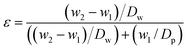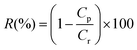From hydrophobic to hydrophilic polyvinylidenefluoride (PVDF) membranes by gaining new insight into material's properties
E. Fontananova*a,
M. A. Bahattabb,
S. A. Aljlilb,
M. Alowairdyb,
G. Rinaldic,
D. Vuonoc,
J. B. Nagyc,
E. Drioliac and
G. Di Profioa
aInstitute on Membrane Technology of the National Research Council of Italy (ITM-CNR), University of Calabria, Via P. Bucci, cubo 17/C, 87036 Rende (CS), Italy. E-mail: e.fontananova@itm.cnr.it
bKing Abdulaziz City for Science and Technology (KACST), P.O. Box 6086, Riyadh-11442, Saudi Arabia
cDepartment of Environmental and Chemical Engineering (DIATIC), University of Calabria, 87036 Rende (CS), Italy
First published on 19th June 2015
Abstract
This work provides an easy and versatile strategy to manufacture novel polyvinylidenefluoride (PVDF) membranes by solution casting and phase separation techniques displaying tailored physicochemical and microstructural features depending on the opportune combination of functionalization by blending chemical additives (multiwalled carbon nanotubes, MWCNTs) and manufacturing procedure. The systematic study of the effect of (i) polymer concentration, (ii) use of pore forming additives (LiCl), and (iii) type and concentration of MWCNTs, on the PVDF crystalline composition and membrane microstructure, highlights the strong relationships of these parameters with the wettability, fouling and transport attributes of the formed membranes. The results provide the key to discriminate membrane preparation conditions favoring hydrophilic, low fouling, and highly selective PVDF–MWCNTs membranes, for water-treatment applications in pressure-driven membrane operations, from conditions favoring the formation of hydrophobic and waterproof membranes, to be used in the membrane contactors field. Also, they open exciting perspectives for a more effective development of PVDF-based nanostructured membranes for advanced separations based on a comprehensive investigation and understanding of material's properties.
Introduction
Modern chemical industry requires advanced separations to boost production efficiency and product quality according to a process intensification strategy.1 In this context, improved membrane materials, displaying tailored chemical functionalities and an engineered structure at multi-scale level, would provide improved separation ability (selectivity), enhanced transmembrane flux and reduced fouling (productivity), compared to traditional ones. Among the different membrane-forming materials, one of the most used is polyvinylidenefluoride (PVDF) (Fig. 1). PVDF is a semicrystalline material with four possible conformations named as α, β, γ, and δ phase.2–5 The C–F bonds are polar and the highest dipole moment is obtained with the alignment of all dipoles of the polymer in the same direction, corresponding to the β-phase of the PVDF. The dipole moments of α crystallites are oriented in opposite directions, resulting in a zero net polarization. PVDF has outstanding properties in terms of thermal stability, chemical resistance and processability to form membranes by casting solution methods.3–5 Thank to these features, applications of PVDF membranes are currently found in pressure-driven water- and waste-treatment treatment (e.g., microfiltration, ultrafiltration and membrane bioreactor), and membrane contactors operations (e.g., membrane distillation, acid gas absorption and stripping, volatile organic compounds removal).3Despite its attractiveness as high performing polymer material, the performances of PVDF membranes are substantially limited by fouling in the case of pressure-driven membrane operations,6,7 and wetting in membrane contactors applications.3,8 To mitigate these problems, hydrophilic modification of PVDF has been currently adopted with the aim of reducing fouling, while enhanced hydrophobicity would improve wetting-resistance. These modifications can be achieved during membrane synthesis by blending the polymer with chemical modifiers. Hydrophilic polymer (e.g. polyvinylpyrrolidone) or inorganic particles (e.g. TiO2, ZrO2 and carbon nanotubes, (CNTs) can be used to enhance hydrophilicity.9–12 More hydrophobic polymers (e.g. perfluoropolymers) or co-polymers with higher fluorine content than the homopolymer (e.g. polyvinylidenefluoride-co-hexafluoropropylene) can be used to increase membrane hydrophobicity.3,13 In alternative, PVDF membranes can be post-treated after manufacture. This can be achieved by hydrophilization through physical surface modification (e.g. coating with a hydrophilic polymer layer) or chemical treatment (e.g. plasma grafting of polar groups).4,13–16 Hydrophobicization by coating with a superhydrophobic layer or grafting of fluorinated species is also possible.3 Furthermore, surface roughness modulation is known to provide additional options to tailor the hydrophobic/hydrophilic character of the membrane surface.17,18
Despite the numerous progresses in the production and modification of PVDF membranes, a comprehensive study on the combined effects of the polymer crystalline phase and surface roughness on hydrophobic/hydrophilic behaviour and transport properties of PVDF membranes, at the best of our knowledge, has not been report yet. This is particularly important in the case membranes containing low percentage of functional additive (typically <3 wt%). Often the increase of hydrophilicity of composite or mixed matrix membranes prepared by blending inorganic filler with the polymer is attributed to the presence of polar groups on these additives. It is interesting to note that graphitic carbon nanomaterials like CNTs and graphene oxide (GO) are usually introduced at lower content (≤2 wt%) in composite membrane in comparison to three-dimensional inorganic nanofillers like TiO2 and ZrO2 (usually blended at loading ≥5 wt%, up to 60 wt%), because of their high specific surface, elevated aspect ratio, and the intrinsic properties of graphitized structure.19–22 In addition, the relatively easy functionalization of the surface of carbon nanomaterials render them ideal candidate to tailor the polymer/nanofiller interface in mixed matrix membranes.
