The tolerance of growth and clonal propagation of Phragmites australis (common reeds) subjected to lead contamination under elevated CO2 conditions
Na Zhang†
a,
Jixiang Lin†b,
Yuheng Yanga,
Zhuolin Lic,
Ying Wangd,
Luyao Chenga,
Yujie Shia,
Yuting Zhanga,
Junfeng Wanga and
Chunsheng Mu*a
aKey Laboratory of Vegetation Ecology, Ministry of Education, Institute of Grassland Science, Northeast Normal University, Changchun 130024, China. E-mail: mucs821@nenu.edu.cn; Fax: +86 431 85687517; Tel: +86 431 85098113
bKey Laboratory of Saline-Alkali Vegetation Ecology Restoration in Oil Field Ministry of Education, Alkali Soil Nature Environmental Science Center, Northeast Forestry University, Harbin 150040, China
cDepartment of Environmental Science, East China Normal University, Shanghai 200241, China
dKey Laboratory of Songliao Aquatic Environment, Ministry of Education, Jilin Jianzhu University, Changchun 130118, China
First published on 16th June 2015
Abstract
Phragmites australis is a rhizomatous perennial plant with extensive distribution and tolerance. To explore plant growth and clonal propagative tolerance to lead contamination under elevated CO2, they were exposed to combinations of five Pb levels (0, 300, 500, 1500, 3000 mg kg−1) and two CO2 concentrations (380 ± 20 and 760 ± 20 μmol mol−1) in phytotron. Biomass, photosynthetic parameters and rhizome growth were significantly inhibited, while number of axillary shoot buds and daughter apical rhizome shoots were increased by Pb additions. ∼80% of daughter shoots was from daughter axillary shoots, representing a phalanx growth pattern. Under elevated CO2, photosynthetic parameters (excluding stomatal conductance and transpiration rate), growth of clonal modules were increased, facilitating plant biomass accumulation, phalanx growth and spreading strategy. The results suggest that elevated CO2 might improve growth and clonal propagative resistance to Pb contamination through increasing photosynthetic, phalanx growth and population expansion of Phrgagmites australis.
Introduction
Due to deforestation and sustained use of fossil fuels, the concentration of atmospheric CO2 has increased from pre-industrial levels of 280 ppm to approximately 380 ppm, and is predicted to be possibly doubled by the end of the 21st century.1,2 Elevated CO2 generally causes reduction in stomatal conductance (gs) and transpiration rate (E), but increases water use efficiency (WUE) and net photosynthetic rate (Pn).3–5 However, it has been ambiguous about the results regarding effects of elevated CO2 on plant biomass allocation. Numerous researches have demonstrated that elevated CO2 leads to increased photosynthetic production allocation to roots by increase in branched root systems, which may stimulate water and nutrient absorption by plant.6–8 Inversely, some scholars have suggested that elevated CO2 promotes biomass accumulation in stems and leaves instead of root systems.9In recent decades, heavy metals contaminations have been a serious problem around the world. The levels of heavy metal contaminations in soils range from trace to as high as 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1.10 Among the heavy metal-contaminated soils, lead (Pb) is one of the most toxic ones and its phytotoxicity may cause a wide range of adverse effects on the plant growth and physiology. Photosynthesis is considered as one of the most sensitive metabolic processes to Pb toxicity. Substantial literatures have shown that the reasons for inhibitory effects of Pb on photosynthesis include stamotal closure, damaged chloroplast ultrastructural organization, restrained synthesis of chlorophyll, obstructed electron transport, and inhibited activities of Calvin cycle enzymes.11,12 Such changes of key processes may eventually lead to an inhibition in plant growth and biomass production.13 It is noteworthy that, under global elevated CO2 scenario, the effects of heavy metals on the plant physiology, growth and development may alter. Elevated CO2 has been shown to alleviate the adverse damage induced by metals through increasing antioxidant enzyme activity and photosynthesis, which increases biomass accumulation.14–16 Biomass accumulation exhibits plant growth, but biomass allocation is an important strategy that is used to maintain and extend plant population and to fight against stress or bad environment.17–19 Therefore, it is necessary to focus on biomass allocation of plants subjected to heavy metals under atmosphere elevated CO2 scenario, and on what the causes of that biomass changes could be.
000 mg kg−1.10 Among the heavy metal-contaminated soils, lead (Pb) is one of the most toxic ones and its phytotoxicity may cause a wide range of adverse effects on the plant growth and physiology. Photosynthesis is considered as one of the most sensitive metabolic processes to Pb toxicity. Substantial literatures have shown that the reasons for inhibitory effects of Pb on photosynthesis include stamotal closure, damaged chloroplast ultrastructural organization, restrained synthesis of chlorophyll, obstructed electron transport, and inhibited activities of Calvin cycle enzymes.11,12 Such changes of key processes may eventually lead to an inhibition in plant growth and biomass production.13 It is noteworthy that, under global elevated CO2 scenario, the effects of heavy metals on the plant physiology, growth and development may alter. Elevated CO2 has been shown to alleviate the adverse damage induced by metals through increasing antioxidant enzyme activity and photosynthesis, which increases biomass accumulation.14–16 Biomass accumulation exhibits plant growth, but biomass allocation is an important strategy that is used to maintain and extend plant population and to fight against stress or bad environment.17–19 Therefore, it is necessary to focus on biomass allocation of plants subjected to heavy metals under atmosphere elevated CO2 scenario, and on what the causes of that biomass changes could be.
