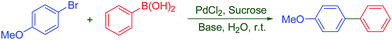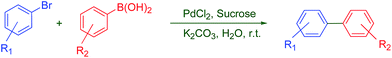‘On-water’ organic synthesis: a green, highly efficient, low cost and reusable catalyst system for biaryl synthesis under aerobic conditions at room temperature†
Bishwajit Saikia*,
Preeti Rekha Boruah,
Abdul Aziz Ali and
Diganta Sarma
Department of Chemistry, Dibrugarh University, Dibrugarh-786004, Assam, India. E-mail: bishwajitsaikia@gmail.com; Tel: +91 9954314676
First published on 2nd June 2015
Abstract
A simple, greener, efficient, low cost and mild protocol has been developed for sucrose assisted palladium-catalyzed Suzuki reactions in water at room temperature under aerobic conditions. The results demonstrate that the sucrose played a crucial role making this protocol highly efficient. The PdCl2/sucrose/K2CO3/H2O system showed superb catalytic activity towards the Suzuki reaction of a wide range of aryl/heteroaryl halides with different substituted phenylboronic acids. This method offers an attractive alternative to the existing protocols since the reaction proceeds in aqueous media at room temperature with operational simplicity, shorter reaction time, cost-effectiveness and provides the products in high yields. The catalytic system is highly recyclable, allowing the reuse of the palladium catalyst in subsequent catalytic runs without significant loss of activity.
New advances in cross-coupling techniques have a made very significant contribution to organic synthesis as well as pharmaceutical, agricultural and advanced functional materials.1 The recent literature findings reveal that the Suzuki–Miyaura cross-coupling reaction is one of the highly attractive and useful protocols for carbon–carbon bond formation due to its extensive range of functional group tolerance, commercial availability of organoboron reagents and low toxicity of boron by-products.2 At the present time, synthetic researchers have a growing awareness in developing greener processes, as the sustainability has become a very crucial matter in all kind of human activity.3 Among the principles of green chemistry, the choice of solvents is a key factor, since it might gives rise to hazards, toxicity, pollution and waste are generated and mixed with the effluent water.4 With consideration of safety, economy and environment problems, most industries and pharmaceutical companies are currently approaching green chemistry and considering water as a potentially useful and safe alternative5 because it is an extremely abundant, non-toxic, non-corrosive and non-flammable green solvent. As a result, the use of water as a sole reaction medium is one of the growing challenges for modern researchers, particularly in chemistry.6 To date, a lot of efforts have been made by researchers to carry out organic reactions in neat water.7 Among those developed transformations, the palladium-catalyzed Suzuki–Miyaura cross-coupling reaction is an unique example, which is one of the most versatile and powerful tools for the construction of biaryls.8 In this regard, the progress of catalytic system in pure water look like particularly appropriate for the Suzuki–Miyaura reaction due to the excellent stability of boronic acids in aqueous medium.
The problems related with the use of water as a sole reaction media are the solubility of substrate as well as stability of metal catalyst. However, to some extent, these problems have been overcome by using different alternatives such as additives,9 organic co-solvent,10 phase-transfer catalyst,11 surfactant,12 microwave heating,13 ultrasound,14 heterogeneous catalysts,15 ligand-free approach and water soluble catalysts or ligands etc.16 Although, majority of these strategies were found to be effective in carrying out this transformation, most of these systems are associated with several drawbacks such as low reactivity, long reaction time, requirement of large amount of catalysts, cost, harsh reaction conditions, toxic and moisture sensitive nature, tedious procedure for the synthesis of ligand (few of them also involve two or more steps), use of expensive reagents etc. In addition, there are just few reported catalytic systems for the Suzuki–Miyaura cross-coupling of highly challenging heteroaryl halides with arylboronic acids in aqueous media.17 Therefore, the development of Pd-catalyzed Suzuki–Miyaura cross-coupling reaction of aryl/heteroaryl halides with arylboronic acids in water using very cheap and abundant water soluble ligand is very much attractive. Similarly, the design of efficient catalysts producing high yields under aerobic conditions continues to be an important challenge.
