicles: detecting drug release using fluorescence
Malinda Salima,
Osama K. Abou-Zied*b,
H. Udani Kulathungab,
Asweni Baskaranc,
Umah R. Kuppusamyd and
Rauzah Hashim*a
aCenter of Fundamental Science of Self-Assembly, Department of Chemistry, Faculty of Science, University of Malaya, 50603, Kuala Lumpur, Malaysia. E-mail: rauzah@um.edu.my
bDepartment of Chemistry, Faculty of Science, Sultan Qaboos University, P.O. Box 36, Postal Code 123, Muscat, Sultanate of Oman. E-mail: abouzied@squ.edu.om
cMushroom Research Centre, Institute of Biological Sciences, Faculty of Science, University of Malaya, 50603, Kuala Lumpur, Malaysia
dDepartment of Biomedical Science, Faculty of Medicine, University of Malaya, 50603 Kuala Lumpur, Malaysia
First published on 19th June 2015
Abstract
The use of alkyl glycosides as alternatives to other surfactants for pharmaceutical applications has received much interest due to their specific carbohydrate interactions, low inherent toxicity, and biodegradability. We have formulated sugar-based vesicles that carry mono- and disaccharide groups using octyl glucoside and maltoside, respectively. The physicochemical properties of the vesicles including size, surface charge, shape, and effect of the cholesterol content on the vesicle stability were investigated using dynamic light scattering and transmission electron microscopy. Stable glucoside (C8G) and maltoside (C8M) vesicles were achieved by incorporation of 20 mol% cholesterol, and 8 mol% dicetyl phosphate charge additives. A model small hydrophilic methylene blue (MB) dye was encapsulated into C8G and C8M, and the leakage of the dye was determined by examining the corresponding fluorescence signal in frequency and time domains. The results indicate a tight binding of MB in C8G, as reflected in the short fluorescence lifetime (τ < 0.10 ns) compared to MB in C8M (τ = 0.28 ns). This was in agreement with the leakage efficiency of MB which was measured at physiological temperature to be almost zero in MB–C8G, while a ca. 17% leakage was detected for MB–C8M after 30 minutes. The latter shows a ca. 44% leakage after 60 minutes. The larger head group in C8M increases the membrane fluidity, leading to a more flexible vesicle with more leakage efficiency. A local basic environment was observed in dye-encapsulated vesicles, and was attributed to through space interactions between MB and each of the sugar polar sites and the localized water molecules. The vesicles were non-cytotoxic at low concentrations. The current work shows the usefulness of using C8M as a carrier intended for fast release of hydrophilic drugs.
1. Introduction
Nano-sized vesicles formed from self-assembly of amphiphilic lipids or surfactant molecules are known for their ability to encapsulate and deliver various types of active agents such as proteins, peptides, nucleic acids, and other small molecules. These carriers have been investigated in various biological applications such as drug deliveries and disease diagnosis.1–3 Selectivity of vesicles to target cells is generally achieved via modification with various ligands including antibodies,4 peptides,5 proteins,6 and carbohydrates.7 It has been widely recognized that carbohydrate-conjugated nanoparticles can be used to bind lectin (a carbohydrate-binding protein) overexpressed in numerous cancer cell surfaces.8,9 Besides specific cell-recognition, these molecules have also been shown to exhibit prolonged plasma circulation, reduced non-specific biomolecule interactions and macrophage uptake.10,11Although carbohydrate-modified vesicles are commonly produced through conjugation of sugar moieties to phospholipid vesicles termed liposomes, there are increasing studies in vesicle formulation solely using glycolipids or sugar surfactants and cholesterol due to the susceptibility of phospholipids to oxidation and their high costs of production.12,13 A major class of synthetic sugar surfactants that are derived from renewable sources, and are readily biodegradable with low inherent toxicity and immunogenicity, is alkyl glycosides.14,15 Self-assembled alkyl glycoside vesicles based on its pure form (e.g. alkyl monoglucosides, alkyl diglucosides) or mixtures of different sugar units (alkyl polyglycosides), have been tested for their drug-carrying capabilities in pharmaceutical and cosmetic applications. Kiwada et al. synthesized and tested the feasibility of monoalkylated glucoside vesicles as drug carriers.16 High vesicle stability in plasma with comparable 14C-sucrose encapsulation efficiency to egg phosphatidylcholine was observed. The same group also showed specific liver targeting using alkyl galactoside vesicles.17 More recently, Muzzalupo and co-workers fabricated sugar vesicles using alkyl monoglucosides, and studied the encapsulation as well as in vitro release of a hydrophobic methotrexate anti-cancer drug.18 The authors showed high drug entrapment efficiencies, and low hemolytic toxicities of the sugar-surfactant vesicles.
