Graphene/SrTiO3 nanocomposites used as an effective electron-transporting layer for high-performance perovskite solar cells
Abstract
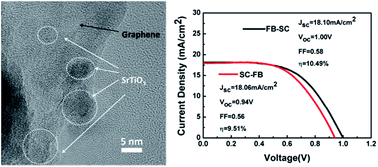
Organic–inorganic perovskite solar cells based on binary oxides have been studied for a long time and have obtained an impressive advance in performance. However, studies using ternary oxides as the electron-transporting layer are scarce and there are still many problems to be solved. The ternary oxide SrTiO3 with a perovskite structure is well matched with the perovskite absorber layer in the crystal structure. Although the device based on mesoporous-SrTiO3 (mp-SrTiO3) showed a high Voc, its average Jsc is still too low compared with mp-TiO2 based devices. In this work, we used graphene/SrTiO3 nanocomposites as an effective electron-transporting layer. Due to the superconductivity of the graphene combined with tuning the amount of the starting graphene to increase the light harvesting of the absorber layer and decrease recombination centers, we got a great achievement. The device based on graphene/SrTiO3 nanocomposites exhibited a PCE of 10% with a Jsc of 18.08 mA cm−2, increased by 46.0 and 45.6% respectively compared with the mp-SrTiO3 based device, indicating incorporation of graphene is an effective way to improve the Jsc of mp-SrTiO3 based perovskite solar cells.

 Please wait while we load your content...
Please wait while we load your content...