At the moment, we are interested in developing a simple and general protocol for the palladium-catalyzed and aerobic Suzuki reaction in pure water. Herein, we describe a very mild, efficient, simple and highly cost-effective one-pot protocol for the Suzuki–Miyaura cross-coupling reactions of aryl/heteroaryl halides with various arylboronic acids at room temperature in water using truly nontoxic, very cheap and economically important disaccharide ‘Sucrose’ as a water soluble ligand. Most importantly sucrose is enormously available at price comparable to those of synthetic organic base chemicals. Sucrose is the ordinary table sugar which we eat every day, it is the most abundant non-polymeric biogenic raw material consequently its use as a ligand in Suzuki reaction is considered as highly environmental friendly in terms of abundance and availability as a feedstock. This interesting finding gives us an opportunity to make known the catalytic performance of PdCl2–sucrose for the Suzuki–Miyaura cross-coupling reaction in water at room temperature (Scheme 1). To the best of our knowledge this is the first ‘On-water’ one-pot Pd-catalyzed aerobic Suzuki–Miyaura cross-coupling reaction assisted by ‘Sucrose’ that requires neither heating nor the addition of any additives. The procedural simplicity using most abundant and renewable resource sucrose is an advantage and we believed that, the present work surpass the criteria in terms of novelty. Therefore, the present synthetic method would be extremely beneficial and more efficient to synthetic chemistry community.
Considering the key role of base in the Suzuki–Miyaura cross-coupling reactions, different bases were screened in the presence of PdCl2 and sucrose in water at room temperature (Table 1, entries 1–14) and it was found that base had a significant influence on the cross-coupling efficiency (i.e. the nature of the bases plays an important role in Suzuki reaction). Generally, a strong base stimulates side reactions lowering the yield of the desired product, and a weak base remains unable to activate boronic acids. A model reaction of 4-bromoanisole and phenylboronic acid was chosen to screen the reaction conditions and the results are summarized in Table 1. It was found that, in comparison to the other bases, metal carbonates provide higher yields with excellent reaction rate. Among the various bases screened in water, K2CO3 provided the highest cross-coupling yield of 96% in 1 h (Table 1, entry 3) and we believed that the ability to dissolve bases in water for activating arylboronic acid has enhanced the rate of the reaction in an aqueous medium. It is also important to mention that during the presented experiments, homo coupling of phenylboronic acid to unsubstituted biphenyl was negligible. Triethylamine, a typical organic base, was tested in this system, providing a negative result of <5% isolated yield of the cross-coupled product in 12 h (Table 1, entry 11). Moreover, this reaction was unsuccessful in the absence of base (Table 1, entry 12), which confirmed the essentiality of base for the smooth Suzuki–Miyaura cross-coupling reaction.
| Entry | Base | Time (h) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 4-bromoanisole (1 mmol), phenylboronic acid (1.2 mmol), PdCl2 (0.01 mmol), sucrose (0.005 mmol), base (1.2 mmol) in H2O (3 mL) at room temperature.b Isolated yield after chromatography.c Without base.d Without sucrose.e Without PdCl2. | |||
| 1 | Na2CO3 | 2 | 90 |
| 2 | NaHCO3 | 6 | 60 |
| 3 | K2CO3 | 1 | 96 |
| 4 | Cs2CO3 | 2 | 86 |
| 5 | Na3PO4·12H2O | 6 | 28 |
| 6 | Na2HPO4 | 6 | 32 |
| 7 | NaOtBu | 6 | 25 |
| 8 | K3PO4 | 6 | <10 |
| 9 | NaOH | 8 | 28 |
| 10 | KOH | 8 | 25 |
| 11 | Et3N | 12 | <5 |
| 12 | — | 24 | No reactionc |
| 13 | K2CO3 | 12 | 25d |
| 14 | K2CO3 | 12 | No reactione |
Additionally we had performed two controlled experiments to demonstrate the need of PdCl2 as a catalyst and sucrose as a ligand in this reaction. The coupling reaction of phenylboronic acid and 4-bromoanisole without using sucrose was very sluggish and took 12 h to achieve only 25% yields of 4-methoxybiphenyl in neat water (Table 1, entry 13). From this experiment, now it is clear that sucrose played a crucial role in the high efficiency of this system. But there was no product formation between phenyl boronic acid and 4-bromoanisole in the absence of PdCl2 under this reaction condition (Table 1, entry 14).
With the optimized conditions in hand, we further studied the generality of the cross-coupling reactions between aryl halides and arylboronic acids in our ‘On-water’ one-pot protocol (PdCl2/sucrose/K2CO3/r.t.) and the results are presented in Table 2. As shown in Table 2, the Suzuki reactions of aryl bromides with phenylboronic acid proceeded very smoothly under aerobic conditions in H2O at room temperature to afford the corresponding coupled products in high yields. A wide range of electronically and structurally diverse aryl halides (including the deactivated aryl halides), were readily converted to the corresponding coupled products with different substituted aryl boronic acids under this green reaction condition. Various electron-donating and electron-withdrawing groups such as –CH3, –OCH3, –CN, –COCH3, –CHO and –NO2, –CF3 were well tolerated to give the desired unsymmetrical biaryls in excellent yields (Table 2, entries 1–18). However, various substituted arylboronic acids, bearing either electron-donating or electron-withdrawing groups, such as –CH3, –OMe, –COCH3, –CF3 and –NO2, provided the corresponding products in 98% to 48% isolated yields (Table 2, entries 3, 9, 10, 12–17). These results show that the stronger the electron-donating group in arylboronic acid, the higher the catalytic activity. This is because an electron-rich arylboronic acid could accelerate the rate of the transmetalation step in the Suzuki–Miyaura cross-coupling reaction.
| Entry | ArX | ArB(OH)2 | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: aryl/heteroaryl halide (1 mmol), arylboronic acid (1.2 mmol), PdCl2 (0.01 mmol), sucrose (0.005 mmol), K2CO3 (1.2 mmol) in H2O (3 mL) at room temperature.b Isolated yield after chromatography.c 1st run.d 2nd run.e 3rd run. | ||||
| 1 |  |
 |
1c, 1d, 1e | 96c, 96d, 95e |
| 2 |  |
 |
1c, 1d, 1e | 96c, 96d, 96e |
| 3 |  |
 |
1c, 1d, 1e | 98c, 98d, 98e |
| 4 |  |
 |
1c, 1.5d, 1.5e | 91c, 91d, 89e |
| 5 |  |
 |
2c, 2d, 2e | 90c, 90d, 90e |
| 6 |  |
 |
2c, 2d, 2e | 92c, 89d, 90e |
| 7 |  |
 |
2.5c, 2.5d, 2.5e | 90c, 88d, 90e |
| 8 |  |
 |
1.5c, 1.5d, 1.5e | 88c, 88d, 87e |
| 9 |  |
 |
2c, 2d, 2e | 91c, 91d, 90e |
| 10 |  |
 |
2.5c, 2.5d, 2.5e | 90c, 90d, 88e |
| 11 |  |
 |
1c, 1d, 1.5e | 92c, 90d, 90e |
| 12 |  |
 |
1.5c, 2d, 1.5e | 90c, 92d, 90e |
| 13 |  |
 |
1c, 1d, 1e | 94c, 94d, 91e |
| 14 |  |
 |
2c, 2d, 2e | 91c, 90d, 90e |
| 15 |  |
 |
3c, 3d, 3e | 48c, 48d, 47e |
| 16 |  |
 |
2c, 2d, 2e | 91c, 90d, 90e |
| 17 |  |
 |
2.5c, 2.5d, 2.5e | 90c, 90d, 88e |
| 18 |  |
 |
3c, 3d, 3e | 79c, 79d, 75e |
Encouraged by such promising results, we next turned our attention to the Suzuki reaction of phenylboronic acid with challenging heteroaryl halides, and the results are summarized in Table 2. The reactions carried out using 3-bromothiophene and 2-bromopyridine with arylboronic acids under this protocol, afforded good to excellent isolated yields of the desired products in a very short reaction time (Table 2, entries 17 and 18). For our satisfaction we performed all the reactions three times to verify the reproducibility of results and also yield of the desired cross-coupled products (Table 2, entries 1–18).
Literature report revealed that in carbohydrate chemistry, the general route of reaction control with transition metal catalysis is mostly unclear.18 The key reason seems to be the low crystallization tendency of carbohydrate-transition metal complexes which may be partly due to the lack of a predominant metal-binding site of carbohydrates. The lack of proper understanding of the mode of bonding between carbohydrate and transition metal ions results in the limited use of metal catalysis. The exact mechanism for the PdCl2 catalyzed sucrose assisted Suzuki cross-coupling reaction is not clear. In view of the fact that the molecule of sucrose has several pathetic coordination sites19 as well as palladium(II) is particularly suitable for the complexation of various types of diols and therefore, it is believed that PdCl2 units are attached to 1,2-(D-glucopyranoside-O3′,O4′) and 1,3-(D-fructofuranosyl-O1,O3) diolate groups of sucrose to form five- and six-membered chelate rings20 during the course of a metal-mediated reaction leading to the significant improvement of the catalytic efficiency of the system.
The reusability of transition metal catalysts are the trends of the catalysis industry along with the development of green chemistry, not only for lowering costs, but also for avoiding environmental pollution. Recycling experiments were performed using the standard 4-bromoanisole/phenylboronic cross-coupling test reaction. After the first reaction cycle diethyl ether was added to the system followed by centrifugation, the clearly separated diethyl ether layer has been removed from the system and dried to get the desired crude product. After each run, the catalyst system was washed with diethyl ether to completely remove the organic substrates and then used for the next run without any further treatment (Fig. 1). Fresh substrates were added again to the reused catalyst system, which was stirred for another 1 h (Table 3). After the first recycling step full catalytic activity is preserved and a slight loss in activity is observed for only after the 5th run. The average yield over 5 runs was 93% (Table 3, entries 1–5). Therefore, in that order several cycles of catalyst use and reuse are possible and the results are summarized in Table 3.
In conclusion, we report a highly environmentally friendly reaction protocol for aqueous Suzuki–Miyaura cross-coupling catalysis under air at room temperature, which is applicable to a broad range of substrates. The ability to use water as the reaction medium and sucrose as ligand greatly increases the green credentials of the method. Correspondingly, recycling of the active catalytic species can be performed several times without significant loss in catalytic activity. We anticipate that this approach will offer an alternative synthetic strategy for the practical construction of biaryl/heterobiaryl compounds in near future. Studies aimed at extending the scope of this catalyst system to other types of cross-coupling and related reactions are currently ongoing in our laboratory.
Acknowledgements
The authors acknowledge the Department of Science and Technology, New Delhi for financial assistance to the Department of Chemistry, Dibrugarh University, Dibrugarh, Assam, INDIA.Notes and references
- (a) J. P. Corbet and G. Mignani, Chem. Rev., 2006, 106, 2651–2710 CrossRef CAS PubMed; (b) S. R. Chemler, D. Trauner and S. J. Danishefsky, Angew. Chem., Int. Ed., 2001, 40, 4544–4568 CrossRef CAS; (c) A. F. Littke and G. C. Fu, Angew. Chem., Int. Ed., 2002, 41, 4176–4211 CrossRef CAS; (d) L. Botella and C. Najera, Angew. Chem., Int. Ed., 2002, 41, 179–181 CrossRef CAS; (e) J. P. Stambuli, R. Kuwano and J. F. Hartwig, Angew. Chem., Int. Ed., 2002, 41, 4746–4748 CrossRef CAS PubMed; (f) O. Navarro, R. A. Kelly and S. P. Nolan, J. Am. Chem. Soc., 2003, 125, 16194–16195 CrossRef CAS PubMed.
- (a) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS; (b) N. Miyaura and A. Suzuki, Chem. Commun., 1979, 866–867 RSC; (c) A. Suzuki, Angew. Chem., Int. Ed., 2011, 50, 6723–6737 Search PubMed; (d) K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2001, 40, 4442–4489 Search PubMed; (e) S. R. Chemler, D. Trauner and S. J. Danishefsky, Angew. Chem., Int. Ed., 2001, 40, 4544–4568 CrossRef CAS; (f) J. Magano and J. R. Dunetz, Chem. Rev., 2011, 111, 2177–2250 CrossRef CAS PubMed; (g) A. Molnár, Chem. Rev., 2011, 111, 2251–2320 CrossRef PubMed; (h) L. Yin and J. Liebscher, Chem. Rev., 2007, 107, 133–173 CrossRef CAS PubMed.
- (a) M. Eissen, Chem. Educ. Res. Pract., 2012, 13, 103–111 RSC; (b) I. Eilks and F. Rauch, Chem. Educ. Res. Pract., 2012, 13, 57–58 RSC; (c) J. Liu, Science, 2010, 328, 50 CrossRef CAS PubMed; (d) D. Rowe, Science, 2007, 317, 323–324 CrossRef CAS PubMed.
- (a) P. T. Anastas and J. C. Warner, in, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 1998 Search PubMed; (b) M. O. Simon and C. J. Li, Chem. Soc. Rev., 2012, 41, 1415–1427 RSC; (c) P. T. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC; (d) M. Carril, R. SanMartin and E. Dominguez, Chem. Soc. Rev., 2008, 37, 639–647 RSC; (e) K. H. Shaughnessy, Eur. J. Org. Chem., 2006, 1827–1835 CrossRef CAS PubMed; (f) J. M. DeSimone, Science, 2002, 297, 799–803 CrossRef CAS PubMed.
- W. J. W. Watson, Green Chem., 2012, 14, 251–259 RSC.
- (a) V. Polshettiwar, A. Decottignies, C. Len and A. Fihri, ChemSusChem, 2010, 3, 502–522 CrossRef CAS PubMed; (b) M. Lamblin, L. Nassar-Hardy, J.-C. Hierso, E. Fouquet and F.-X. Felpin, Adv. Synth. Catal., 2010, 352, 33–79 CrossRef CAS PubMed; (c) C.-J. Li, Chem. Rev., 2005, 105, 3095–3166 CrossRef CAS PubMed; (d) C.-J. Li, Acc. Chem. Res., 2002, 35, 533–538 CrossRef CAS PubMed.
- (a) A. Modak, J. Mondal, M. Sasidharan and A. Bhaumik, Green Chem., 2011, 13, 1317–1331 RSC; (b) C. I. Herrerías, X. Yao, Z. Li and C.-J. Li, Chem. Rev., 2007, 107, 2546–2562 CrossRef PubMed; (c) N. Jiang and A. J. Ragauskas, Tetrahedron Lett., 2006, 47, 197–200 CrossRef CAS PubMed; (d) I. P. Beletskaya and A. V. Cheprakov, in Organic Synthesis in Water, ed. P. A. Grieco, Blackie Academic & Professional, London, 1998, ch. 5, p. 141 Search PubMed; (e) I. P. Beletskaya and A. V. Cheprakov, in Handbook of Organopalladium Chemistry for Organic Synthesis, ed. E.-I. Negishi and A. de Meijere, Wiley, New York, 2002, vol. 2, ch. 10, p. 2957 Search PubMed.
- (a) A. Decottignies, A. Fihri, G. Azemar, F. Djedaini-Pilard and C. Len, Catal. Commun., 2013, 32, 101–107 CrossRef CAS PubMed; (b) V. Polshettiwar, C. Len and A. Fihri, Coord. Chem. Rev., 2009, 253, 2599–2626 CrossRef CAS PubMed; (c) G. Hervé, G. Sartori, G. Enderlin, G. Mackenzie and C. Len, RSC Adv., 2014, 4, 18558–18594 RSC; (d) S. Gallagher-Duval, G. Hervé, G. Sartori, G. Enderlin and C. Len, New J. Chem., 2013, 37, 1989–1995 RSC; (e) G. Sartori, G. Enderlin, G. Hervé and C. Len, Synthesis, 2012, 767–772 CAS; (f) G. Sartori, G. Hervé, G. Enderlin and C. Len, Synthesis, 2013, 330–333 CAS; (g) P. R. Boruah, A. A. Ali, B. Saikia and D. Sarma, Green Chem., 2015, 17, 1442–1445 RSC; (h) B. Saikia, A. A. Ali, P. R. Boruah, D. Sarma and N. C. Barua, New J. Chem., 2015, 39, 2440–2443 RSC.
- (a) M. Mondal and U. Bora, Green Chem., 2012, 14, 1873–1876 RSC; (b) P. R. Boruah, M. J. Koiri, U. Bora and D. Sarma, Tetrahedron Lett., 2014, 55, 2423–2425 CrossRef CAS PubMed.
- (a) F. Li and T. S. A. Hor, Adv. Synth. Catal., 2008, 350, 2391–2400 CrossRef CAS PubMed; (b) L. R. Moore, E. C. Western, R. Cracium, J. M. Spruell, D. A. Dixon, K. P. O’Halloran and K. H. Shaughnessy, Organomettallics, 2008, 27, 576–593 CrossRef CAS; (c) P. Capek, R. Pohl and M. Hocek, Org. Biomol. Chem., 2006, 4, 2278–2284 RSC; (d) B. Saikia, P. R. Boruah, A. A. Ali and D. Sarma, Tetrahedron Lett., 2015, 56, 633–635 CrossRef CAS PubMed.
- (a) B. Li, C. Wang, G. Chen and Z. Zhang, J. Environ. Sci., 2013, 25, 1083–1088 CrossRef CAS; (b) S. L. Mao, Y. Sun, G. A. Yu, C. Zhao, Z. J. Han, J. Yuan, X. Zhu, Q. Yang and S. H. Liu, Org. Biomol. Chem., 2012, 10, 9410–9417 RSC.
- (a) B. H. Lipshutz, S. Ghorai, A. R. Abela, R. Moser, T. Nishikata, C. Duplais, A. Krasovskiy, R. D. Gaston and R. C. Gadwood, J. Org. Chem., 2011, 76, 4379–4391 CrossRef CAS PubMed; (b) J. Zhi, D. Song, Z. Li, X. Lei and A. Hu, Chem. Commun., 2011, 47, 10707–10709 RSC; (c) B. H. Lipshutz, T. B. Petersen and A. R. Abela, Org. Lett., 2008, 10, 1333–1336 CrossRef CAS PubMed.
- (a) V. Polshettiwar and R. S. Varma, Acc. Chem. Res., 2008, 41, 629–639 CrossRef CAS PubMed; (b) B. A. Roberts and C. R. Strauss, Acc. Chem. Res., 2005, 38, 653–661 CrossRef CAS PubMed; (c) N. E. Leadbeater, Chem. Commun., 2005, 2881–2902 RSC; (d) C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250–6284 CrossRef CAS PubMed; (e) C. J. Li, Angew. Chem., Int. Ed., 2003, 42, 4856–4858 CrossRef CAS PubMed.
- (a) A. L. F. Souza, L. C. Silva, B. L. Oliveir and O. A. C. Antunes, Tetrahedron Lett., 2008, 49, 3895–3898 CrossRef PubMed; (b) V. Polackova, M. Hut'ka and S. Toma, Ultrason. Sonochem., 2005, 12, 99–102 CrossRef CAS PubMed.
- (a) J. Bastos-Arrieta, A. Shafir, A. Alonso, M. Munoz, J. Macanas and D. N. Muraviev, Catal. Today, 2012, 193, 207–212 CrossRef CAS PubMed; (b) S. Bhunia, R. Sen and S. Koner, Inorg. Chim. Acta, 2010, 363, 3993–3999 CrossRef CAS PubMed.
- (a) B. Basu, K. Biswas, S. Kundu and S. Ghosh, Green Chem., 2010, 12, 1734–1738 RSC; (b) S. Shi and Y. Zhang, Green Chem., 2008, 10, 868–872 RSC; (c) T. Maegawa, Y. Kitamura, S. Sako, T. Udzu, A. Sakurai, A. Tanaka, K. Endo, U. Bora, T. Kurita, A. Kozaki, Y. Monguchi and H. Sajiki, Chem.–Eur. J., 2007, 13, 5937–5947 CrossRef CAS PubMed.
- (a) A. N. Marziale, D. Jantke, S. H. Faul, T. Reiner, E. Herdtweck and J. Eppinger, Green Chem., 2011, 13, 169–177 RSC; (b) K. M. Dawood and M. M. El-Deftar, ARKIVOC, 2010, 319–330 CrossRef CAS.
- E. Avela, S. Aspelund, B. Holmbom and B. Melander, in Sucrochemistry, ACS Symp. Ser. 41, ed. J. L. Hickson, ACS, Washington, 1977, pp. 62–76 Search PubMed.
- S. Herdin, G. Kettenbach and P. Kluefers, Z. Naturforsch., 2004, 59, 134–139 CAS.
- R. Ahlrichs, M. Ballauff, K. Eichkorn, O. Hanemann, G. Kettenbach and P. Klüfers, Chem.–Eur. J., 1998, 4, 835–844 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra09102k |
| This journal is © The Royal Society of Chemistry 2015 |