The above-ground shoots origin from below-ground bud bank for perennial plants that have predominantly clonal reproduction. The best strategy to resist various disturbances is that they can produce more ramets (daughter shoots) through clonal propagation or below-ground bud bank to increase plant productivity.20–22 Zhang et al. (2015)23 regarded that the Phragmites australis in well-watered environment had stronger clonal propagative ability, exhibiting in more number of buds and daughter shoots. Several scholars have studied effects of elevated CO2 on the plant clonal growth, and they found that elevated CO2 improved vegetative propagative ability through enhancing rhizome elongation and growth of tiller ramets.24,25 However, lack research attention to data that have focused on the combined impacts of elevated CO2 and heavy metals on below-ground buds or clonal propagation of perennial plants.
In addition, the heavy metal accumulation in plant organs might be altered by atmosphere elevated CO2. Several literatures have demonstrated that elevated CO2 has stimulatory effects on heavy metal accumulation.26,27 Some recent researches however, have documented that elevated CO2 has no effect or reduce heavy metal uptake by plants.14,26 It is so far not agreement on the effects of elevated CO2 on heavy metal accumulation of plants.
Phragmites australis is a typical rhizomatous perennial plant with high biomass production and phytoremediation ability of heavy metal.28,29 Its population expansion mainly depends on clonal propagation (e.g. vegetation tillering and rhizome spread), because seeding establishment occurs rarely in the field.30 It remains unclear and has not been reported so far that how P. australis grown in Pb contaminated soil will respond to elevated CO2 in term of biomass allocation, photosynthesis and clonal reproduction. In addition, for soils with pH > 6.5, the toxicity threshold of soil Pb is 500 mg kg−1, according to the environmental quality standard for soil, GB15618-1995, issued by the State Environmental Protection Administration of China. Typically, each plant species' tolerance of Pb can be best assessed with toxicity assays across a range of concentrations.31 Thus, we designed pot experiments to imitate Pb contamination with a gradient of five Pb concentrations (0, 500, 1000, 1500 and 3000 mg kg−1), and two CO2 concentrations (380 ± 20 and 760 ± 20 μmol mol−1) in artificial climate chambers. The objective of present study is to investigate the tolerance of growth and clonal propagation of P. australis exposed to Pb contamination under elevated CO2, measuring the biomass, photosynthesis and clonal propagation parameters as well as Pb accumulation in organs. We hypothesized that (i) elevated CO2 might promote photosynthesis and change biomass allocation on organs of P. australis subjected to Pb contamination; (ii) to adapt to Pb contaminated environment, some clonal reproduction strategy might be adopted by P. australis under elevated CO2 condition (iii) elevated CO2 might change the Pb translocation or allocation to organs.
Materials and methods
Preparation of Pb contaminated soils
Soil for use in the pot experiment was collected from the surface layer (0–20 cm) in grassland near the Northeast Normal University field experiment station, located in Changling County, Jilin Province, China (123° 44′E, 44° 44′N, 167 m a.s.l.). Its total nitrogen (N) was 6.9%, organic carbon was 0.4%, pH was 8.6, electric conductivity was 91 μs cm−1, field capacity was 200 g kg−1, and Pb concentration was 5.9 mg kg−1. The fresh, collected soil was mixed homogeneously and allowed to air dry. It was then passed through a 1 mm sieve, after which it was divided into 3 kg subsamples. Specified concentrations of Pb (NO3)2 solution (520 ml) were added and thoroughly mixed into the soil subsamples to obtain five levels of Pb contamination: 0, 300, 500, 1500, 3000 mg Pb kg−1. The relative water content of the spiked soils reached precisely 70% of field capacity. The contaminated soil subsamples were transferred into plastic pots (16 cm diameter × 14 cm height). All the soil-filled pots were placed in a room with ventilation in darkness for 45 days (from June 1 to July 15, 2013). At the time, a small amount of spiked soils was sampled randomly and analyzed for Pb concentrations. The concentrations of Pb in the artificially contaminated soils was 5.9 ± 0.2, 304 ± 4.38, 508 ± 7.89, 1513 ± 37.28, 3020 ± 120.41 mg kg−1, respectively, which was very close to the five targeted levels of added Pb.Plant cultures and treatments
Seeds of P. australis were collected from mature wild plants in a wetland, where is located west of Changchun city, Jilin province, China (125° 1′E, 43° 56′N, 188 m a.s.l.). Seeds were sown in plastic tanks (80 × 50 × 30 cm) that contained 20 cm of humus soil, which was watered daily to keep it moist. The tanks were housed in a greenhouse at 14–24 °C, on the campus of Northeast Normal University, Jilin City, China (43° 51′N, 125° 19′E, 236 m a.s.l.). After 50 days had elapsed since sowing (from May 26 to July 15, 2013), uniform plants ∼8 cm high, with 3 or 4 leaves, were transplanted into the pots, and then placed in the artificial climate chamber (LT/ACR-2002 Phytotron System, E-Sheng Tech., Beijing, China). Knowing that some weak seedlings were unlikely to survive being transplanted, we initially placed twenty seedlings into each pot, and allowed them to grow for 5 days under weak light. Then, the weakest plants were removed to keep 10 strong seedlings in each pot.These pots with 10 strong seedlings were placed randomly and equally in two phytotrons. There were five levels of Pb treatments for a total of 30 pots (6 replicated pots per treatment) per phytotron. One phytotron was maintained ambient CO2 at 380 ± 20 μmol mol−1. The other phytotron was assigned to a doubled level at 760 ± 20 μmol mol−1. The CO2 was supplied from a steel can and delivered through 0.64 cm tubing, and the concentrations were monitored every 5 s and adjusted every 10 s for the whole day. The light and temperature regimes in the phytotrons were set according to the diurnal/nocturnal periods and temperature changes of June to September in Northeast China.32 The high-stress sodium lamps (Philips) with photosynthetically active radiation provided light at a rate of 500 mmol m−2 s−1 from 5:30–19:30 for 14 h per day, and they were shut in other time. The relative humidity was maintained at 40–60% in the phytotrons. The temperature regimes were 22 °C from 5:30–8:30, 25 °C from 8:30–11:30, 28 °C from 11:30–14:30, 25 °C from 14:30–17:30, 22 °C 17:30–19:30 and 18 °C from 19:30–5:30. Air temperature in each chamber was monitored and adjusted every 10 s for 24 h a day, and maintained within ±1 °C of set points. The pots were watered to 3.42–3.48 kg using an electronic scale (KaiFeng group co. Ltd, ACS-30, China) at 5:00 pm each day, to maintain the soil water content at 70–80% of field capacity. In order to ensure that each plant experienced similar light conditions, the pot positions were randomly changed every 2 d in the same phytotron during the treatment period. Furthermore, since there were no chamber replicates in this study, we switched the pots between the two chambers every 2 weeks, changing the environmental settings so that all pots were undergo as similarly as possible during the experiment. The total time of CO2 enrichment was 60 days (from July 20 to September 19, 2013).