Besides monosaccharide vesicles, disaccharide-modified nanoparticles could also be used for targeted deliveries due to the presence of disaccharide-binding lectins in several types of tumor cells.19,20 In a recent publication, Song et al. synthesized disaccharide-modified liposomes through conjugation of aminated sucrose and maltose to the carboxyl end of PEGylated phospholipid, where they reported an enhanced tumor intracellular uptake through lectin-mediated endocytosis.21 Alkyl diglucoside such as dodecyl maltoside in its free form also appears to be a potential cancer-preventive agent as it may delay tumor progression by the inhibiting tumor necrosis factor alpha gene expression and its release; this is a phenomenon not observed in alkyl glucoside.22 Moreover, disaccharide-functionalized vesicles have been shown to possess higher binding affinity towards lectin receptors compared to their monosaccharide counterpart.23
Previously, we have reported the preparation of vesicle drug carriers using Guerbet branched chain maltoside.24 To form a stable vesicle, the maltoside glycolipid was mixed with anionic surfactants sodium dodecyl sulphate (SDS) and aerosol OT (AOT). The AOT/glycolipid mixture was found to give a broad polydispersity feature, while the SDS/glycolipid mixture gave a translucent dispersion with a main hydrodynamic radius of 40 nm. The latter formed a single unilamellar vesicle (SUV) that was confirmed by cryo-TEM. While the stability of the glycolipid vesicle dispersions was investigated, drug encapsulation was not attempted in the previous study.24
In the current work, we continue our investigation to study the direct formation of disaccharide-based vesicle without functionalization using octyl maltoside (Fig. 1). The vesicle was prepared using a thin film hydration method.25 The physicochemical properties and storage stability of octyl maltoside vesicles (C8M) were characterized and compared to the monosaccharide-sugar surfactant octyl glucoside (Fig. 1) vesicles (C8G). Dye encapsulation efficiencies of a model small hydrophilic methylene blue (MB, see Fig. 1) dye in the vesicles were examined and compared by following the change in its fluorescence signal in frequency and time domains. Leakage of the water-soluble dye from vesicles in buffered solutions at room and physiological temperatures was investigated to understand the effect of temperature on dye release. Finally, 3[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay was conducted to determine the in vitro cytotoxicity of vesicles towards macrophage and cancer cells.
2. Experimental section
2.1 Formation of vesicles
Octyl β-D-glucopyranoside (≥98%) and maltopyranoside (≥99%) were purchased from Sigma-Aldrich, and used as received. The vesicles (C8G and C8M) were formulated at 10 mM in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v chloroform
1 v/v chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol solution with 10–20 mol% cholesterol and 0–8 mol% dicetyl phosphate (DCP). DCP was commonly added to induce negative charge and minimize the aggregation between neutral uncharged molecules.26 The organic solvents were then removed by rotary evaporation (Buchi R-210), of which sample thin films were formed on walls of the round bottom flasks. To rehydrate the thin lipid film, 4 ml phosphate buffered saline (PBS, 10 mM, pH 7.4) solution was heated to temperatures greater than the solid–liquid crystal phase transition temperatures of C8M and C8G at 80 °C, and added to each sample. The resulting multilamellar-vesicle solutions were vortex mixed, and passed through an N2-driven extruder (10 ml Lipex, Northern Lipids Inc.) for more than 10 cycles through 100 nm diameter pore size polycarbonate filters to obtain small unilamellar vesicles of homogeneous size distribution.
methanol solution with 10–20 mol% cholesterol and 0–8 mol% dicetyl phosphate (DCP). DCP was commonly added to induce negative charge and minimize the aggregation between neutral uncharged molecules.26 The organic solvents were then removed by rotary evaporation (Buchi R-210), of which sample thin films were formed on walls of the round bottom flasks. To rehydrate the thin lipid film, 4 ml phosphate buffered saline (PBS, 10 mM, pH 7.4) solution was heated to temperatures greater than the solid–liquid crystal phase transition temperatures of C8M and C8G at 80 °C, and added to each sample. The resulting multilamellar-vesicle solutions were vortex mixed, and passed through an N2-driven extruder (10 ml Lipex, Northern Lipids Inc.) for more than 10 cycles through 100 nm diameter pore size polycarbonate filters to obtain small unilamellar vesicles of homogeneous size distribution.
2.2 Physical characterization of the vesicles
Dynamic light scattering (DLS) measurements, performed using Zetasizer Nano ZS (Malvern) equipped with a 633 nm He–Ne laser light source, was used to confirm the vesicle formation. DLS was also used to determine the average hydrodynamic size and the size distribution of C8G and C8M. The samples were also subjected to zeta potential measurements using the same instrument at 25 °C. The stability of different vesicles (formulated with different cholesterol content) at room temperature and 4 °C storage was evaluated by size measurement determination.The morphology of the vesicles was analyzed using transmission electron microscopy (TEM, JEOL JEM-2100F operating at 200 kV accelerating voltage). 5 μl of the vesicle sample was placed on a carbon-coated copper grid and the excess sample was completely aspirated after 5 minutes incubation. The sample was then stained by adding 2 μl of 1% (w/v) phosphotungstic acid solution, followed by a 2 minute incubation and excess solution removal.
2.3 Cytotoxicity test
MTT colorimetric assay was carried out to evaluate the cytotoxicity of C8G and C8M vesicles of varying concentrations on macrophage RAW 264.7 and colorectal HCT116 cancer cell lines. In the assay, yellow tetrazolium salt is reduced to insoluble purple formazan crystals by the mitochondrial dehydrogenases of viable cells. The cells (5 × 104 cells per well) were seeded in a 96-well flat-bottomed micro-titre plate and incubated at 37 °C overnight in a humidified environment of 5% CO2 and 95% air to allow cell adherence. Cells were then treated with 0.0005 μM to 500 μM of C8G and C8M vesicles for another 24 h. Subsequently, 5 mg ml−1 of MTT solution was added into each well and incubated for 4 h for formazan crystal formation. The supernatant was then carefully removed, and 100 μl of dimethyl sulfoxide (Sigma-Aldrich) was added into each well to dissolve the MTT formazan crystal. The absorbance was measured at 540 nm with a spectrophotometer (BioTek Instruments). The complete growth medium served as negative control, and cells incubated in medium only without the vesicles were used as positive control.2.4 Dye encapsulation
MB was encapsulated into the vesicles based on a passive loading approach, of which 80 μM MB (≥82%, Sigma-Aldrich) was added to the PBS solution during rehydration for dye encapsulation. The excess non-encapsulated dyes were separated from the vesicles using Sephadex G-50 spin columns (USA Scientific). Encapsulation efficiencies of the dye markers were assessed by the ratio of the dye incorporated into the vesicles to the total amount of dye introduced (eqn (1)):27
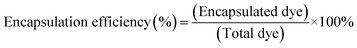 | (1) |
Drug-loading efficiency is determined by the mass ratio of MB in the glucoside vesicle samples to the vesicles (eqn (2)):27
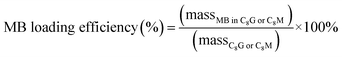 | (2) |
2.5 Dye release study
Self-quenching method28 was used to analyze the in vitro release or leakage of the dye markers from vesicles by diluting 10 μl of the encapsulated vesicles in 110 μl of PBS solutions at room (23 °C) and physiological (37 °C) temperatures. Fluorescence intensities of the samples were measured in 30 minute intervals for 90 minutes.The steady-state fluorescence spectra were measured using a Shimadzu RF-5301 PC spectrofluorophotometer. Time-resolved fluorescence transients were performed using a TimeMaster fluorescence lifetime spectrometer obtained from Photon Technology International. Excitation was done at 650 nm using a laser diode and emission was detected using a cut-off filter (λdetection ≥ 665 nm). The instrument response function (IRF) was measured from the scattered light and estimated to be approximately 1.5 ns (full width at half-maximum). The measured transients were fitted to multiexponential functions convoluted with the system response function. The fit was judged by the value of the reduced chi-squared (χ2). The experimental time resolution (after deconvolution) was approximately 100 ps, using stroboscopic detection.29 In all the experiments, samples were measured in a 1 cm path-length quartz cell at 23 ± 1 °C.
The percentage of the dye released from the vesicles was calculated based on eqn (3):
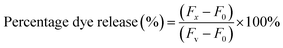 | (3) |
3. Results and discussion
3.1 Vesicle characterization
Vesicles were prepared using monosaccharide- and disaccharide-containing single chain surfactants. Incorporation of cholesterol (CHL) into these surfactants was found to affect the physical stability of the formed vesicles, as shown in Fig. 2.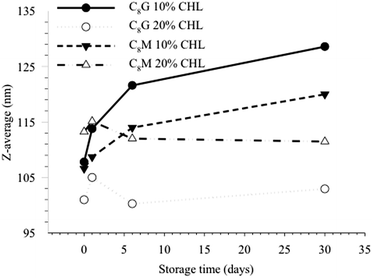 | ||
| Fig. 2 Effect of CHL content on the average hydrodynamic diameter of the C8G and C8M vesicles, and their time-dependent storage stability. Lines are added for visual aid. | ||
The stability of the C8G and C8M vesicles was found to improve when the cholesterol content is raised. At 10 mol% cholesterol content, there was an increase in the vesicle size while no significant changes were observed in 20 mol% formulation after more than one month of room temperature storage. The average hydrodynamic diameters of the C8G and C8M vesicles before and after 30 day storage at 20 mol% cholesterol were 105 and 111 nm, respectively, with a low polydispersity index (PDI < 0.1). This suggests a homogeneous particle size distribution, as seen in Fig. 3. In addition, the diameters remained the same for more than 3 months. Our observation corroborates previous findings, where intercalation of cholesterol (often in the range of 20–50 mol%) to non-ionic surfactant vesicles could enhance the physical stability of the vesicles against intravesicular aggregation.30 However, storage of the vesicles at 4 °C triggered the formation of solid white precipitates that may be caused by lipid-CHL phase separation. As C8G and C8M lamellar vesicles were formed using extrusion technique, where the samples were passed through a defined polycarbonate membrane pore size, effects of CHL on the vesicle size could not be observed. Formulation of stable vesicles using 20 mol% CHL was therefore used for further examinations and dye leakage studies. Subsequently, similar average hydrodynamic diameters for the MB-loaded C8G (107 nm) and C8M (120 nm) vesicles was obtained due to extrusion technique, with narrow size distribution where PDI was less than 0.13.
 | ||
| Fig. 3 Particle size distribution of C8G and C8M vesicles before and after 30 days of room temperature storage, measured using dynamic light scattering. | ||
Table 1 compares some properties of the current vesicle formulations with those of our previous Guerbet branched chain glycolipids.24 We note that the methods of preparation in both cases are different. Previously, we had used the “ultrasonication” method does not usually produce stable, reproducible, and homogeneous vesicle systems.31 On the other hand, the thin film hydration technique combined with extrusion (used in the current work) assists in the formation of stable vesicles with high reproducibility. Furthermore, we also note the glycolipids used in both cases are of different alkyl chain groups, namely octyl versus branched hexyl-decyl (derived from Guerbet alcohol32) units. In the previous work, the glucoside formulation did not produce stable vesicles, and hexosome emulsion was obtained instead. Anionic co-surfactants such as AOT and SDS were added to the maltoside to stabilize the vesicle formations. Comparing these to the current formulations that contain DCP as a co-surfactant and CHL, we produced more defined size and stable vesicle systems for both lipids with glucose and maltose units.
| Monoalkylated glycolipids [current work] | ||
|---|---|---|
| C8G/DCP/CHL | C8M/DCP/CHL | |
| Preparation method | Thin film hydration and extrusion | Thin film hydration and extrusion |
| Self- assembly | SUV vesicle | SUV vesicle |
| Size | 105 nm | 110 nm |
| Stability | >3 months | >3 months |
| Guerbet glycolipids24 | ||
|---|---|---|
| C10C6-M/AOT | C10C6-M/SDS | |
| Preparation method | Ultrasonication | Ultrasonication |
| Self- assembly | Polydisperse vesicle | SUV vesicle |
| Size | SUV 20–100 nm | 80 nm |
| LUV 100–200 nm | ||
| Stability | A few days | 1 week |
Evaluation of the vesicle surface charge based on zeta potential (ζ) technique shows that the particles have a net negative charge due to the presence of DCP, with an average ζ of −19 ± 5 mV (C8M vesicles) and −32 ± 4 mV (C8G vesicles) in 10 mM PBS solution of pH 7.4. It was generally accepted that colloids with high absolute ζ values of >30 mV are electrically stabilized, while lower ζ particles have the tendency to aggregate due to a reduced electrostatic repulsion. C8M vesicles could therefore be considered less stable compared to C8G; and its reduced negative charge may be caused by masking of the surface charge due to the larger disaccharide head group.
Direct visualization of the formation of vesicular nanostructures was confirmed using TEM as depicted in Fig. 4. The observed vesicles are however not perfectly spherical, and polygonal shape transformations occurred, which could be caused by the effects of extrusion and osmotic stress.33,34
3.2 Cytotoxicity assay
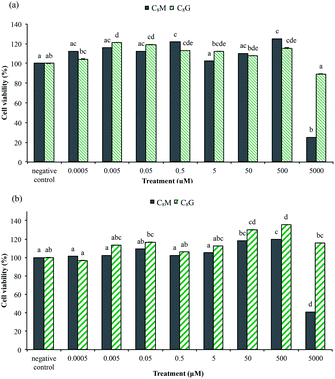
An assessment of the potential cytotoxicity of the C8G and C8M vesicles was carried out using an MTT cell viability assay. Fig. 5a and b shows the percentage of cell viability of RAW 264.7 macrophage cells and colorectal HCT116 cancer cells incubated with various concentrations of vesicles. Untreated cells (negative control) was taken as having 100% viability.Exposure of RAW 264.7 and HCT116 cells to C8G vesicles from 0.0005 μM to 5000 μM showed no cytotoxic effect, with significant increase (p < 0.05) in cell proliferation observed between 0.005–500 μM for macrophage, and between 50–500 μM for cancer cells. As seen from Fig. 5b, C8M vesicles are also not cytotoxic over a large range of concentrations from 0.0005 μM to 500 μM in both RAW 264.7 and HCT116 cells. There was a significant (p < 0.05) reduction in the percentage of cell viability at high C8M concentration of 5000 μM, although an induced cell proliferation was observed at 0.5–500 μM (macrophage) and 50–500 μM (cancer cells).
It has been previously reported that tumour cells exposed to empty vesicles that consist of phosphatidylcholine, cholesterol and ganglioside glycolipid would continue to proliferate normally; whereas positively charged vesicles showed non-proliferative behaviours.35 In addition, Li et al. noted that long (48 hours) incubation time of tumour cells with empty (phospholipid/cholesterol/glutamic acid lipid) vesicles was needed to be able to observe the effects of cytotoxicity.36 We therefore conclude that C8G and C8M vesicles are biocompatible at a large range of concentrations. However, despite the ability of the vesicles to encapsulate anti-cancer drugs, their use in cancer cells can be precarious due to the induced cell proliferation.
3.3 Dye encapsulation
Hydrophilic drugs have been used as chemotherapeutic agents, but their deliveries remain a challenge. Encapsulation of drug molecules in vesicles has proven to be useful in reducing the drug toxicity and improving its therapeutic efficacy. Furthermore, localization of the drug in vesicle (hydrophobic on lipid bilayer and hydrophilic in inner aqueous vesicle compartment depending on solubility) can protect the active compound from the outer environment thus providing an increased stability.37 We herein seek to investigate and understand the encapsulation, interaction and release properties of a hydrophilic low molecular weight MB dye in the mono- and disaccharide-based synthetic sugar surfactant vesicles.MB is a cationic photosensitizer that has been used in a variety of clinical applications including photodynamic therapy for cancer treatment.38 It has been shown to induce apoptotic cell death through generation of cytotoxic reactive oxygen species (ROS) when irradiated by light of specific wavelengths. When encapsulated in, for instance, alginate nanoparticles, an enhanced ROS production was observed compared to free MB.39 In the current study, MB was encapsulated in C8G and C8M vesicles with low entrapment efficiencies of ca. 15 and 20%. Low loading efficiencies of MB in both C8G and C8M was also observed at ca. 0.1 and 1.1%. Relative poor encapsulation efficiencies and loading efficiencies (<15%) of water-soluble compounds have been generally observed using thin film hydration vesicle preparation technique and passive drug loading, where there is random entrapment of drug into the inner aqueous compartment of the vesicles during aqueous rehydration.40,41
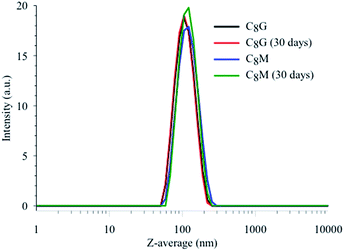
Release of MB into the outer aqueous solution results in an increase in its fluorescence peak intensity at 680 nm. Fig. 6 shows the fluorescence spectra of MB encapsulated in the C8G and C8M vesicles at different incubation temperatures and time intervals. The spectrum of MB in buffer is included for comparison (normalized for clarity). Since the MB concentration may be different in each vesicle, we will compare the change in the fluorescence intensity in C8G and C8M separately during the release process. Despite starting the load process of MB in C8G and C8M with the same concentration, the fluorescence intensity in C8G (red curve) is much lower than that in C8M (pink curve) at 23 °C. This result reflects either a poor loading of MB in C8G, or a tight binding and more shielding of the MB molecules from buffer in C8G. The latter case is evident in the fluorescence lifetime values (Table 2) in which the lifetime of free MB in buffer was measured to be 0.35 ns, whereas the lifetime values in vesicles are <0.10 and 0.28 ns for MB in C8G and C8M, respectively. The shorter lifetimes in vesicles point to a limited flexibility of the local environment, with C8G being less flexible. Tight binding and less flexible environment enhances the nonradiative decay contribution to the overall excited state deactivation process which reduces both the fluorescence intensity and lifetime.
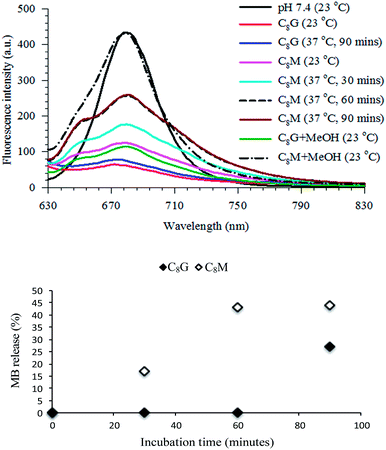 | ||
| Fig. 6 Top: fluorescence spectra of MB in buffer and vesicles. λex = 600 nm. Concentration of MB was 9 μM in buffer and estimated to be around 9 μM in vesicles using eqn (1). The spectrum of MB in buffer was normalized for ease of spectral shape comparison. Bottom: percentage release of MB from MB–C8G (solid fill) and MB–C8M (no fill) vesicles from 0 to 90 minutes incubation time at 37 °C. | ||
| τ (ns)a | |
|---|---|
| a Uncertainty in measurements is ± 0.01 ns. λex = 650 nm. Fluorescence was observed using a cut-off filter ≥665 nm. | |
| Buffer (pH 7.4) | 0.35 |
| Methanol | 0.59 |
| Acidic (pH 1.8) | 0.31 |
| Basic (pH 13.2) | 0.28 |
| C8G (23 °C) | <0.10 |
| C8G (37 °C, 90 min) | <0.10 |
| C8G + methanol | 0.33 |
| C8M (23 °C) | 0.28 |
| C8M (37 °C, 90 min) | 0.31 |
| C8M + methanol | 0.35 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
MB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) glucose molar ratio: glucose molar ratio: |
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.35 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
0.35 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
MB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) maltose molar ratio: maltose molar ratio: |
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.37 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
0.37 |
Leakage of MB at 37 °C starts only after 90 minutes in MB–C8G with ca. 27% release relative to methanol lysis (vide infra), whereas in the MB–C8M complex the release of MB starts after 30 minutes (ca. 17%). The release of MB from C8M approaches a constant value (ca. 44%) after 60 minutes (compare the two spectra in Fig. 6 after 60 and 90 minutes). Less than 10% MB release was observed after one day of room temperature incubation for both complexes which demonstrates a greater temperature effect than osmotically induced release (due to sample dilution) on dye leakage. The release of MB (a hydrophilic dye) from the C8G vesicle was slower compared to the release of a hydrophobic dye, where ∼60% leakage of methotrexate was observed after 2 hours of incubation at 37 °C.18 This could be attributed to the longer dye diffusion path from the aqueous interior of the vesicle.
Maximum release of MB from vesicle can be induced by adding methanol, which causes vesicle rupture.42 Fig. 6 displays the spectra of the MB–C8G and MB–C8M complexes after the addition of methanol. The fluorescence intensity of MB–C8M indicates efficient release of MB (ca. 98%). On the other hand, the effect of methanol on the MB–C8G complex shows only a ca. 30% release. The results emphasize the lower flexibility of C8G vesicle which tends to strongly bind the MB dye. The lifetime values, after methanol addition, point to exposure of MB to buffer which is more in the case of MB–C8M (Table 2). The measured lifetime of free MB in methanol is longer than that of MB in buffer as shown in Table 2. The observed lifetimes after vesicle rupture indicate that the effect of methanol is on the vesicle only and that the released MB molecules are in a mainly buffer environment. This conclusion is consistent with our steady-state fluorescence data in which the fluorescence peak of MB in methanol was observed at 670 nm which is blue-shifted by 10 nm compared to that in buffer (data not shown). Fig. 6 shows that the peak position of MB after vesicle rupture matches that of MB free in buffer.
The spectra in Fig. 6 show a blue shoulder at 650 nm when encapsulated in vesicle, which is clear for the MB–C8M complex in all stages of dye release. In order to unravel the origin of this peak, we measured the steady-state and time-resolved fluorescence of MB in acidic and basic media. Fig. 7 shows the results for the fluorescence spectra, and the corresponding lifetimes are included in Table 2. It is clear that MB in vesicles shows some similarity with that in a basic medium. We have shown recently that lipids similar to the current vesicles have a slight basic effect on the encapsulated probes in different phases.43,44 In order to clarify the role of the sugar units, we measured the fluorescence signals for MB mixed with glucose and maltose in the same buffer (data are shown in Fig. 8 and Table 2).
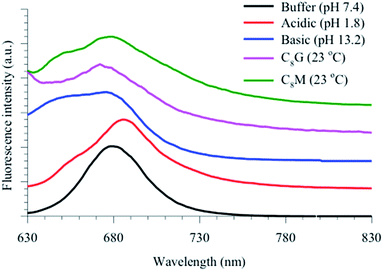 | ||
| Fig. 7 Fluorescence spectra of MB in aqueous solutions of different pH values and in vesicles. The spectra were spaced for clarity. λex = 600 nm. | ||
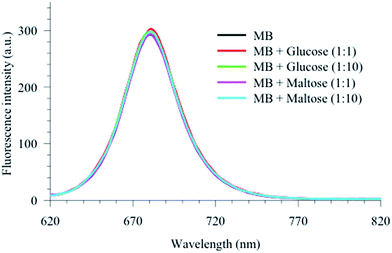 | ||
| Fig. 8 Fluorescence spectra of MB in buffer in the absence and presence of glucose and maltose as indicated. λex = 600 nm. | ||
The results show no effect of the sugar units on the MB spectroscopy up to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 molar ratio (MB
10 molar ratio (MB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sugar). The basic effect must then derive from the microenvironment packing of the vesicles in which through space interactions between the encapsulated MB molecules and the sugar units are dominant. This tight packing may force water to be close to the OH groups of the sugar units which may influence the local environment. It has been suggested that water near the cavity rim of β-cyclodextrin is modified by the extensive network of the OH groups of the sugar units in such a way as to increase its basicity.45 The basicity is then a consequence of the behavior of water near the sugar units which is different from bulk environment. The constrained local environment around the MB molecules in the vesicles may produce a similar effect as reported for β-cyclodextrin in which the water molecules are forced to be very close to the rich OH groups of the sugar units. Such packing will lead to strong interactions between the MB molecules with both the sugar units and the local water molecules so that the effect of basicity is expected to dominate the MB local environment.
sugar). The basic effect must then derive from the microenvironment packing of the vesicles in which through space interactions between the encapsulated MB molecules and the sugar units are dominant. This tight packing may force water to be close to the OH groups of the sugar units which may influence the local environment. It has been suggested that water near the cavity rim of β-cyclodextrin is modified by the extensive network of the OH groups of the sugar units in such a way as to increase its basicity.45 The basicity is then a consequence of the behavior of water near the sugar units which is different from bulk environment. The constrained local environment around the MB molecules in the vesicles may produce a similar effect as reported for β-cyclodextrin in which the water molecules are forced to be very close to the rich OH groups of the sugar units. Such packing will lead to strong interactions between the MB molecules with both the sugar units and the local water molecules so that the effect of basicity is expected to dominate the MB local environment.
The blue shoulder is also observed in the spectra of MB in vesicles after methanol addition, being less in C8M (Fig. 6). These results point to the close proximity of MB to the sugar units after vesicle rupture, most likely due to hydrogen bond interaction between MB and both sugar moiety and water molecules.
In order to test the extent of cationic MB adsorption to the outer surface of the anionic vesicles, free dye was added to the bare C8G vesicle, and passed through size exclusion columns for excess dye removal. No distinctive MB fluorescence emission peak at 680 nm was observed, although there was a very slight background intensity increase compared to the fluorescent signals of the bare vesicle. These results point to the presence of electrostatic interaction between the positively charged MB and the outer surface of C8G, albeit not significant. This further confirms that the fluorescence intensities obtained in the dye release experiments were attributed mainly to the encapsulated dye and not to the surface-MB physical interactions.4
4. Conclusions
Alkyl glucoside and maltoside can be used to formulate stable sugar-based vesicles which are non-cytotoxic at low concentrations, with potential applications in active compound deliveries. Encapsulation of hydrophilic MB in C8G and C8M vesicles showed varying release properties depending on the incubation time and temperature. There was a stronger interaction between MB and C8G vesicles due to a less flexible lipid packing, and this resulted in low MB release rate accompanied by incomplete release upon methanol lysis. On the other hand, C8M vesicle shows more flexibility with a higher release rate which could favor applications where faster compound release is desired.Acknowledgements
The authors would like to thank The Research Council of Oman (Grant no. RC/SCI/CHEM/14/01) and the University of Malaya and the Ministry of Higher Education High Impact Research Grant (Grant no. UM.C/625/1/HIR/MOHE/05) for financial support.References
- G. Gregoriadis and A. T. Florence, Drugs, 1993, 45, 15–28 CrossRef CAS PubMed.
- M. J. Lawrence, Chem. Soc. Rev., 1994, 23, 417–424 RSC.
- M. Salim, H. Minamikawa, A. Sugimura and R. Hashim, MedChemComm, 2014, 5, 1602–1618 RSC.
- L. D. Leserman, J. Barbet, F. Kourilsky and J. N. Weinstein, Nature, 1980, 288, 602–604 CrossRef CAS PubMed.
- A. Ito, K. Ino, T. Kobayashi and H. Honda, Biomaterials, 2005, 26, 6185–6193 CrossRef CAS PubMed.
- T. D. Heath and F. J. Martin, Chem. Phys. Lipids, 1986, 40, 347–358 CrossRef CAS.
- M. N. Jones, Adv. Drug Delivery Rev., 1994, 13, 215–249 CrossRef CAS.
- A. Nag and P. C. Ghosh, J. Drug Targeting, 1999, 6, 427–438 CrossRef CAS PubMed.
- M. Yamamoto, K. Ichinose, N. Ishii, T. Khoji, K. Akiyoshi, N. Moriguchi, J. Sunamoto and T. Kanematsu, Oncol. Rep., 2000, 7, 107–118 CAS.
- A. Chonn and P. R. Cullis, J. Liposome Res., 1992, 2, 397–410 CrossRef CAS.
- T. Allen, Adv. Drug Delivery Rev., 1994, 13, 285–309 CrossRef CAS.
- D. A. van Hal, J. A. Bouwstra, A. van Rensen, E. Jeremiasse, T. de Vringer and H. E. Junginger, J. Colloid Interface Sci., 1996, 178, 263–273 CrossRef CAS.
- S. Beugin, K. Edwards, G. Karlsson, M. Ollivon and S. Lesieur, Biophys. J., 1998, 74, 3198–3210 CrossRef CAS.
- W. von Rybinski, Curr. Opin. Colloid Interface Sci., 1996, 1, 587–597 CrossRef CAS.
- D. Balzer and H. Lüders, Nonionic surfactants: alkyl polyglucosides, CRC Press, 2000 Search PubMed.
- H. Kiwada, H. Niimura, Y. Fujisaki, S. Yamada and Y. Kato, Chem. Pharm. Bull., 1985, 33, 753–759 CrossRef CAS.
- H. Kiwada, H. Niimura and Y. Kato, Chem. Pharm. Bull., 1985, 33, 2475–2482 CrossRef CAS.
- R. Muzzalupo, L. Tavano and C. La Mesa, Int. J. Pharm., 2013, 458, 224–229 CrossRef CAS PubMed.
- R. Lotan, Y. Matsushita, D. Ohannesian, D. Carralero, D. M. Ota, K. R. Cleary, G. L. Nicolson and T. Irimura, Carbohydr. Res., 1991, 213, 47–57 CrossRef CAS.
- R. Lotan, H. Ito, W. Yasui, H. Yokozaki, D. Lotan and E. Tahara, Int. J. Cancer, 1994, 56, 474–480 CrossRef CAS PubMed.
- C. K. Song, S. H. Jung, D.-D. Kim, K.-S. Jeong, B. C. Shin and H. Seong, Int. J. Pharm., 2009, 380, 161–169 CrossRef CAS PubMed.
- S. Okabe, M. Suganuma, Y. Tada, Y. Ochiai, E. Sueoka, H. Kohya, A. Shibata, M. Takahashi, M. Mizutani and T. Matsuzaki, Cancer Sci., 1999, 90, 669–676 CrossRef CAS PubMed.
- J. Park, L. H. Rader, G. B. Thomas, E. J. Danoff, D. S. English and P. DeShong, Soft Matter, 2008, 4, 1916–1921 RSC.
- N. Ahmad, R. Ramsch, J. Esquena, C. Solans, H. A. Tajuddin and R. Hashim, Langmuir, 2012, 28, 2395–2403 CrossRef CAS PubMed.
- A. Bangham, Annu. Rev. Biochem., 1972, 41, 753–776 CrossRef CAS PubMed.
- I. F. Uchegbu and S. P. Vyas, Int. J. Pharm., 1998, 172, 33–70 CrossRef CAS.
- K. Rege and I. L. Medintz, in Methods in Bioengineering: Nanoscale Bioengineering and Nanomedicine, ed. K. Rege and I. L. Medintz, Artech House, 2009, p. 212 Search PubMed.
- J. Weinstein, S. Yoshikami, P. Henkart, R. Blumenthal and W. Hagins, Science, 1977, 195, 489–492 CAS.
- D. R. James, A. Siemiarczuk and W. R. Ware, Rev. Sci. Instrum., 1992, 63, 1710–1716 CrossRef CAS PubMed.
- A. Manosroi, P. Wongtrakul, J. Manosroi, H. Sakai, F. Sugawara, M. Yuasa and M. Abe, Colloids Surf., B, 2003, 30, 129–138 CrossRef CAS.
- R. A. Parente and B. R. Lentz, Biochemistry, 1984, 23, 2353–2362 CrossRef CAS.
- A. J. O'Lenick Jr, J. Surfactants Deterg., 2001, 4, 311–315 CrossRef PubMed.
- J. Pencer, G. F. White and F. R. Hallett, Biophys. J., 2001, 81, 2716–2728 CrossRef CAS.
- H. Naito, M. Okuda and O.-Y. Zhong-Can, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1996, 54, 2816 CrossRef.
- J. K. Dunnick, R. F. Kallman and J. P. Kriss, Biochem. Biophys. Res. Commun., 1976, 73, 619–624 CrossRef CAS.
- T. Li and S. Takeoka, Int. J. Nanomed., 2013, 8, 3855–3866 Search PubMed.
- S. Vrignaud, J.-P. Benoit and P. Saulnier, Biomaterials, 2011, 32, 8593–8604 CrossRef CAS PubMed.
- J. P. Tardivo, A. Del Giglio, C. S. de Oliveira, D. S. Gabrielli, H. C. Junqueira, D. B. Tada, D. Severino, R. de Fátima Turchiello and M. S. Baptista, Photodiagn. Photodyn. Ther., 2005, 2, 175–191 CrossRef CAS.
- Y. Chen, W. Zheng, Y. Li, J. Zhong, J. Ji and P. Shen, Cancer Sci., 2008, 99, 2019–2027 CAS.
- T. Ohsawa, H. Miura and K. Harada, Chem. Pharm. Bull., 1984, 32, 2442–2445 CrossRef CAS.
- S. Sur, A. C. Fries, K. W. Kinzler, S. Zhou and B. Vogelstein, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 2283–2288 CrossRef CAS PubMed.
- V. Gupta, N. Gupta, I. H. Shaik, R. Mehvar, I. F. McMurtry, M. Oka, E. Nozik-Grayck, M. Komatsu and F. Ahsan, J. Controlled Release, 2013, 167, 189–199 CrossRef CAS PubMed.
- N. I. Zahid, O. K. Abou-Zied and R. Hashim, J. Phys. Chem. C, 2013, 117, 26636–26643 CAS.
- O. K. Abou-Zied, N. I. Zahid, M. F. Khyasudeen, D. S. Giera, J. C. Thimm and R. Hashim, Sci. Rep., 2015, 5(8699), 1–9 Search PubMed.
- J. E. Hansen, E. Pines and G. R. Fleming, J. Phys. Chem., 1992, 96, 6904–6910 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |