A surfactant free synthesis and formation mechanism of hollow Cu2O nanocubes using Cl− ions as the morphology regulator†
Abstract
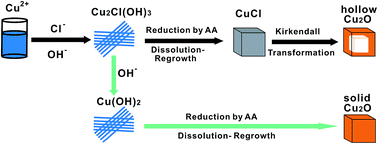
Hollow nanomaterials have attracted intense attention due to their special structures and potential applications in many fields. In this paper, we report a surfactant free synthesis of hollow Cu2O nanocubes by reducing Cu2+ precursors using Cl− ions as the morphology regulator at room temperature. It is found that in the presence of Cl− ions, hollow Cu2O nanocubes can be easily synthesized by directly reducing Cu2+ precursors with ascorbic acid. Through well-designed experiments, we propose that, in this surfactant free synthetic route, the formation of hollow Cu2O nanocubes results from a reaction activated Kirkendall diffusion process of cubic CuCl intermediates, which are formed in the reaction process and act as self-sacrificial templates. The amounts of Cl− ions and NaOH are two key factors to determine whether hollow Cu2O nanocubes are formed or not.

 Please wait while we load your content...
Please wait while we load your content...