Determination of photosynthetic parameters
Before sampling, photosynthetic parameters were measured with an LI-6400 gas exchange system (LI-6400XT, Li-Cor, Inc., Lincoln, NE, USA) on the youngest available fully expanded leaves (3 leaves per pot, 6 pots per treatment). The net photosynthetic rate (Pn), intercellular CO2 concentration (Ci), stomatal conductance (gs) and transpiration rate (E) were determined under ambient CO2 (380 μmol mol−1) and elevated CO2 (760 μmol mol−1) under a light intensities of 500 μmol m−2 s−1 to equal the light of the phytotron. Leaves were allowed to acclimate for a few minutes until the Pn stabilized and the coefficient of variation was below 0.5. The water use efficiency (WUE) was calculated from the ratios of Pn to E.Classification of below-ground buds and above-ground daughter shoots
In this study, plants developed from seeds were defined as “parent shoots”, and those that resprouted from buds of rhizomes or basal nodes of parent shoots were considered as “daughter shoots”.As proposed by Zhang et al. (2009),33 the three categories of below-ground buds were (i) axillary shoot buds, (ii) axillary rhizome buds, and (iii) apical rhizome buds; the three categories of daughter shoots were (i) daughter axillary shoots, (ii) daughter axillary rhizome shoots, and (iii) daughter apical rhizome shoots. The axillary shoot bud at a basal node of a shoot can grow upwards to form a daughter axillary shoot, or horizontally into a rhizome. The axillary rhizome buds attached to rhizome nodes grow upwards to form daughter axillary rhizome shoots, or horizontally into rhizomes. The apical rhizome buds originating from the end of a rhizome grow upwards to form a daughter apical rhizome shoot, or continue to horizontally extend the rhizome.
Measurement of clonal parameters and biomasses
Each plant per pot was separated from the contaminated soil along with its root systems. We then immediately counted the number of each type of bud >1 mm in length, the number of each type of daughter shoot and the number of rhizomes, and measured the total length of rhizomes. The rhizome length and number of buds, daughter shoots and rhizomes were expressed per parent shoot. Finally, each plant was washed gently with tap water, three times with deionized water, and then divided into leaves, stems and root systems (including root and rhizome) before being oven-dried at 75 °C to a constant weight. The dry weights were measured, and the dried tissues then were ground into fine powder by ball mill (Retsch, MM400, Germany) for Pb concentration measurement.Measurement of Pb concentration
The homogeneous soil samples and plant powder (0.15 g) were digested in a microwave oven (ANALYX, CEM Mars5, USA) with solution of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 HNO3
1 HNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HF and HNO3
HF and HNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCLO4 (v/v) for 120 minutes respectively until clear and transparent liquid. Then, they were continued to digest in electronic heating digestion apparatus (LabTech, DigiBlock ED36, USA) until approximate 0.5 cm height of solutions under high temperature 180 °C. The cooled solutions were added up to 50 ml of total volume respectively. Concentrations of Pb were determined using a Graphite Furnace Atomic Absorption Spectrometer (Varian, SpectrAA Z220, USA). The detection limits for Pb are from 10–100 ppm, and some extracts were further diluted for determination of Pb concentrations within the detection limits.
HCLO4 (v/v) for 120 minutes respectively until clear and transparent liquid. Then, they were continued to digest in electronic heating digestion apparatus (LabTech, DigiBlock ED36, USA) until approximate 0.5 cm height of solutions under high temperature 180 °C. The cooled solutions were added up to 50 ml of total volume respectively. Concentrations of Pb were determined using a Graphite Furnace Atomic Absorption Spectrometer (Varian, SpectrAA Z220, USA). The detection limits for Pb are from 10–100 ppm, and some extracts were further diluted for determination of Pb concentrations within the detection limits.
Statistical analysis
We used two-ways ANOVA to evaluate the response of plants to Pb, CO2 concentrations and their interaction. Data were analyzed as a split-plot design with CO2 treatments being the main plot and Pb concentrations being the subplot. As the two-ways ANOVA showed significant effects of Pb and CO2 treatments, a separate ANOVA was used to evaluate the effects of Pb under the same CO2 level, with Pb as a fixed factor. An independent sample T-test was used to evaluate the effect of CO2 on these parameters under the same Pb level, using CO2 levels as a grouping variable.Before the ANOVA was conducted, data were checked for their normality and homogeneity of variance, and were squared root-transformed as necessary to meet those assumptions. Multiple comparisons of means were performed using LSD tests at α = 0.05 when ANOVA results were significant. All statistical analyses were performed with the SPSS v.13.0 statistical package (SPSS Inc., Chicago, USA), and figures were plotted with SIGMAPLOT 11.0 (Systat Software, Inc., San Jose, CA, USA).
Results
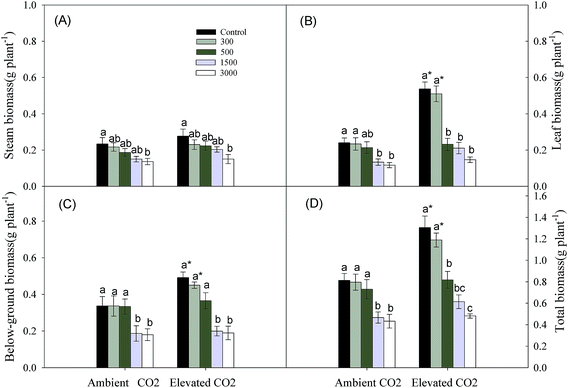
Biomasses in organs
Leaf, below-ground and total biomasses were affected significantly by CO2 and Pb concentrations, and their interaction affected total biomass significantly (Table 1). Increasing soil Pb concentrations caused a continuous reduction in the stem, leaf and below-ground biomasses and total biomass (Fig. 1). Elevated CO2 increased biomasses of different organs resulting in an increase in total biomass compared to ambient CO2 control (Fig. 1).| CO2 | Pb | CO2 × Pb | ||||
|---|---|---|---|---|---|---|
| F-value | P | F-value | P | F-value | P | |
| a Note: * = significance at P ≤ 0.05; ** = significance at P ≤ 0.01; *** = significance at P ≤ 0.001; ns = no significance. | ||||||
| Biomass | ||||||
| Stem | 1.66 | 0.21ns | 2.69 | 0.05* | 0.13 | 0.97ns |
| Leaf | 11.03 | 0.00*** | 6.37 | 0.00*** | 2.73 | 0.06ns |
| Below-ground | 3.73 | 0.02* | 9.74 | 0.00*** | 0.74 | 0.57ns |
| Total | 18.30 | 0.00*** | 19.83 | 0.00*** | 3.14 | 0.04* |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Photosynthetic parameters | ||||||
| Pn | 108.60 | 0.00*** | 87.79 | 0.00*** | 1.98 | 0.14ns |
| gs | 10.39 | 0.00*** | 2.44 | 0.04* | 0.17 | 0.95ns |
| Ci | 249.20 | 0.00*** | 39.66 | 0.00*** | 6.54 | 0.00*** |
| E | 158.81 | 0.00*** | 3.79 | 0.02* | 0.10 | 0.98ns |
| WUE | 139.20 | 0.00*** | 5.19 | 0.00*** | 0.70 | 0.60ns |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| No. of buds and daughter shoots | ||||||
| Axillary shoot buds | 24.76 | 0.00*** | 8.80 | 0.00*** | 1.64 | 0.20ns |
| Axillary rhizome buds | 9.02 | 0.01* | 8.85 | 0.00*** | 1.65 | 0.20ns |
| Apical rhizome buds | 3.30 | 0.08ns | 27.51 | 0.00*** | 0.18 | 0.95ns |
| Total | 19.53 | 0.00*** | 25.94 | 0.00*** | 1.90 | 0.15ns |
| Daughter axillary shoots | 0.04 | 0.84ns | 1.26 | 0.32ns | 0.50 | 0.74ns |
| Daughter apical rhizome shoots | 1.33 | 0.26ns | 5.90 | 0.00*** | 0.26 | 0.90ns |
| Total | 0.49 | 0.49ns | 1.62 | 0.21ns | 0.34 | 0.85ns |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Rhizome | ||||||
| No. of rhizomes | 4.75 | 0.04* | 15.29 | 0.00*** | 0.36 | 0.83ns |
| Rhizome length | 17.42 | 0.00*** | 34.44 | 0.00*** | 0.77 | 0.56ns |
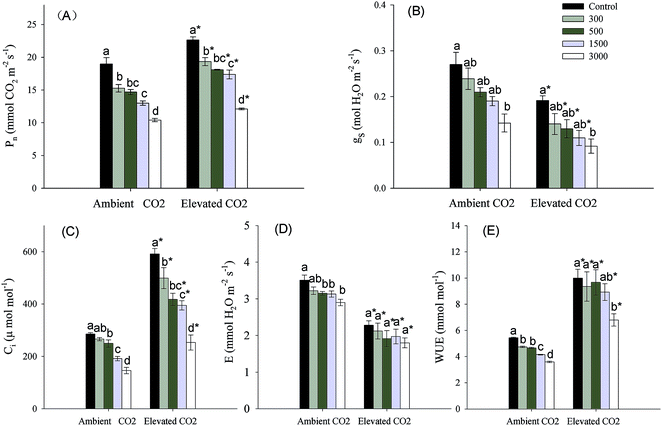
Photosynthetic parameters
CO2 and Pb concentrations had significant effects on photosynthetic parameters (Pn, gs, Ci, E) and WUE, and Pb × CO2 affected Ci significantly (Table 1). At each CO2 level, Pn, gs, Ci, E, WUE were significantly reduced by Pb treatments (Fig. 2). When compared with the ambient CO2 control, elevated CO2 increased Pn, Ci and WUE significantly, but decreased gs, and E significantly at the same Pb level. The increased or reduced percentages tended to drop with increasing Pb concentrations in soils (Fig. 2).Clonal parameters
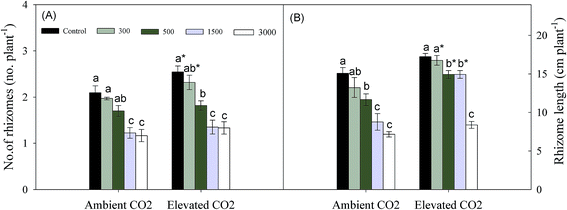
