Hydrogen production by aqueous-phase reforming of ethylene glycol over a Ni/Zn/Al derived hydrotalcite catalyst
Guanyi Chen*abcd,
Ningge Xua,
Xiangping Lia,
Qingling Liua,
Huijun Yanga and
Wanqing Lia
aSchool of Environmental Science and Engineering/State Key Laboratory of Engines, Tianjin University, Tianjin 300072, China. E-mail: Chen@tju.edu.cn
bSchool of Science, Tibet University, No. 36 Jiangsu Street, Lhasa, 850012, Tibet Autonomous Region, China
cKey Laboratory of Biomass-based Oil and Gas (Tianjin University), China Petroleum and Chemical Industry Federation, China
dKey Laboratory of Efficient Utilization of Low and Medium Grade Energy (Tianjin University), Ministry of Education, Tianjin 300072, China
First published on 1st July 2015
Abstract
Ni/Zn/Al hydrotalcites (Ni/Zn/Al-HT) with different Ni/Zn ratios have been prepared by a coprecipitation method. The properties and microstructure of Ni/Zn/Al-HT precursors and derived catalysts were characterized by X-ray diffraction (XRD), H2-temperature programmed reduction (TPR), N2 physical adsorption analysis (BET), scanning electron microscopy (SEM) and particle size distribution. The results exhibited that the as-prepared samples consisted of a hydrotalcite phase and a ZnO phase, and Zn2+ was introduced into the layers. At the ratio of Ni/Zn = 1, the ZnAl2O4 phase emerged after calcination; Ni still remained in its original state after reaction, and ZnO always existed during the whole process. In aqueous-phase reforming (APR) of ethylene glycol, the H2 production rate over Ni/Zn/Al-HT was high, and the selectivity of H2 can almost reach 100% with a high conversion rate exceeding 99%.
Introduction
Hydrogen is nonpolluting and efficient as a potential desirable energy source. However, nearly 95% of the world hydrogen energy is produced from fossil fuels at present.1 With the depletion of fossil fuels, using renewable sources for hydrogen production is an attractive alternative. What is more, the production of H2 from renewable biomass sources is environmentally-friendly due to its carbon-neutral nature.Aqueous-phase reforming (APR) is a lower energy consumption technology for conversion of biomass to hydrogen than steam reforming or gasification which usually occurs at high temperature and is accompanied by many side reactions. It needn't vaporize water and minimizes undesirable decomposition reactions, meanwhile, the water-gas shift reaction (WGS) is active at the low temperature, which is possible to realize generating H2 and CO2 in a single step with low levels of CO.
Many oxygenated compounds derived from biomass, such as methanol, ethanol, sorbitol, glycerol and ethylene glycol have been used in APR process.2–8 The mechanism of hydrogen production of ethylene glycol had been researched by Shabaker et al.4,9,10 Ethylene glycol in which each carbon atom bonds to an oxygen atom is capable of being converted to more H2 and CO2 by means of C–C scission and WGS reaction. There is not only the process of the cleavage of C–C bonds in the APR, but also involves C–H scission to form adsorbed species on the catalyst surface, such as CO, which is going through water-gas shift to form CO2. Thus, the catalyst applied to the reaction must be chosen to be favor in the cleavage of C–C and WGS reaction, but should inhibit C–O scission and methanation reactions.
In the past decades, many catalysts (such as supported Pt, Pd, Ru, Rh and Ir) had been tested in APR process. These catalysts with exorbitant price presented good activity and hydrogen selectivity,3–5,11,12 but the expensive price prohibited their extensive use. Currently, non-noble based catalysts used for hydrogen production aroused researchers' widespread interest, especially VIII group transition metals. Just as noble metal, the non-noble metal also possesses superior catalytic activity and ability of C–C scission. Skeletal Ni and Sn modified RANEY®-Ni catalysts showed equivalent catalytic effect,11,13–15 but the steps of synthesizing supported Ni catalysts appeared cumbersome. Thus, it is significantly interesting to develop high efficient catalytic material with low cost and simple prepared method for hydrogen production by APR process of oxygenated hydrocarbons.
Hydrotalcite-like compounds are being the center of interests of academics and industrials as layered double hydroxides (LDHs) which include lamellar materials derived from brucite layers.16–19 These compounds are usually expressed by the formula as: [M2+(1−x)M3+x(OH)2]·[An−]x/n·mH2O; where M2+ is a bivalent cation like Mg2+, Ni2+, Cu2+, Zn2+; M3+ is a trivalent cation like Al3+, Fe3+, Mn3+, Cr3+; and An− is a convertible anion located in the interlayer space for the sake of balancing the positive charge of the layers. The LDHs will become an amorphous metal oxides with small crystal size and high thermal stability by means of heating, which is called ex-LDHs. The ex-LDHs present significant properties such as large surface area, high dispersion of the active metal,16,20–22 the function of memory or the interaction between different cations in the oxides matrix.23,24 Furthermore, the small and thermally stable metallic crystalline will be formed through the reduction treatment. Thus, many kinds of hydrotalcite-like compounds have been widely used for hydrogen production in the aqueous-phase reforming of hydrocarbons or alcohols.7,15,17,25 Pan had demonstrated the catalyst of Ni/Sn/Al derived from hydrotalcite performed well activity in the process of aqueous-phase reforming of ethylene glycol, in which the selectivity of H2 reached 100%. However, Sn2+ wasn't introduced into the layers because of its large ionic radii.25 The researchers also tested the activity of Ni supported catalysts by reduction of Ni/Mg/Al for aqueous-phase reforming. The Ni/Mg/Al catalyst derived hydrotalcite exhibited a rather high activity as well as high H2 selectivity (98.84%), but the catalyst was easily deactivated in the aqueous solutions.26,27 Liu reported that Ni/Zn/Al could form well hydrotalcite crystalline with the proper ratio by coprecipitation so that it possessed all the property that hydrotalcite had,28 but their catalysts didn't show any catalytic performance or effects of preparation process on it, because the catalysts didn't be applied to catalyze any reactions.
Herein, the Ni/Zn/Al derived from hydrotalcite was used as catalyst for hydrogen production by the aqueous-phase reforming of ethylene glycol. The performance of H2 production was tested over Ni/Zn/Al catalysts after reduction. The effects of the atomic ratio Ni/Zn and reaction conditions on the catalytic performance was investigated in detail (Fig. 1).
Experimental
Catalyst preparation
A series of Ni/Zn/Al hydrotalcite-like compounds with different Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn
Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al atomic ratios were synthesized by a coprecipitation method.28 An aqueous solution (50 mL) containing Ni(NO3)2·6H2O, Al(NO3)3·9H2O and Zn(NO3)2·6H2O with the cation concentration of 1 mol L−1 was added dropwise with vigorous stirring into 50 mL of NaOH and NaCO3 solutions (CO32−/Al3+ = 0.375 and OH−/Al3+ = 6.3) at ambient temperature. The pH was controlled between 6 and 8, and the resulting slurry was aged at 333 K for 18 h. The precipitate was washed thoroughly with distill water under 100 °C till the pH of the filtrate up to 7, then the samples were dried at 80 °C overnight, crashed and passed through an 80-mesh sieve. The prepared catalysts precursors were designated as Ni/Zn/Al-HT.
Al atomic ratios were synthesized by a coprecipitation method.28 An aqueous solution (50 mL) containing Ni(NO3)2·6H2O, Al(NO3)3·9H2O and Zn(NO3)2·6H2O with the cation concentration of 1 mol L−1 was added dropwise with vigorous stirring into 50 mL of NaOH and NaCO3 solutions (CO32−/Al3+ = 0.375 and OH−/Al3+ = 6.3) at ambient temperature. The pH was controlled between 6 and 8, and the resulting slurry was aged at 333 K for 18 h. The precipitate was washed thoroughly with distill water under 100 °C till the pH of the filtrate up to 7, then the samples were dried at 80 °C overnight, crashed and passed through an 80-mesh sieve. The prepared catalysts precursors were designated as Ni/Zn/Al-HT.
