Distortion–interaction analysis along the reaction pathway to reveal the reactivity of the Alder-ene reaction of enes†
Abstract
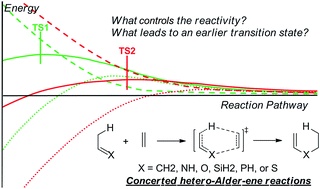
The reactivity of hetero-substituted propylene in uncatalyzed Alder-ene type reactions was investigated using CBS-QB3, G3B3, M11, and B3LYP methods, and the results are interpreted by distortion–interaction analysis of both the transition states and the complete reaction pathways. The reactivity trend for third-period element substituted ene reactants (ethylidenesilane, ethylidenephosphine, and ethanethial) is higher than that of the corresponding second-period element substituted ene reactants (propylene, ethanimine, and acetaldehyde). Theoretical calculations also indicate that for the same period element substituted ene reactants, the reactivity trend is ethylidenesilane > ethylidenephosphine > ethanethial, and propylene > ethanimine > acetaldehyde. Application of distortion–interaction analysis only of the transition states does not give a satisfactory explanation for these reactivities. Using distortion–interaction analysis along the reaction pathways, we found that the reactivity is mainly controlled by the interaction energy. A lower interaction energy along the reaction pathway leads to an earlier transition state and a lower activation energy, which also can be attributed to orbital interaction, closed-shell repulsion, and static repulsion. In some cases, the distortion energy also influences the reactivity.

 Please wait while we load your content...
Please wait while we load your content...