CO2 and Pb concentrations showed remarkable effects on number of axillary shoot buds, axillary rhizome buds and total number of buds as well as number and length of rhizomes (Table 1). We observed different responses of various types of buds and daughter shoots to Pb additions. Number of axillary shoot buds was increased firstly, then decreased along with increasing soil Pb contamination, and was still greater at highest Pb level than control. The similar changes occurred in number of daughter axillary shoots, causing that its proportions (in total number of daughter shoots) were increased by Pb additions. Increasing soil Pb concentrations caused a continuous increase in daughter apical rhizome shoots, but decreased the number of axillary and apical rhizome buds and total number of buds significantly (Table 2). There was no daughter axillary rhizome shoots that originated from axillary rhizome buds, but daughter axillary shoots accounted for ∼80% of total number of daughter shoots at each Pb level. Elevated CO2 triggered an increase in number of each type of buds and daughter shoots, especially for axillary shoot buds of plants grown in higher Pb concentrations in soils and for axillary rhizome buds of plants grown in lower Pb treatments, resulting in a significant increase in total number of buds (Table 2).| Pb concentration(mg kg−1) | |||||
|---|---|---|---|---|---|
| No. of buds and daughter shoots (no. plant−1) | Control | 300 | 500 | 1500 | 3000 |
| a Different letters indicate significant differences (P ≤ 0.05) between different Pb levels (within CO2 one level), and an asterisk indicates significant difference (P ≤ 0.05) between elevated CO2 and ambient CO2 control (within one Pb level). | |||||
| Ambient CO2 | |||||
| Axillary shoot buds | 0.29 ± 0.06b | 0.36 ± 0.07ab | 0.43 ± 0.03ab | 0.51 ± 0.07a | 0.30 ± 0.06b |
| Axillary rhizome buds | 2.97 ± 0.19a | 2.57 ± 0.32a | 1.73 ± 0.20b | 1.42 ± 0.19b | 1.33 ± 0.29b |
| Apical rhizome buds | 1.87 ± 0.24a | 1.57 ± 0.04ab | 1.20 ± 0.20b | 0.72 ± 0.14bc | 0.53 ± 0.07c |
| Total | 5.12 ± 0.28a | 4.49 ± 0.28a | 3.37 ± 0.13b | 2.66 ± 0.06bc | 2.07 ± 0.18c |
| Daughter axillary shoots | 0.80 ± 0.15a | 1.17 ± 0.18a | 1.32 ± 0.12a | 1.19 ± 0.15a | 1.04 ± 0.10a |
| Daughter apical rhizome shoots | 0.23 ± 0.04b | 0.40 ± 0.06ab | 0.50 ± 0.12ab | 0.50 ± 0.06ab | 0.63 ± 0.07a |
| Total | 1.39 ± 0.20b | 1.70 ± 0.00ab | 1.80 ± 0.12a | 1.69 ± 0.12ab | 1.43 ± 0.09ab |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Elevated CO2 | |||||
| Axillary shoot buds | 0.38 ± 0.05b | 0.43 ± 0.12b | 0.73 ± 0.09a* | 0.87 ± 0.09a* | 0.57 ± 0.09b* |
| Axillary rhizome buds | 3.60 ± 0.55a* | 3.41 ± 0.38a* | 3.20 ± 0.55a* | 1.97 ± 0.52ab | 1.40 ± 0.29b |
| Apical rhizome buds | 2.20 ± 0.20a | 1.81 ± 0.23ab | 1.32 ± 0.06b | 0.84 ± 0.15bc | 0.67 ± 0.15c |
| Total | 6.18 ± 0.43a* | 5.65 ± 0.38a* | 5.25 ± 0.55a* | 3.67 ± 0.72b | 2.30 ± 0.31b |
| Daughter axillary shoots | 1.10 ± 0.22a | 1.30 ± 0.06a | 1.30 ± 0.21a | 1.25 ± 0.28a | 1.17 ± 0.20a |
| Daughter apical rhizome shoots | 0.34 ± 0.08b | 0.51 ± 0.11ab | 0.52 ± 0.06ab | 0.54 ± 0.06ab | 0.67 ± 0.09a |
| Total | 1.39 ± 0.17a | 1.68 ± 0.27a | 1.82 ± 0.06a | 1.76 ± 0.26a | 1.77 ± 0.20a |
At each CO2 level, the number and length of rhizomes were significantly decreased with increasing soil Pb concentrations. At lower levels of Pb contaminations, elevated CO2 increased number of rhizomes significantly (Fig. 3a). Rhizome length was increased significantly by elevated CO2 at each Pb (<3000 mg kg−1) level, and the increases were promoted with increasing Pb concentration (Fig. 3b).
