Light-mediated cascade transformation of activated alkenes: BiOBr nanosheets as efficient photocatalysts for the synthesis of α-aryl-β-trifluoromethyl amides†
Cuibo Liu and
Bin Zhang*
Department of Chemistry, School of Science, Tianjin University, and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300072, P. R. China. E-mail: bzhang@tju.edu.cn
First published on 7th July 2015
Abstract
A facile light-induced, BiOBr nanosheet promoted one-pot tandem transformation of activated alkenes is presented. A wide variety of acyclic α-aryl-β-trifluoromethyl amides are synthesized via the consecutive trifluoromethylation/aryl migration/desulfonylation and N–H bond formation process.
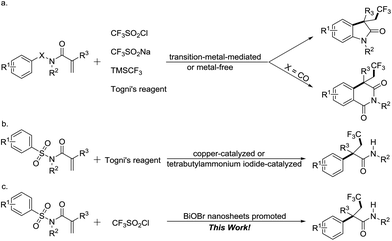
Tandem or cascade transformation has been proved to be an efficient and atom economic strategy in organic synthesis as it enables a rapid increase in molecular complexity from readily available starting materials.1 Additionally, the advantage of such transformation is the simultaneous formation of two or more bonds in a single manipulation process. Alkenes are privileged motifs for tandem reactions and have been intensively studied to date. However, transformations involving construction of C–CF3 bonds are still limited to halotrifluoromethylation,2 hydrotrifluoromethylation,3 aminotrifluoromethylation,4 and oxytrifluoromethylation.5 Recently, aryltrifluoromethylation of alkenes has spurred intense interest from synthetic chemists.6 Following the pioneering work of Liu and co-workers,7 alternative methods to enable the introduction of aryl and trifluoromethyl groups across the double bond of alkenes by transition-metal-mediated or metal-free protocols have been independently developed. Although these strategies are effective, in most cases, these transformations proceed in an intramolecular fashion and some β-trifluoromethylated oxindoles derivatives with various biologically activity are synthesized from N-aryl acrylamide substrates (Scheme 1a). In sharp contrast, the reports on the formation of the products in an acyclic manner through the cascade aryltrifluoromethylation of alkenes are relatively rare. Very recently, the Nevado group described respectively the copper- and tetrabutylammonium iodide-catalyzed aryltrifluoromethylation of conjugated tosyl amides via a one-pot trifluoromethylation/aryl migration/desulfonylation sequence by employing Togni's reagent as the CF3 source, and a series of linear α-aryl-β-trifluoromethyl amides bearing a quaternary stereocenter were well established (Scheme 1b).8 Despite effectiveness of Nevado's methods, increasing the diversity of available methodologies that realize the successful formation of acyclic α-aryl-β-trifluoromethyl amides from activated alkenes is still of considerable interest.
Light-driven chemical transformations including photoredox catalysis are becoming one of the efficient and sustainable tools in synthetic chemistry.9 Undoubtedly, searching for particularly useful catalysts that can promote the organic transformations effectively is a key in this field. Over the past decade various photocatalysts or sensitizers have been synthesized. In general, they can be mainly classified into three types: organic dyes, ruthenium(II) or iridium(III) or copper(I) metal complexes, and inorganic semiconductors. Among them, inorganic semiconductors have been recognized as the highly promising type due to their unique characteristics, such as easy-preparation, cheap, nontoxic, efficient, recyclable and so on. Usually, TiO2, ZnO, ZnS and CdS are typical semiconductors for organic chemical transformations because of their unique wide band gaps.10 In recent years, inorganic bismuth-containing nanomaterials have been found to be the potential photocatalysts, which show strong absorption in UV or visible-light region.11 Apart from their photocatalytic applications for the degradation of organic dyes and splitting of water into hydrogen and oxygen, the use of them for photocatalytic synthesis of organic molecules has also been reported. For example, Pericàs and König groups reported a light-driven asymmetric α-alkylation of aldehydes by combining Bi2O3 or PbBiO2Br as the low-band-gap photocatalyst with the MacMillan imidazolidinone as the chiral catalyst.12 Later, the Pericàs group demonstrated that Bi2O3 could be used as efficient photocatalysts for the atom transfer radical addition (ATRA) reaction of organobromides to diversely functionalized terminal olefins.13 The Fu et al. made an advance on finding a surface-chlorinated BiOBr/TiO2 hybrid composites to realize the selective Csp3–H bonds functionalization of alkanes.14 The asymmetric reactions, difunctionalization of alkenes, and C–H bonds functionalizations are the current hot research topics in chemistry and materials science, revealing that the inorganic bismuth semiconductors are a kind of potential and alternative photocatalysts for chemical transformations. Therefore, the development of bismuth-based photocatalysts for new organic reactions, particularly the tandem transformations, is highly desirable.
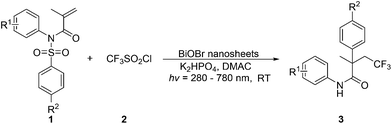
Herein, we present a light-mediated, BiOBr nanosheet promoted one-pot cascade trifluoromethylation/1,4-aryl migration/desulfonylation and N–H bond formation reaction of conjugated tosyl amides, and a series of α-aryl-β-trifluoromethyl amides with diverse functional groups are successfully prepared (Scheme 1c). These trifluoromethyl functionalized organic compounds maybe find potential applications in pharmaceuticals, agrochemicals, and functional materials.15
The as-prepared BiOBr is a layer-structured semiconductor.16 Inorganic nanosheets are considered as one kind of efficient photocatalysts due to the dramatically improving intrinsic catalytic properties of nanosheets over other correspond inorganic counterparts.17 The reductive potential of electrons in the conduction band of BiOBr semiconductor is −0.27 V (vs. SCE),11a which is higher than CF3SO2Cl (−0.18 V vs. SCE).18 It means that the photogenerated electron of BiOBr can effectively reduce CF3SO2Cl and thus produce the CF3 radical, which be used for subsequent transformation of alkenes. Our group has recently reported the trifluoromethylation/arylation of N-aryl acrylamide using BiOBr nanosheets as photocatalysts.16 We speculate that this photocatalytic method may also facilitate the trifluoromethylation/aryl migration/desulfonylation of the conjugated tosyl amide substrates. To validate the hypothesis, N-phenyl-N-tosylmethacrylamide 1a and CF3SO2Cl 2 are selected as model substrates in conjunction with BiOBr nanosheets, K2HPO4 and HSiEt3 in DMF under light excitation (280–780 nm) for 6 h. It is found that the one-pot sequential transformation of 1a definitely occurs, and 74% yield of α-aryl-β-trifluoromethyl amide is obtained (Table S1,† entry 1). When DMF is replaced with DMAC, the yield of the product is slightly increased (Table S1,† entry 2). However, when the reaction is carried out in the absence of HSiEt3, a comparable yield of 3a is obtained (Table S1,† entry 3). Although the reaction efficiency is a little improved due to the addition of HSiEt3, in order to reduce the complexity of the operation, we determine that the HSiEt3 is not a necessary additive. Encouraged by this result, the solvent are screened next. We observe that only DMAC gives the best result and the others are inferior or even noneffective. These results suggest a significant solvent dependence for this photocatalytic reaction (Table S1,† entries 4–10). Notably, no product 3a is detected either in the dark or in the absence of BiOBr nanosheets, strongly illustrating that the light and the photoredox catalysts are important to induce this reaction (Table S1,† entries 11 and 12). We have attempted to use CdS nanosheets and Ru(bpy)3Cl2·6H2O as alternative photocatalysts, which are demonstrated exceptional photocatalytic properties for organic transformations, however, dissatisfactory results are obtained (Table S1,† entries 13 and 14).
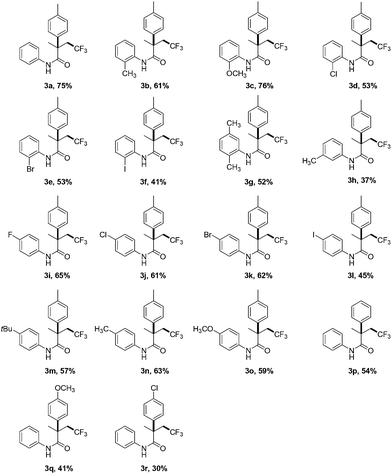
Under the optimized reaction conditions, the scope of this transformation is explored and displayed in Table 1. We firstly examine the substitution pattern on the aromatic ring directly bound to the N atom. When the substituents at the ortho position, these one-pot sequential reactions can produce the corresponding aryltrifluoromethylated amides in moderate to good yields (Table 1, 3b–e). The 1,4-dimethyl substituted tosyl amide does not hinder the reaction, and the product 3g can be isolated in 52% yield. Substrate 1h with a methyl on the meta position of phenyl ring is also amenable to the standard conditions, and 37% yield of the product is obtained. The introduction of either electron-donating or electron-withdrawing groups at the para position is well tolerated, and 57–65% yields of the β-trifluoromethylated amides are constructed (Table 1, 3i–k, 3m–o). In contrast, the presence of iodine seems to decrease the reaction efficiency no matter where the iodine atom lies on the aryl ring (Table 1, 3f and 3l). Next, the influence of the substituents on the aromatic ring of the sulfonamide group is also investigated. Replacement of the methyl group by either a hydrogen, methoxyl, or chlorine giving rise to the corresponding products in slightly lower yields (Table 1, 3p–r). All the obtained results indicate that our photocatalytic methodology is particularly universal, and may provide a new facile access to the synthesis of CF3-containing pharmaceutical blocks.
| a Reaction conditions: 1a (0.2 mmol), 2 CF3SO2Cl (1.0 mmol), K2HPO4 (0.6 mmol), BiOBr nanosheets (20 mg), DMAC (1.0 mL), 300 W Xe arc lamp, RT. |
|---|
 |
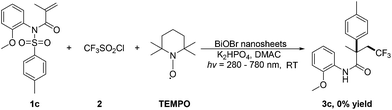
In order to verify whether these reactions proceed through a radical pathway, the control experiment is performed (Scheme 2). Treatment of 1c with 2 under standard conditions with the addition of radical trapping agent 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) delivers no desirable product, further supports that a radical process is involved in our photocatalytic arylation/trifluoromethylation of alkenes.
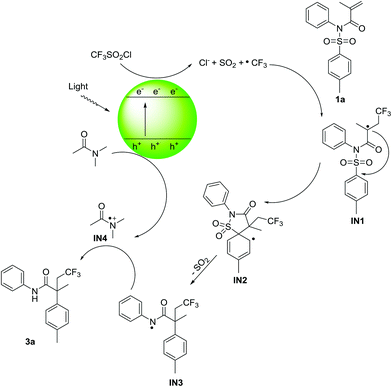
Based on the above results and related photocatalytic literatures,6–8,10b a plausible reaction mechanism is proposed in Scheme 3. Light absorption of BiOBr nanosheets promotes the electron to transfer from the valence band (VB) to the conduction band (CB), which is a key photochemical step. CF3SO2Cl is then reduced by the photogenerated electron to generate the corresponding free radical anion of triflyl chloride, which experiences fast collapses to form the relative stable CF3 radical with sulfur dioxide and chloride anion.18 Subsequently, the CF3 radical interacts with alkene 1a affording the activated radical intermediate IN1. A dearomatization/5-ipso cyclization then takes place on the aromatic ring generating aryl radical IN2. Rearomatization of IN2 with concomitant desulfonylation leads to amidyl radical IN3. The photogenerated hole obtains an electron from the solvent DMAC via a single-electron transfer process to close the catalytic cycle and meanwhile give the radical cation IN4. The amide functions as an efficient electron donor could be found in many reactions.19 Finally, the formed intermediate IN3 abstracts the hydrogen radical released from IN4 to deliver the desired product 3a. The preliminary mechanism may need further theoretical understanding.
Conclusions
In conclusion, we have developed a facile light-induced, BiOBr nanosheet promoted one-pot tandem trifluoromethylation/1,4-aryl migration/desulfonylation and N–H bond formation reaction of conjugated N-tosyl amides. This strategy enables a practical access to a series of α-aryl-β-trifluoromethyl amides bearing a quaternary stereocenter in moderate to good yields. In this heterogeneous reaction media, electron-exchange occurs between the organic intermediates in solution and the semiconductor surface. Control experiments illustrate that the synergistic effect of photogenerated electrons and holes plays a key role for the reaction efficiency. This low-cost, green and efficient photocatalytic strategy can be considered as a viable alternative to the previously mentioned methods. Further explorations on light-mediated cascade reactions and mechanistic understanding are currently being investigating in our laboratory.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (no. 21422104).Notes and references
- (a) J. Zhou, Chem.–Asian J., 2010, 5, 422 CrossRef CAS PubMed; (b) L. Chen, Y. Du, X.-P. Zeng, T.-D. Shi, F. Zhou and J. Zhou, Org. Lett., 2015, 17, 1557 CrossRef CAS PubMed; (c) J.-C. Wasilke, S. J. Obrey, R. T. Baker and G. C. Bazan, Chem. Rev., 2005, 105, 1001 CrossRef CAS PubMed; (d) K. C. Nicolaou, D. J. Edmonds and P. G. Bulger, Angew. Chem., Int. Ed., 2006, 45, 7134 CrossRef CAS PubMed; (e) M. J. Gaunt, C. C. C. Johansson, A. McNally and N. T. Vo, Drug Discovery Today, 2007, 12, 8 CrossRef CAS PubMed; (f) Y. Xia, Y. Zhang and J. B. Wang, ACS Catal., 2013, 3, 2586 CrossRef CAS.
- (a) C.-J. Wallentin, J. D. Nguyen, P. Finkbeiner and C. R. J. Stephenson, J. Am. Chem. Soc., 2012, 134, 8875 CrossRef CAS PubMed; (b) J. Ignatowska and W. Dmowski, J. Fluorine Chem., 2007, 128, 997 CrossRef CAS PubMed; (c) D. B. Bagal, G. Kachkovskyi, M. Knorn, T. Rawner, B. M. Bhanage and O. Reiser, Angew. Chem., Int. Ed., 2015, 54, 6999 CrossRef CAS PubMed.
- (a) D. J. Wilger, N. J. Gesmundo and D. A. Nicewicz, Chem. Sci., 2013, 4, 3160 RSC; (b) X. Wu, L. L. Chu and F.-L. Qing, Angew. Chem., Int. Ed., 2013, 52, 2198 CrossRef CAS PubMed; (c) Y. Yasu, T. Koike and M. Akita, Org. Lett., 2013, 15, 2136 CrossRef CAS PubMed; (d) S. Mizuta, S. Verhoog, K. M. Engle, T. Khotavivattana, M. O. Duill, K. Wheelhouse, G. Rassias, M. Médebielle and V. Gouverneur, J. Am. Chem. Soc., 2013, 135, 2505 CrossRef CAS PubMed.
- (a) H. Egami, S. Kawamura, A. Miyazaki and M. Sodeoka, Angew. Chem., Int. Ed., 2013, 52, 7841 CrossRef CAS PubMed; (b) F. Wang, X. X. Qi, Z. L. Liang, P. H. Chen and G. S. Liu, Angew. Chem., Int. Ed., 2014, 53, 1881 CrossRef CAS PubMed; (c) S. Kawamura, H. Egami and M. Sodeoka, J. Am. Chem. Soc., 2015, 137, 4865 CrossRef CAS PubMed; (d) Y. Yasu, T. Koike and M. Akita, Org. Lett., 2013, 15, 2136 CrossRef CAS PubMed; (e) A. Carboni, G. Dagousset, E. Magnier and G. Masson, Org. Lett., 2014, 16, 1240 CrossRef CAS PubMed.
- (a) Y. Yasu, T. Koike and M. Akita, Angew. Chem., Int. Ed., 2012, 51, 9567 CrossRef CAS PubMed; (b) R. Tomita, Y. Yasu, T. Koike and M. Akita, Angew. Chem., Int. Ed., 2014, 53, 7144 CrossRef CAS PubMed; (c) H. Egami, R. Shimizu and M. Sodeoka, Tetrahedron Lett., 2012, 53, 5503 CrossRef CAS PubMed; (d) R. Zhu and S. L. Buchwald, J. Am. Chem. Soc., 2012, 134, 12462 CrossRef CAS PubMed; (e) P. G. Janson, I. Ghoneim, N. O. Iichenko and K. J. Szabó, Org. Lett., 2012, 14, 2882 CrossRef CAS PubMed; (f) Y. Li and A. Studer, Angew. Chem., Int. Ed., 2012, 51, 8221 CrossRef CAS PubMed; (g) C. Feng and T.-P. Loh, Chem. Sci., 2012, 3, 3458 RSC.
- (a) E. Merino and C. Nevado, Chem. Soc. Rev., 2014, 43, 6598 RSC; (b) L. L. Shi, X. B. Yang, Y. Y. Wang, H. J. Yang and H. Fu, Adv. Synth. Catal., 2014, 356, 1021 CrossRef CAS PubMed; (c) W. J. Fu, F. J. Xu, Y. Q. Fu, C. Xu, S. H. Li and D. P. Zou, Eur. J. Org. Chem., 2014, 709 CrossRef CAS PubMed; (d) L. Li, M. Deng, S.-C. Zheng, Y.-P. Xiong, B. Tan and X.-Y. Liu, Org. Lett., 2014, 16, 504 CrossRef CAS PubMed; (e) F. Yang, P. Klumphu, Y.-M. Liang and B. H. Lipshutz, Chem. Commun., 2014, 50, 936 RSC; (f) W. Wei, J. W. Wen and H. Wang, J. Org. Chem., 2014, 79, 4225 CrossRef CAS PubMed; (g) X.-J. Tang, C. S. Thomoson and W. R. Dolbier, Org. Lett., 2014, 16, 4594 CrossRef CAS PubMed; (h) H. Egami, R. Shimizu and M. Sodeoka, J. Fluorine Chem., 2013, 152, 51 CrossRef CAS PubMed; (i) P. Xu, J. Xie, Q. C. Xue, C. D. Pan, Y. X. Cheng and C. J. Zhu, Chem.–Eur. J., 2013, 19, 14039 CrossRef CAS PubMed; (j) S. Tang, Z. H. Li, M. W. Wang, Z. P. Li and R. L. Sheng, Org. Biomol. Chem., 2015, 13, 5285 RSC; (k) H. Egami, R. Shimizu, S. Kawamura and M. Sodeoka, Angew. Chem., Int. Ed., 2013, 52, 4000 CrossRef CAS PubMed; (l) F. Wang, D. H. Wang, X. Mu, P. H. Chen and G. S. Liu, J. Am. Chem. Soc., 2014, 136, 10202 CrossRef CAS PubMed; (m) X. W. Liu, F. Xiong, X. P. Huang, L. Xu, P. F. Li and X. X. Wu, Angew. Chem., Int. Ed., 2013, 52, 6962 CrossRef CAS PubMed.
- X. Mu, T. Wu, H. Y. Wang, Y. L. Guo and G. S. Liu, J. Am. Chem. Soc., 2012, 134, 878 CrossRef CAS PubMed.
- (a) W. Q. Kong, M. Casimiro, E. B. Merino and C. Nevado, J. Am. Chem. Soc., 2013, 135, 14480 CrossRef CAS PubMed; (b) W. Q. Kong, M. Casimiro, N. Fuentes, E. Merino and C. Nevado, Angew. Chem., Int. Ed., 2013, 52, 13086 CrossRef CAS PubMed.
- Selected examples see: (a) Q.-Y. Meng, J.-J. Zhong, Q. Liu, X.-W. Gao, H.-H. Zhang, T. Lei, Z.-J. Li, K. Feng, B. Chen, C.-H. Tung and L.-Z. Wu, J. Am. Chem. Soc., 2013, 135, 19052 CrossRef CAS PubMed; (b) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102 RSC; (c) N. Iqbal, J. Jung, S. Park and E. J. Cho, Angew. Chem., Int. Ed., 2014, 53, 539 CrossRef CAS PubMed; (d) J. Xie, Q. C. Xue, H. M. Jin, H. M. Li, Y. X. Cheng and C. J. Zhu, Chem. Sci., 2013, 4, 1281 RSC; (e) M. Fagnoni, D. Dondi, D. Ravelli and A. Albini, Chem. Rev., 2007, 107, 2725 CrossRef CAS PubMed; (f) J. Xuan and W. J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 6828 CrossRef CAS PubMed; (g) S. Q. Zhu, A. Das, L. Bui, H. J. Zhou, D. P. Curran and M. Rueping, J. Am. Chem. Soc., 2013, 135, 1823 CrossRef CAS PubMed; (h) H. Jiang, Y. Z. Cheng, R. Z. Wang, M. M. Zheng, Y. Zhang and S. Y. Yu, Angew. Chem., Int. Ed., 2013, 52, 13289 CrossRef CAS PubMed; (i) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed; (j) L. J. Allen, P. J. Cabrera, M. Lee and M. S. Sanford, J. Am. Chem. Soc., 2014, 136, 5607 CrossRef CAS PubMed; (k) S. Fukuzumi and K. Ohkubo, Chem. Sci., 2013, 4, 561 RSC; (l) F. Z. Su, S. C. Mathew, L. Möhlmann, M. Antonietti, X. C. Wang and S. Blechert, Angew. Chem., Int. Ed., 2011, 50, 657 CrossRef CAS PubMed.
- Selected examples see: (a) X. J. Lang, X. D. Chen and J. C. Zhao, Chem. Soc. Rev., 2014, 43, 473 RSC; (b) M. Cherevatskaya and B. König, Russ. Chem. Rev., 2014, 83, 183 CrossRef PubMed; (c) M. Rueping, J. Zoller, D. C. Fabry, K. Poscharny, R. M. Koenigs, T. E. Weirich and J. Mayer, Chem.–Eur. J., 2012, 18, 3478 CrossRef CAS PubMed; (d) T. Caronna, C. Gambarotti, L. Palmisano, C. Punta and F. Recupero, Chem. Commun., 2003, 2350 RSC; (e) A. Villa, G. M. Veith, D. Ferri, A. Weidenkaff, K. A. Perry, S. Campisia and L. Prati, Catal. Sci. Technol., 2013, 394 RSC; (f) H. Kisch, Adv. Photochem., 2001, 26, 93 CAS; (g) T. Mitkina, C. Stanglmair, W. Setzer, M. Gruber, H. Kisch and B. König, Org. Biomol. Chem., 2012, 10, 3556 RSC; (h) W. W. Zhao, C. B. Liu, L. M. Cao, X. G. Yin, H. L. Xu and B. Zhang, RSC Adv., 2013, 3, 22944 RSC.
- (a) Z. Jiang, F. Yang, G. D. Yang, L. Kong, M. O. Jones, T. C. Xiao and P. P. Edwards, J. Photochem. Photobiol., A, 2010, 212, 8 CrossRef CAS PubMed; (b) Z. K. Xu, L. Han, B. H. Lou, X. W. Zhang and S. J. Dong, Nanoscale, 2014, 6, 145 RSC; (c) H. J. Zhang, Y. X. Yang, Z. Zhou, Y. P. Zhao and L. Liu, J. Phys. Chem. C, 2014, 118, 14662 CrossRef CAS.
- (a) P. Riente, A. M. Adams, J. Albero, E. Palomares and M. A. Pericàs, Angew. Chem., Int. Ed., 2014, 53, 9613 CrossRef CAS PubMed; (b) M. Cherevatskaya, M. Neumann, S. Füldner, C. Harlander, S. Kümmel, S. Dankesreiter, A. Pfitzner, K. Zeitler and B. König, Angew. Chem., Int. Ed., 2012, 51, 4062 CrossRef CAS PubMed.
- P. Riente and M. A. Pericàs, ChemSusChem, 2015, 8, 1841 CrossRef CAS PubMed.
- R. S. Yuan, S. L. Fan, H. X. Zhou, Z. X. Ding, S. Lin, Z. H. Li, Z. Z. Zhang, C. Xu, L. Wu, X. X. Wang and X. Z. Fu, Angew. Chem., Int. Ed., 2013, 52, 1035 CrossRef CAS PubMed.
- Selected examples see: (a) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881 CrossRef PubMed; (b) T. Furuya, A. S. Kamlet and T. Ritter, Nature, 2010, 473, 470 CrossRef PubMed; (c) X.-H. Xu, K. Matsuzaki and N. Shibata, Chem. Rev., 2015, 115, 731 CrossRef CAS PubMed; (d) J. Nie, H. C. Guo, D. Cahard and J.-A. Ma, Chem. Rev., 2011, 111, 455 CrossRef CAS PubMed; (e) O. Tomashenko and V. V. Grushin, Chem. Rev., 2011, 111, 4475 CrossRef CAS PubMed; (f) S. Purser, P. R. Moore, S. Swallow and V. Governeur, Chem. Soc. Rev., 2008, 37, 320 RSC; (g) V. Matoušek, A. Togni, V. Bizet and D. Cahard, Org. Lett., 2011, 13, 5762 CrossRef PubMed.
- C. B. Liu, W. W. Zhao, Y. Huang, H. M. Wang and B. Zhang, Tetrahedron, 2015, 71, 4344 CrossRef CAS PubMed.
- (a) M. L. Guan, C. Xiao, J. Zhang, S. J. Fan, R. An, Q. M. Cheng, J. F. Xie, M. Zhou, B. J. Ye and Y. Xie, J. Am. Chem. Soc., 2013, 135, 10411 CrossRef CAS PubMed; (b) Y. Xu, W. W. Zhao, R. Xu, Y. M. Shi and B. Zhang, Chem. Commun., 2013, 49, 9803 RSC; (c) L. C. Liu, Z. Y. Ji, W. X. Zou, X. R. Gu, Y. Deng, F. Gao, C. J. Tang and L. Dong, ACS Catal., 2013, 3, 2052 CrossRef CAS.
- D. A. Nagib and D. W. C. MacMillan, Nature, 2011, 480, 224 CrossRef CAS PubMed.
- (a) Z. C. Zhang, Y. F. Chen, X. B. Xu, J. C. Zhang, G. L. Xiang, W. He and X. Wang, Angew. Chem., 2014, 126, 439 CrossRef PubMed; (b) L. C. He, Y. Liu, J. Z. Liu, Y. S. Xiong, J. Z. Zheng, Y. L Liu and Z. Y. Tang, Angew. Chem., Int. Ed., 2013, 52, 3741 CrossRef CAS PubMed; (c) Y. L. Hu, J. Liao, D. M. Wang and G. K. Li, Anal. Chem., 2014, 86, 3955 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Optimization of the reaction conditions, experimental procedures and full spectroscopic data for all compounds. See DOI: 10.1039/c5ra08996d |
| This journal is © The Royal Society of Chemistry 2015 |