Catalyst characterization
The XRD patterns were obtained on a Bruker D8 Advance X-ray diffractometer using Ni-filtered Cu Kα radiation (1.5418 Å) with a scanning angle (2θ) of 10–90°. The voltage was 40 kv, and the current was 40 mA. The Scherrer equation was used to estimate the crystallite size. The textual characteristics, such as BET specific area and pore volume (BJH method), were determined by N2 physisorption at 77 K. Prior to the analysis the samples were outgassed at 423 K under a N2 flow for 6 h. The reducibility of the catalysts was analyzed by temperature programmed reduction (TPR), carried out in Autosorb-iQ-C-TCD-MS apparatus. Scanning electron microscope (SEM) measurements were performed with a ZEISS MERLIN Compact microscope. For the sake of removing the surface contaminants, 50 mg of the catalyst was pretreated at 300 °C in a pure N2 for 3 h, then cooled to the room temperature, and then kept it under 90% H2/Ar in the range of 23–900 °C at a heating rate of 10 °C min−1. The amount of H2 consumed was quantified with a thermal conductivity detector (TCD) equipped with a 5 Å molecular sieve to remove H2 from the effluent gas. To determine their particle size distribution, the samples were pretreated by sonication in water for 2 h and then analyzed with a nanoparticle analyzer (DelsaNano C, Beckman Coulter, Fullerton, CA).Catalytic activity tests
The catalytic activity test for aqueous-phase reforming (APR) of ethylene glycol was carried out in an autoclave reactor with a capacity of 100 mL, in which there were an 20 wt% aqueous solution and 0.2 g catalyst. Before the test, the catalyst was reduced at 500 °C for 4 h under H2 flow (50 mL min−1), using heating rate of 10 °C min−1. The reaction took place at 210–240 °C, and the pressure in the reactor was regulated to 1.95–3.4 MPa with nitrogen gas. The gas products were collected and analyzed by gas chromatography (Beifen GC-3420A), equipped with 3 m Porapak Q column and 2 m 5 Å molecular sieve (TCD and FID). The products detected were H2, CO2, CO, CH4 and C2H6 in the gas phase, which were calculated without considering water for their selectivity. The liquid phases were analyzed by Agilent 7890A 60 m DB-Wax capillary column. The analysis result presented that liquid products mainly contained un-reacted ethylene glycol, methanol and acetic acid. The data were collected for up to 3 h for each set of reaction condition taking into account the performance of APR in order to assure that the catalyst system reached steady state.H2 selectivity was calculated by hydrogen amounts of H2/total carbon amounts of gas products ×  where R was the H2/CO2 reforming ratio of 5/2 for ethylene glycol; alkane selectivity was calculated by carbon amounts of alkane/total carbon amounts of gas products.
where R was the H2/CO2 reforming ratio of 5/2 for ethylene glycol; alkane selectivity was calculated by carbon amounts of alkane/total carbon amounts of gas products.
Results and discussion
Structure analysis of hydrotalcite precursors
It's of vital importance to detect that whether the as prepared Ni/Zn/Al-HT samples form the hydrotalcite phase, so the XRD patterns of it are shown in Fig. 2 (Fig. 2: (Ni + Zn)/Al = 4, and Ni/Zn = 0.25, 0.5, 1, 2, 4 respectively). Only the precursor of Ni/Zn = 4 formed a single hydrotalcite phase, while in the case that Ni/Zn ≤ 2, a ZnO phase was observed obviously and accompanied with a hydrotalcite phase. Liu reported that the precursors could form a hydrotalcite phase when the ratio of Ni/Zn was between 0.25 and 4, at the same time the ratio of (Ni + Zn)/Al equaled to 4–6,28 Fig. 2 shows the similar result. The formation of ZnO can be ascribed to that more spare Zn2+ formed Zn(OH)2 when the ratio of Ni/Zn is smaller than the ratio in the desired hydrotalcite, Zn(OH)2 is converted to ZnO through drying out because of its easy dehydration.29 The interlayer space (dc) of Ni/Zn/Al-HT (Ni/Zn = 0.25, 0.5, 1, 2, 4) is 7.7402, 7.6448, 7.7330, 7.7221, 7.7017 Å, respectively. Thus it can be seen that the value of dc decreases with the content of Zn2+ decreasing, which illustrates that the Zn2+ is introduced in the layer. Several parameters of hydrotalcite can be calculated by the follow functions:30
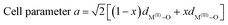 | (1) |
 | (2) |
 | ||
| Fig. 2 XRD patterns of Ni/Zn/Al-HL precursors with different Ni/Zn ratio. (a) Ni0.8Zn3.2Al; (b) Ni1.33Zn2.66Al; (c) Ni2Zn2Al; (d) Ni2.66Zn1.33Al; (e) Ni3.2Zn0.8Al, (◆): hydrotalcite; (●): ZnO. | ||
The results of H2-TPR analysis of catalysts with different Ni/Zn ratio are shown in Fig. 3. It is apparent that every catalyst has an obvious main reductive peak around between 217 and 513 °C. As is shown in Fig. 3, the low temperature reductive peaks are around 282, 320, 314 and 304 °C for the four catalysts except Ni2.66Zn1.33Al. However, the H2 consumption reductive peak of Ni2.66Zn1.33Al appears at 419 and 473 °C with a shoulder peaks around 349 °C. Wang reported that single NiO would reduce at 375 °C with H2 consumed;32 for our catalysts, the reductive temperature of NiO shows lower than that. This case is supposed that the NiO reduced already in our catalysts is divided into two kinds: one is free or weak interaction with other oxides, so it can be reduced easily under low temperature; and the other interacts with other oxides strongly under enough content of Ni in catalysts, which needs higher reductive temperature. Moreover, ZnAl2O4 will be showed up in calcined catalysts when the content of Ni is adequate.33 Thus, the low temperature reductive peaks between 217 and 435 °C in every catalyst ascribe to the reduction of the first kind of NiO demonstrated before; the high temperature reductive peaks at 361, 530, 473 and 386 °C in the four catalysts except Ni2Zn2Al are due to the reduction of the other kind of NiO, it's likely that all the NiO had been reduced at the same time period in Ni2Zn2Al so as to forming the single reductive peak of NiO with the strongest intensity. The shoulder peaks at 527, 530, 573, 559 and 526 °C in the every catalyst is attributed to the reduction of Al3+ probably. More content of Ni causes the intensity of H2 consumption peak at 304 °C of Ni3.2Zn0.8Al becoming weak, because the quantity of NiO that possessed reduction ability decreased, the Ni2+ was introduced into ZnAl2O4 formed as demonstrated before instead of Zn2+, thereupon it got hardly reduced as the same as Zn2+.
 | ||
| Fig. 3 H2-TPR profiles of Ni/Zn/Al hydrotalcite. (a) Ni0.8Zn3.2Al; (b) Ni1.33Zn2.66Al; (c) Ni2Zn2Al; (d) Ni2.66Zn1.33Al; (e) Ni3.2Zn0.8Al. | ||
Table 1 listed the BET (BJH method) results of Ni/Zn/Al-HT catalysts with different Ni/Zn ratio. Ni0.8Zn3.2Al has the biggest surface area, pore volume and pore size. The surface area decreases gradually with the increase of Ni content firstly, and then increasing when the ratio of Ni/Zn is up to 4.
| Samples | BET (m2 g−1) | Pore volume (cm3 g−1) | Pore size (nm) |
|---|---|---|---|
| Ni0.8Zn3.2Al | 37.40 | 0.16 | 53.08 |
| Ni1.33Zn2.66Al | 26.08 | 0.09 | 15.90 |
| Ni2Zn2Al | 7.42 | 0.03 | 21.72 |
| Ni2.66Zn1.33Al | 3.58 | 0.01 | 17.14 |
| Ni3.2Zn0.8Al | 18.91 | 0.08 | 21.72 |
Catalytic performance
The aqueous-phase reforming reaction of ethylene glycol is mainly through the pathway of C–C cleavage and WGS reaction to produce H2 as shown in Fig. 4. Meanwhile, some side reactions also happen, which generate side products like C2H6, C2H5OH and so on. | ||
| Fig. 4 Part reaction pathways from reaction of ethylene glycol with water (*represents a surface metal site).3 | ||
In our reaction, the H2 production and selectivity increase from 72.74% first, and then decline to 67.05% with the contents of Ni increasing, as is shown in Table 2. However, the yield of CO2 and alkanes selectivity present opposite tendency. The phenomenon can be attributed to the high content of metal, which may contribute to catalyst deactivation because of the aggregation of metallic particles partly. It can be seen that the alkanes selectivity reaches the most (23.93%) when the Ni content increases to Ni/Zn = 4, which illustrates that methanation reaction will be strengthened with enough Ni. Optimistically, all conversion of ethylene glycol is over 99%. It is obvious that the catalyst with Ni/Zn = 1 possesses the best catalytic ability. The H2 selectivity reaches 100%, the alkanes selectivity is as low as 7.36% and the content of H2 in the gas product is 79.95%. The case is probably supposed that moderate content of Ni will promote the cleavage of C–C to the greatest extent, and weaken the methanation reaction as well. In addition, some crystal may form, which is also to the benefit of H2 production. So it is chosen for further investigation.
| Samples | Gas phase composition | H2 sel% | Alkanes sel% | Conversion% | |||
|---|---|---|---|---|---|---|---|
| H2% | CO2% | CH4% | C2H6% | ||||
| a Reactant variety: 20 wt% solution of ethylene glycol; reaction temperature and pressure: 225 °C, 2.6 MPa. | |||||||
| Ni0.8Zn3.2Al | 72.74 | 20.28 | 0 | 1.74 | 95.58 | 21.35 | 99.85 |
| Ni1.33Zn2.66Al | 73.20 | 22.28 | 1.95 | 0.77 | 100.00 | 12.70 | 99.74 |
| Ni2Zn2Al | 79.95 | 18.10 | 0.77 | 0.36 | 100.00 | 7.36 | 99.27 |
| Ni2.66Zn1.33Al | 70.24 | 28.13 | 0.33 | 0.21 | 93.77 | 2.50 | 99.82 |
| Ni3.2Zn0.8Al | 67.05 | 25.54 | 6.71 | 0.67 | 79.75 | 23.93 | 99.87 |
XRD, SEM and particle size distribution analysis of catalysts
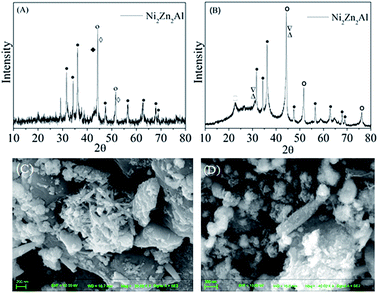
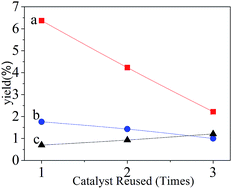
Three oxides had been formed in the Ni2Zn2Al derived hydrotalcite after calcination: the wurtzite type phase (ZnO), the rock salt type phase (NiO) and the spinel phase (ZnAl2O4), which corresponds to the report given by Resini. C.20 At the same time, the crystalline of Al2O3 was highly dispersed in the whole calcining process.28 The component of NiO in the calcined catalyst probably was reduced to Ni thereby playing a great part in promoting hydrogen production.25 The result of XRD patterns after reduction is shown in Fig. 5(A). It can be clearly seen that metal Ni was formed as expected at 39.45, 46.84, 71.3° with the crystalline of AlNi and AlNi3 alloy formed at the same time. As demonstrated before, ZnO is hardly to reduce Zn2+, so the phase of ZnO can still be detected. The H2 production and catalytic activity are probably facilitated by ZnO as well.However, some crystalline may change with the reaction happening. Fig. 5(B) shows the XRD result of Ni2Zn2Al derived hydrotalcite after reaction. The composition of catalyst contains mainly Ni, ZnO, H2O and AlNi alloy with different ratios. The catalyst still remains the component activated to H2, which is considered as a factor to have ability to recycle as shown in Fig. 6. With increasing the number of repeated use, it can be seen that the catalytic performance of the recycling catalysts is weakening gradually. But a certain amount of H2 is still generated by reusing catalysts for two times. The SEM pattern of Ni2Zn2Al derived hydrotalcite after reduction is exhibited in Fig. 5(C). The structure is composed of three phase: porous Ni-rich compact regions occupied much of the pattern, nanorod of ZnO regions around the Ni-rich regions and crystalline regions sticking to the Ni-rich regions containing AlNi alloy with different ratios.34,35 As suggested in Fig. 5, the Ni-rich regions still remained after exposure to aqueous-phase reforming reaction conditions, which displays in Fig. 5(D). However, the nanorod of ZnO is adhered to much crystalline of AlNi and Al0.96Ni1.04 alloy, which is difficult to be observed. The SEM results are corresponded to the XRD patterns apparently and the particle size distribution patterns shown in Fig. 6. From Fig. 6 and Table 3, it is obvious that the average particle size of the catalysts after reduction and reaction are 604.1 and 550.5 nm, respectively. But the catalysts happened to aggregate probably after reaction, so that the polydispersity index is bigger than that after reduction.
| D10 (nm) | D50 (nm) | D90 (nm) | Average (nm) | Polydispersity index | |
|---|---|---|---|---|---|
| After reduction | 490.00 | 579.00 | 686.30 | 604.10 | 0.127 |
| After reaction | 464.20 | 539.60 | 627.80 | 550.50 | −2.163 |
Effects of reaction conditions over Ni2Zn2Al-HT
The influences of reaction temperature and pressure on the yield rate of H2, CO2 and CH4 over Ni2Zn2Al hydrotalcite derived catalyst is shown in Fig. 7 and 8.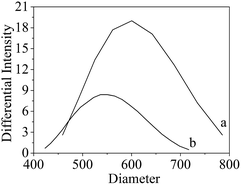 | ||
| Fig. 7 Particle size distribution patterns of Ni2Zn2Al derived hydrotalcite. (a) After reduction; (b) after reaction. | ||
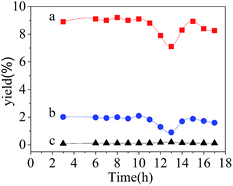
Fig. 8 indicates that the best result appears at 225 °C. The content of H2 in the gas product grows up from 1.68 to 8.9% with the temperature increasing from 210 to 225 °C, then declines to 6.4% when the reaction temperature increases to 240 °C. The H2 selectivity reaches nearly 100%, and the methane selectivity increases from 7.36 to 32.95%, following on dropping to 5.38%. The tendency is similar to the report given by Pan.25 Davda also reported that the H2 selectivity would decrease when the reaction temperature increased over Pt/Al2O3, and the alkanes selectivity exhibited the increasing trend.3 With increasing of reaction temperature, it appears that CO and CO2 in the gas product both take the methanation reaction with H2; meanwhile, the water-gas shift reaction is weakened.
As illustrated in Fig. 9, H2 production decreases from 8.9 to 1.45% when the pressure increases from 2.6 to 3.0 MPa. The H2 selectivity remains nearly 79%, and alkanes selectivity reaches 12%. With reaction pressure rising, the partial pressure of H2 increases in the gas products, thus, the equilibrium of chemical reaction is broken and moves reversely, the rate of reaction decreases in consequence; moreover, the increasing of pressure results in the rate of H2 and CO decreasing, which are desorbed from the catalyst catalytic site, so it is more likely to take methanation reaction, then leads to H2 production decreasing.36,37
The catalytic performance versus time on the stream for aqueous-phase reforming of 20 wt% ethylene glycol over Ni2Zn2Al hydrotalcite derived catalyst is exhibited in Fig. 10. It is apparent that the catalytic activity exists the tendency of declining, but the deactivation phenomenon doesn't sustain too long, otherwise the degree of deactivation isn't dramatic either. The H2 production also existed decreasing trend after 10 h over Pt/Mn and Pt/Al2O3 given by Kim.38 Resini C reported that the catalytic activity of Ni50ZnAl-HT decreased after 500 min in the process of ethanol steam reforming.20 Therefore, further studies are needed to investigate the mechanism of deactivation and the effect of Ni/Zn ratio.
Conclusion
Ni/Zn/Al hydrotalcite (Ni/Zn/Al-HT) catalysts with different ratios of Ni/Zn are synthesized by a coprecipitation method. The results present that Zn2+ was introduced in the layers. XRD patterns suggest that the as-prepared samples was composed by a hydrotalcite phase and a ZnO phase. ZnO may be in favor of H2 selectivity and catalytic activity in aqueous-phase reforming of ethylene glycol. A high H2 production yield, a good H2 selectivity of 100% and a high conversion in excess of 99% are showed over Ni2Zn2Al. Ni/Zn/Al-HT appears very interesting in potential application for aqueous-phase reforming reaction due to its easy fabrication and good performance.Acknowledgements
This paper is financially supported by National Basic Research Program of China through 973 Program (2012CB215303).References
- D. Seth, Int. J. Hydrogen Energy, 2002, 27, 235–264 CrossRef
.
- R. D. Cortright, R. R. Davda and J. A. Dumesic, Nature, 2002, 418, 964–967 CrossRef CAS PubMed
.
- R. R. Davda, J. W. Shabaker, G. W. Huber, R. D. Cortright and J. A. Dumesic, Appl. Catal., B, 2005, 56, 171–186 CrossRef CAS PubMed
.
- J. W. Shabaker, R. R. Davda, G. W. Huber, R. D. Cortright and J. A. Dumesic, J. Catal., 2003, 215, 344–352 CrossRef CAS
.
- J. W. Shabaker, G. W. Huber, R. D. Cortright and J. A. Dumesic, Catal. Lett., 2003, 88, 1–7 CrossRef CAS
.
- R. R. Soares, D. A. Simonetti and J. A. Dumesic, Angew. Chem., Int. Ed., 2006, 45, 3982–3985 CrossRef CAS PubMed
.
- I. O. Cruz, N. F. P. Ribeiro, D. A. G. Aranda and M. M. V. M. Souza, Catal. Commun., 2008, 9, 2606–2611 CrossRef CAS PubMed
.
- P. V. Tuza, R. L. Manfro, N. F. P. Ribeiro and M. M. V. M. Souza, Renewable Energy, 2013, 50, 408–414 CrossRef CAS PubMed
.
- J. W. Shabaker and J. A. Dumesic, Angew. Chem., Int. Ed., 2004, 43, 3105–3112 CAS
.
- A. B. Jain and P. D. Vaidya, Energy Fuels, 2015, 29, 361–368 CrossRef CAS
.
- G. W. Huber, J. W. Shabaker and J. A. Dumesic, Science, 2003, 300, 2075–2077 CrossRef CAS PubMed
.
- B. A. Kaya, S. Irmak, A. H. Glu and O. Erbatur, Int. J. Hydrogen Energy, 2015, 40, 3849–3858 CrossRef CAS PubMed
.
- F. Xie, X. Chu, H. Hu, M. Qiao, S. Yan, Y. Zhu, H. He, K. Fan, H. Li, B. Zong and X. Zhang, J. Catal., 2006, 241, 211–220 CrossRef CAS PubMed
.
- J. W. Shabaker, G. W. Huber and J. A. Dumesic, J. Catal., 2004, 222, 180–191 CrossRef CAS PubMed
.
- J. W. Shabaker, D. A. Simonetti, R. D. Cortright and J. A. Dumesic, J. Catal., 2005, 231, 67–76 CrossRef CAS PubMed
.
- F. Cavani, F. Trifiro and A. Vaccari, Catal. Today, 1991, 11, 173–301 CrossRef CAS
.
- W. Feitknecht, Helv. Chim. Acta, 1942, 25, 555–569 CrossRef CAS PubMed
.
- M. Li, X. Wang, S. Li, S. Wang and X. Ma, Int. J. Hydrogen Energy, 2010, 35, 6699–6708 CrossRef CAS PubMed
.
- S. Miyata, Clays Clay Miner., 1983, 31, 305–311 CAS
.
- C. Resini, T. Montanari, L. Barattini, G. Ramis, G. Busca and S. Presto, et al., Appl. Catal., A, 2009, 355, 83–93 CrossRef CAS PubMed
.
- L. Dussault, J. C. Dupin, E. Dumitriu, A. Auroux and C. Guimon, Thermochim. Acta, 2005, 434, 93–99 CrossRef CAS PubMed
.
- S. H. Sarijo, S. A. I. S. M. Ghazali and M. Z. Hussein, J. Porous Mater., 2015, 22, 473–480 CrossRef CAS
.
- V. Mas, M. L. Dieuzeide, M. Jobbágy, G. Baronetti, N. Amadeo and M. Laborde, Catal. Today, 2008, 133, 319–323 CrossRef PubMed
.
- O. Meyer, F. Roessner, R. A. Rakoczy and R. W. Fischer, ChemCatChem, 2010, 2, 314 CrossRef CAS PubMed
.
- G. Pan, Z. Ni, F. Cao and X. Li, Appl. Clay Sci., 2012, 58, 108–113 CrossRef CAS PubMed
.
- G. Pan, Z. Ni, F. Cao and X. Li, J. Chin. Ceram. Soc., 2010, 38, 1328–1332 CAS
.
- G. Pan, F. Cao, Z. Ni, X. Li, H. Chen, B. Tang and M. Xu, Bull. Chin. Ceram. Soc., 2011, 39, 33–37 Search PubMed
.
- J. Liu and X. Xie, J. Fuel Chem. Technol., 2001, 29, 69–72 Search PubMed
.
- T. Chen, M. Zhou, L. Wang and Z. Zhan, Chin. J. Synth. Chem., 2002, 10, 45–48 CAS
.
- Z. Ni, G. Pan, L. Wang, C. Fang and D. Li, Chin. J. Inorg. Chem., 2006, 22, 91–95 CAS
.
- L. Ren, F. Li, J. He and X. Duan, J. Beijing Univ. Chem. Technol., Nat. Sci. Ed., 2002, 29, 77–79 CAS
.
- Y. Wang, M. Sc. thesis, Dalian University of Technology, 2007
.
- Z. Zhou, A. Zhang, M. Gong and Y. Chen, Chem. Res. Appl., 2000, 12, 521–524 CAS
.
- J. Dong, F. Zhang, W. Zhang and Y. Yang, J. Funct. Mater. Devices, 2011, 36, 657–660 CAS
.
- J. Chen and Z. Xu, Shanghai Nonferrous Met., 2003, 24, 12–15 CAS
.
- Y. Bai, C. Lu, L. Ma, P. Chen, Y. Zheng and X. Li, Chin. J. Catal., 2006, 27, 275–280 CAS
.
- J. Xie, X. Yin, D. Su, C. Wu and J. Zhu, Trans. Chin. Soc. Agric. Mach., 2011, 42, 104–110 CAS
.
- H.-D. Kim, H. J. Park, T.-W. Kim, K.-E. Jeong, H.-J. Chae, S.-Y. Jeong, C.-H. Lee and C.-U. Kim, Int. J. Hydrogen Energy, 2012, 37, 8310–8317 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2015 |