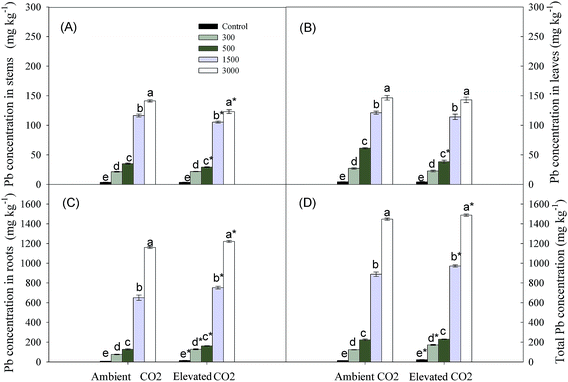
Pb concentrations in organs
Under either ambient or elevated CO2, Pb concentrations in stems, leaves and root systems were significantly increased due to Pb additions in soils, particularly at Pb levels of 1500 and 3000 mg kg−1. The Pb concentration in root systems was greater than that in stems and leaves at the same level of Pb treatments (Fig. 4). Elevated CO2 triggered a vast amount of Pb accumulation in root systems, but its transportation to above-ground organs was inhibited (Fig. 4).Discussion
A large body of literatures has shown that elevated CO2 increases Ci, WUE and Pn significantly,3–5 while the presence of Pb has the reverse effects.11,12 These were similar to our results (Fig. 2a, c and e). The increasing Pn means that elevated CO2 enhances the capacity of carbon assimilation,34 which might cause a significant increasing biomass accumulation of leaves where are main photosynthetic organ at lower levels of Pb concentrations (Fig. 1b). Simultaneously, the increasing Pn due to elevated CO2 also promoted photosynthetic production allocation to below-ground parts (Fig. 1c), which might enhance resources uptake and storage in plants. It is because that plants mainly obtain water and nutrient through roots, and rhizomes are considered to be main storage organs.5,24,35 We also found that the increase in photosynthesis due to relative higher Ci could offset the decreasing carbon assimilation caused by reduced gs (Fig. 2b and d).36 In addition, the decreased percentages of gs were smaller at higher Pb levels than at lower Pb levels under elevated CO2 condition (Fig. 2b). This might be because both elevated CO2 and Pb concentrations caused a reduction in gs to limit E, and elevated CO2 had a minimum effect on gs at higher Pb levels in order to maintain photosynthesis. We can conclude that elevated CO2 could improve plant growth resistance to Pb contaminated environments through biomass allocation to photosynthetic and below-ground resource absorbing organs (i.e. leaves and root systems) due to increasing photosynthesis.For perennial plants, below-ground bud bank may be a main source for vegetative propagation maintaining population maintenance, because germination and population establishment from seeds happen rarely.20,37,38 Meanwhile, the ability of bud emergence is also a main factor that might influence above-ground population density and productivity.39 The research of Zhang et al. (2015)23 has indicated that higher clonal propagative ability plays a critical role in maintaining population stability and expansion of P. australis exposed to Pb stress under well-watered condition. Only a few of scholars has reported effects of elevated CO2 on plant clonal growth. They regarded that elevated CO2 might improve clonal propagative ability through increasing rhizome length and daughter shoot growth.24,25 Clonal modules (e.g. below-ground bud bank) and clonal propagation of perennial plants subjected to heavy metal stress might be affected partly by global elevated CO2. However, there is no report regarding the combined effects of elevated CO2 and heavy metal contamination on below-ground bud bank or clonal reproduction of perennial plants. Previous and our present researches observed that daughter axillary shoots were the main source of above-ground population density.33 We also observed that the proportions of daughter axillary shoots (in total number of daughter shoots) were increased by Pb additions at the same CO2 level (Table 2), which exhibited a phalanx growth pattern.40 The daughter axillary shoots (tiller-based ramets) contribute more to population maintenance for rhizomatous clonal plants.41,42 According to the cost-benefit hypothesis,43 as axillary shoot buds are attached to basal nodes of stem, their emergence into daughter shoots may incur lower cost.44 In contrast, not only do plants supply energy to rhizome elongation and development of rhizome buds from the deep soil layer, but also their high rate of respiration should deplete much of their available energy under anoxic conditions, giving rise to higher cost when rhizome buds emerge from the soil surface.44,45 Therefore, a propagative strategy developed by P. australis, which has dominated clonal propagation, was that they produced more axillary shoot buds and daughter axillary shoots (i.e. phalanx growth form) which incurs lowest coast, in order to maintain population stability under Pb contamination condition. Meanwhile, the increased number of axillary shoot buds due to Pb contamination would contribute more to phalanx growth form during the fowling year under elevated CO2 condition, because each bud can potentially emerge into daughter shoots.46 We also found that the number of daughter apical rhizome shoots tended to increase with increasing soil Pb contamination, particularly at highest Pb level in both ambient and elevate CO2 environment (Table 2). Meanwhile, the rhizome length was significantly increased by elevated CO2 at each Pb level (Fig. 3b). The rhizome-based ramets (e.g. daughter apical rhizome shoots) contribute more to plant population expansion.41,42 The spreading rhizome might provide more space and resources that could be mobilized for bud emergence and regrowth of daughter shoots.47 So, the expanding strategy was also adopted by P. australis to adapt to Pb contamination, especially under elevated CO2 condition. In addition, we also discovered that there was no daughter axillary rhizome shoots that originated from axillary rhizome buds, and similar results were also obtained in previous researches.23,48 We can conclude that axillary rhizome buds mainly grew horizontally into rhizomes in order to continue their expansion, rather than grew upwards to form daughter shoots. This may be related to biological characteristics of P. australis; the growth space must first be expanded, before the number of daughter rhizome shoots can increase.49 In short, P. australis had higher tolerance of clonal propagation to cope with Pb contamination by the phalanx growth and spreading strategy, particularly in elevated CO2 environment.
Pb accumulation in plants was enhanced with increasing soil Pb concentrations, and the majority of absorbed Pb was retained in below-ground part of P. australis (Fig. 4). These results were in line with findings of previous researches.28,50–52 Although a large amount of Pb was accumulated in below-ground organs of P. australis, below-ground biomass accumulations were still increased by elevated CO2 at each Pb level (Fig. 3c and 4c). This indicated that below-ground organs had stronger tolerance under elevated CO2 condition. In addition, elevated CO2 inhibited Pb translocation to above-ground stems and leaves (Fig. 4a and b). The above-ground shoots are considered as the important parts where plants conduct photosynthesis and other metabolisms. Therefore, the Pb allocation form might protect photosynthetic tissues and promote photosynthesis, improving biomass accumulation under elevated CO2 condition. The Pb allocation strategy can be considered as an adaptive mechanism of plants responded to Pb contamination under elevated CO2.
Previous work has shown that increasing heavy metal uptake in roots under elevated CO2 condition is correlated to high metals bioavailability. The high bioavailability is attributed to the decreasing soil pH caused by greater root exudation of carbonic acid, and to the increasing dissolved organic carbon (DOC) concentration released from plant roots due to elevated CO2;16,27,53 More amounts of heavy metals are released from sediments and soil both through DOC–metal complexation reaction and through reducing absorption of heavy metals into soil organic matter as well as clay mineral particles due to reduced pH.54,55 These two processes account for increasing metals bioavailability leading to more heavy metals uptake by root system under elevated CO2 condition. Furthermore, Guo et al. (2011)26 regarded that elevated CO2 increased Cd uptake by rice and wheat, but reduced Cu accumulation. Conversely, several scholars suggested that Cd concentrations in Lolium mutiforum and Lolium perenne were reduced due to elevated CO2.14 Li et al. (2010)56 discovered that various Cd accumulation patterns occurred in different varieties of rice, and they ascribed this variation to difference in exudation rates and spectrum of organic acids released by plants. We concluded that the inconsistency in heavy metal absorption or accumulation under elevated CO2 condition might be closely related to various factors, including plant species and growth media, types of metals and their concentrations in soils, as well as the composition and quantity of plant exudates, etc.
In short, a propagative strategy adopted by P. australis to resist high Pb contaminations was that they could develop phalanx growth pattern through increasing formation and output of axillary shoot buds which incurs lowest coast to maintain population stability. Elevated CO2 might enhance clonal propagation and space expansion of P. australis population in Pb contaminated environment through increasing phalanx growth and spreading strategy. Meanwhile, the inhibiting Pb translocation to photosynthetic organs could be beneficial to photosynthesis, promoting biomass allocation to leaves and root system under elevated CO2 condition.
Conclusions
The phalanx growth form would help P. australis resist to Pb contamination. Elevated CO2 tended to increase photosynthesis, leading to increasing biomass of leaves and root systems. Elevated CO2 also stimulated increase in number of various types of buds and daughter shoots, enhancing clonal propagative ability of P. australis grown in Pb contaminated soils. Clearly, elevated CO2 could ameliorate Pb toxicity and improve resistance capacity of P. australis to Pb contamination. This study would help us better understanding how rhizomatous perennial plants respond to heavy metal contaminations and climate changes. To the best of our knowledge, our experiment represents the first study of the combined effects of elevated CO2 and Pb stress on clonal propagation of P. australis.Acknowledgements
The research was funded by the National “973” Programme of China (grant no. 2015CB150801). We sincerely acknowledge the technical assistance of Jinwei Zhang, Yu Zheng and Dafu Yu in the laboratory.References
- J. Hansen, M. Sato, P. Kharecha, G. Russell, D. W. Lea and M. Siddal, Philos. Trans. R. Soc., A, 2007, 365, 1925–1954 CrossRef CAS PubMed.
- PCC, in ed. S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marqui, K. B. Averyt, M. Tignor and H. L. Miller, Cambridge University Press, 2007, p.137 Search PubMed.
- F. Eller, C. Lambertini, L. X. Nguyen and H. Brix, Global Change Biol., 2014, 20, 531–543 CrossRef PubMed.
- B. A. Kimball, K. Kobayashi and M. Bindi, Adv. Agron., 2002, 77, 293–368 Search PubMed.
- A. L. Rogers and S. W. Humphries, Global Change Biol., 2000, 6, 1005–1011 CrossRef.
- D. G. Milchunas, J. A. Morgan, A. R. Mosier and D. R. Lecain, Global Change Biol., 2005, 11, 1837–1855 CrossRef PubMed.
- H. Poorter, K. J. Niklas, P. B. Reich, J. Oleksyn, P. Poot and H. A. Torbert, New Phytol., 2012, 193, 30–50 CrossRef CAS PubMed.
- S. A. Prior, G. B. Runion, S. C. Marble, H. H. Rogers, C. H. Gilliam and H. A. Torbert, HortScience, 2011, 46, 158–162 CAS.
- R. S. Nowak, D. S. Ellsworth and S. D. Smith, New Phytol., 2004, 162, 253–280 CrossRef PubMed.
- J. M. Blaylock, J. W. Huang, Phytoextraction of metals, John Wiley and Sons, New York, 2000, pp. 53–71 Search PubMed.
- E. Islama, D. Liu, T. Q. Li, X. E. Yang, X. F. Jin and Q. Mahmood, J. Hazard. Mater., 2008, 154, 914–926 CrossRef PubMed.
- P. Sharma and R. S. Dubey, J. Plant Physiol., 2005, 17, 35–52 CAS.
- P. C. Nagajyoti, K. D. Lee and T. V. M. Sreekanth, Environ. Chem. Lett., 2010, 8, 199–216 CrossRef CAS PubMed.
- Y. Jia, S. Tang, R. G. Wang, X. H. Ju, Y. Z. Ding, S. X. Tu and D. L. Smith, J. Hazard. Mater., 2010, 180, 384–394 CrossRef CAS PubMed.
- X. H. Ju, S. Tang, Y. Jia, J. K. Guo, Y. Z. Ding, Z. G. Song and Y. J. Zhao, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2011, 879, 1717–1724 CrossRef CAS PubMed.
- H. B. Wu, S. R. Tang, X. M. Zhang, J. K. Guo, Z. G. Song, S. Tian and D. L. Smith, J. Hazard. Mater., 2009, 170, 861–870 CrossRef CAS PubMed.
- B. Liu, Z. M. Liu, L. X. Wang and Z. N. Wang, Plant Soil, 2014, 380, 389–398 CrossRef CAS.
- N. Marbà and C. M. Duarte, Mar. Ecol.: Prog. Ser., 1998, 174, 269–280 CrossRef.
- Z. Z. Xu, L. M. Huang, X. P. Huang, A. J. Zhu and S. Zhang, Bull. Mar. Sci., 2009, 11, 53–61 Search PubMed.
- E. J. Benson and D. C. Hartnett, Plant Ecol., 2006, 187, 163–178 CrossRef.
- H. J. Dalgleish and D. C. Hartnett, New Phytol., 2006, 171, 81–89 CrossRef PubMed.
- J. F. Wang, S. Gao, J. X. Lin, Y. G. Mu and C. S. Mu, Crop Pasture Sci., 2010, 61, 670–676 CrossRef.
- N. Zhang, J. W. Zhang, Y. H. Yang, X. Y. Li, J. X. Lin, Z. L. Li, L. Y. Cheng, J. F. Wang, C. S. Mu and A. X. Wang, Plant Biol., 2015 DOI:10.1111/plb.12317.
- S. H. Kinmonth and S. H. Kim, Funct. Plant Biol., 2011, 38, 797–807 CrossRef.
- D. T. Tissue and W. C. Oechel, Ecology, 1987, 68, 401–410 CrossRef.
- H. Y. Guo, J. Z. Zhu, H. Zhou, Y. Y. Sun, Y. Yin, D. P. Pei, R. Ji, J. C. Wu and X. R. Wang, Environ. Sci. Technol., 2011, 45, 6997–7003 CrossRef CAS PubMed.
- S. Kim and H. Kang, Water, Air, Soil Pollut., 2011, 219, 365–375 CrossRef CAS.
- Z. H. Ye, A. J. M. Baker, M. H. Wong and A. J. Willis, Ann. Bot., 1997, 80, 363–370 CrossRef CAS.
- W. J. Zheng, X. P. Zheng and C. L. Zhang, Physiol. Plant., 2000, 110, 201–208 CrossRef CAS.
- B. Liu, Z. M. Liu and L. X. Wang, Ecol. Eng., 2012, 44, 344–347 CrossRef PubMed.
- L. Windham, J. S. Weis and P. Weis, Mar. Pollut. Bull., 2001, 42(10), 811–816 CrossRef CAS.
- Z. L. Li, Y. T. Zhang, D. F. Yu, N. Zhang, J. X. Lin, J. W. Zhang, J. H. Tang, J. F. Wang and C. S. Mu, PLoS One, 2014, 9(8), 1–9 Search PubMed.
- J. T. Zhang, C. S. Mu, D. L. Wang, J. F. Wang and G. X. Chen, Can. J. Bot., 2009, 87, 1242–1249 CrossRef.
- J. E. Wands, Y. G. Midgle and M. H. Jones, Global Change Biol., 1999, 5, 723–741 CrossRef.
- S. H. Gao and J. P. Guo, Caoye Kexue, 2004, 21, 23–27 Search PubMed.
- C. J. Bernacchi, P. B. Morgan, D. R. Ort and S. P. Long, Planta, 2005, 220, 434–446 CrossRef CAS PubMed.
- E. J. Benson, D. C. Hartnett and K. H. Mann, Am. J. Bot., 2004, 91, 416–421 CrossRef PubMed.
- J. Yi, Q. F. Li, A. L. Gu, Z. H. Men and X. W. He, J. Arid Land Resour. Environ., 2001, 15, 1–16 Search PubMed.
- K. Jitka and K. Leos, Perspect. Plant Ecol., 2007, 8, 115–129 CrossRef PubMed.
- L. D. Humphrey and D. A. Pyke, J. Ecol., 1998, 86, 854–865 Search PubMed.
- Z. W. Wang, A. K. Xu and T. C. Zhu, J. Plant Biol., 2008, 51, 102–107 CrossRef.
- W. M. Bai, X. Q. Sun, Z. W. Wang and L. H. Li, Plant Ecol., 2009, 205, 13–21 CrossRef PubMed.
- P. A. Vesk and M. Westoby, Oikos, 2004, 106, 200–208 CrossRef PubMed.
- J. R. Hendrickson and D. D. Briske, Oecologia, 1997, 110, 584–591 CrossRef.
- L. Klimeš, J. Klimešová and J. Osbornová, Vegetatio, 1993, 109, 153–160 CrossRef.
- J. Klimešová and L. Klimeš, Perspect. Plant Ecol., 2007, 8, 115–129 CrossRef PubMed.
- K. Prach and P. Pyšek, Folia Geobotonica, 1994, 29(2), 307–320 CrossRef.
- Y. F. Yang and H. Q. Lang, Acta Prataculture Sci., 1998, 7, 1–9 Search PubMed.
- J. Mook and J. Van der Toorn, J. Appl. Ecol., 1982, 19, 501–517 CrossRef PubMed.
- G. Bonanno, Ecotoxicol. Environ. Saf., 2011, 74, 1057–1064 CrossRef CAS PubMed.
- K. Fürtig, D. Pavelic, C. Brunold and R. Brandle, Limnologica, 1999, 29(1), 60–63 CrossRef.
- N. Hechmi, N. B. Aissa, H. Abdenaceur and N. Jedidi, Environ. Sci. Pollut. Res., 2014, 21, 1304–1313 CrossRef CAS PubMed.
- Y. Liu, Y. Li, X. Li and Y. Jiang, Waste Manage., 2008, 28, 1126–1136 CrossRef CAS PubMed.
- M. Rajkumar, M. N. V. Prasad, S. Swaminathan and H. Freitas, Environ. Int., 2013, 53, 74–86 CrossRef CAS PubMed.
- J. Yanai, F. J. Zhao, S. P. McGrath and T. Kosaki, Environ. Pollut., 2006, 139, 167–175 CrossRef CAS PubMed.
- Z. Y. Li, S. R. Tang, X. F. Deng, R. G. Wang and Z. G. Song, J. Hazard. Mater., 2010, 177, 352–361 CrossRef CAS PubMed.
Footnote |
| † Both authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |