A novel Fe3C/graphitic carbon composite with electromagnetic wave absorption properties in the C-band†
Abstract
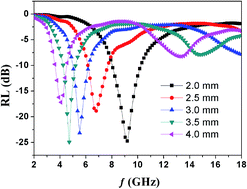
An electromagnetic (EM) wave absorber with characteristic absorption in the millimeter wave range is very important for the EM industry. Especially, absorption properties in the microwave frequency range C-band (4–8 GHz) are necessary in the field of military aircraft. Herein, a Fe3C/graphitic carbon (Fe3C/GC) composite has been synthesized through a low-cost and facile ion-exchange route. The Fe3C/GC could exhibit excellent EM wave absorption properties in the C-band with the frequency range of 4–8 GHz and also exhibits good dielectric loss, which could be attributed to the existence of lightweight GC in the composite. The reflection loss value of the Fe3C/GC composite exceeds −10 dB in the frequency range of 4–8 GHz with an absorber thickness of 2–4 mm, implying its potential application as a lightweight EM wave absorber in the C-band region. Moreover, the Fe3C/GC composite shows better electromagnetic wave absorption properties than other iron compound GC-based composites, such as Fe2N/GC and Fe/GC.

 Please wait while we load your content...
Please wait while we load your content...