Zhang11 prepared PVDF mixed matrix membranes containing 1 wt% of CNTs and/or GO observing a relevant decrease of the water contact angle and an increase of water flux in comparison with the polymeric membranes. These results are attributed mainly to the presence of oxygen containing functional groups on the nanofillers used. Also Silva23 attributed the changes in surface wettability of PVDF membranes containing CNTs to the chemical composition of the additives used at loading ≤1 wt%.
However, it is necessary to consider that the chemical nature of a component present in large defect with respect to the polymer, it could not be sufficient to explain large changes in membrane properties. On the contrary it is well accepted that a functional additive can influence, also if present at low loading, the properties of the main component of the membranes, i.e. the polymer, during the fabrication process in terms of microstructure, as well as, crystalline content in the case of crystalline or semicrystalline polymers.5
In addition to the presence of functional additives in the casting solution, also the membrane preparation conditions (e.g. type of solvent and concentration of the polymer) can influence physicochemical and morphological characteristic of the PVDF membranes, including the polymorphism.24–27 However, in the literature works is not reported a clear correlation between the dominating crystalline phases and the performance in separation processes like fouling tendency of the membranes.
Therefore, it is an important challenge to achieve a more comprehensive understanding of the relationship between the physicochemical and morphological properties of PVDF membranes with their performance.
In this work we propose two different strategies (namely, protocol A and B) to manufacture PVDF-based membranes displaying tailored physicochemical and morphological properties, by selecting the optimum combination between functionalization by blending chemical additives (multiwalled carbon nanotubes (MWCNTs)) and membrane preparation conditions. A systematic study on the effect of: (i) polymer concentration, (ii) use of pore forming additive (LiCl), and (iii) type and concentration of MWCNTs, is performed with the aim to develop PVDF membranes with tuneable properties selected for specific applications, such as water- and waste-treatment by pressure-driven operations (hydrophilic, low fouling, highly selective membranes) or membrane contactors applications (hydrophobic, waterproof membranes).
The results open exciting perspectives for further progress in PVDF membranes design and development for advanced separations based on a deep understanding and a fine modulation of material properties.
Experimental
Membranes preparation
Polyvinylidenefluoride homopolymer Solef 6010 from Solvay Solexis (melting point 170–175 °C, Mw 300–320 kDa, Mw/Mn 2.1–2.6)28 is used as membrane material. N,N-Dimethylformamide (DMF, Sigma-Aldrich) is used as solvent and distilled water as non-solvent in the coagulation bath.The membranes are prepared by non-solvent induced phase separation (NIPS). Two different protocols are developed to prepare porous membranes: in the protocol A, the polymer concentration is 15 wt%; in the protocol B the polymer concentration is 13 wt%. Moreover, in the protocol B 2 wt% of LiCl (Sigma-Aldrich) is added in the casting solution as pore forming (Table 1).
| Sample code | Casting solution composition | Loading of MWCNTs in the prepared membrane [%] | |||
|---|---|---|---|---|---|
| PVDF [wt%] | LiCl [wt%] | MWCNTs [wt%] | DMF [wt%] | ||
| A-P | 15 | — | — | 85 | — |
| A-M-05 | 15 | — | 0.08 | 84.92 | 0.5 |
| A-M-1 | 15 | — | 0.15 | 84.85 | 1 |
| A-M-2 | 15 | — | 0.31 | 84.69 | 2 |
| B–P | 13 | 2 | — | 85 | — |
| B-M-05 | 13 | 2 | 0.07 | 84.93 | 0.5 |
| B-M-1 | 13 | 2 | 0.13 | 84.87 | 1 |
| B-M-2 | 13 | 2 | 0.27 | 84.73 | 2 |
The polymeric membranes are prepared dissolving the polymer in DMF under magnetic stirring at 50 °C. Mixed matrix or composite membranes are prepared using MWCNTs with different properties: pristine (i.e. as synthesized), purified, functionalized by oxidation procedure, and thermally treated. Synthesis, purification and oxidative functionalization of the MWCNTs, are described elsewhere.21 In addition to the procedures previously described, the MWCNTs used in this work are treated by ball milling for 12 hours. Thermal treatment of the MWCNTs is carried out on the purified sample into a quartz tube reactor flushed with nitrogen (460 NmL min−1) for 4 hour at 900 °C.
For the preparation of the composite membranes, the appropriate quantity of MWCNTs is added to the homogeneous polymeric solution and finally sonicated for 2 hours before casting (ultrasonic bath VWR USC-600-TH 45 kHz, 400 W; an ice bath is used to cool the solution against solvent evaporation). Each solution is cast at 350 μm thickness onto a glass plate by using a manual casting knife (Elcometer 3700), at 20 ± 2 °C. The cast solution is immediately immersed in the water coagulation bath with the instantaneous formation of a solid membrane detaching from the glass support. The formed membrane is kept in the coagulation bath for about 2 hours and then removed and immersed in another bath of distilled water for additional 24 h to remove residual traces of solvent and LiCl, if present. Finally, it is stored in distilled water until the use. All the membrane samples are prepared and characterized in duplicate to assess the reproducibility of the results. If not specified, the estimated relative error was ≤5% for all the data reported.
Dispersion test of the MWCNTs in solvents
The pristine, purified, thermally treated, and functionalized MWCNTs are dispersed at a concentration of 0.2 wt% in the following solvents: N,N-dimethylacetamide (DMA), DMF, or N-methyl-2-pyrrolidone (NMP). The dispersions are sonicated for 1 hour before visual observation at different times.Membranes characterization
The membranes cross-section and surface morphology are observed by a FEI Quanta 200 Philips SEM instrument. Cross-sections are prepared by fracturing the membrane samples in liquid nitrogen.TEM pictures are taken on a Philips Tecnai10 instrument using 80 kV accelerating voltage. The membrane samples are encapsulated in an epoxy resin to be cut by an ultramicrotome. The cut membranes are placed on two microscopy grids for the TEM observation.
Surface roughness is assessed by Nanoscope III atomic force microscope (AFM Digital Instruments, VEECO Metrology Group) working in tapping mode. AFM images 10 × 10 μm2 are taken on the up surface of the dried membranes. Roughness analysis is performed by Windows Scanning × Microscope software,29 by calculating average roughness (Ra), root-mean-square roughness (Rms), and maximum height (Rmax).
Advancing and receding contact angles are measured on the up surface of the membranes previously dried at room temperature, by growing/shrinking sessile water drop, using a CAM 200 contact angle meter (KSV Instruments Ltd.) equipped with a microsyringe, automatic dispenser, and software for image acquisition and processing. Advancing contact angle (ACA) is measured with the increase in the volume of the droplet (initial volume 3 μL) by adding water with a constant dosing rate (1 μL s−1) up to 8 μL while capturing images. During the entire measurement, the needle remains attached to the drop so that the portion of the needle inside the drop is maintained as small as possible to minimize droplet adhesion. Receding contact angle (RCA) is measured by reducing the volume of the drop with the same dosing flow rate used in the ACA measurements. Static contact angles (SCA) are measured by depositing a water droplet (5 μL) onto the up surface and measuring the contact angle after equilibration (5 seconds). Contact angles are calculated as the average of five different measurements.
Fourier transform infrared spectroscopy (FT-IR) analyses in attenuated total reflectance (ATR) is performed using a Perkin Elmer Spectrum One (Perkin-Elmer), on the up surface of each membrane.
Raman spectra are recorded using a Jasco NRS-5100 micro Raman Spectrometer. The spectra are acquired in the back-scattering geometry. The 532 nm line of the laser with attenuated power of 0.3 mW, is focused on the sample by means of a 100× objective.
The mean pore diameters is measured by a capillary flow porometer (PMI, Porous Materials Inc. Ithaca, NY) using as wetting liquid 3M Fluorinert™ Electronic Liquid FC-40.
The total porosity of the membrane is measured by the gravimetric method at 25 °C, determining the weight of water contained in the porous part of the membrane. The porosity (ε) is calculated by the following equation:
Evaluation of the transport properties and fouling tendency of the membranes
Pure water permeation test are carried out using a dead-end stirred cell having an active filtration area of 14.6 cm2, pressurized by nitrogen and operating at room temperature (25 ± 3 °C). After about one hour of stabilization under constant transmembrane pressure (TMP), permeate samples are collected at regular time intervals in order to determine the flux (J) as reported in the following equations:where Vp (L) is the permeate volume; t (h) is the permeation time and A (m2) is the filtration area.
Blue Dextran (MW 2000 kDa, Sigma-Aldrich) is used for retention test at a concentration of 0.1 g L−1 in water; the TMP applied during the rejection tests is 5.7 bar for membranes prepared by protocol A and 1 bar for membranes prepared by protocol B. The rejection (R (%)) is calculated using the following equation:
where J1 and J2 are respectively the pure solvent flux before and after rejection test. The relative flux can be used to have an indication about the membrane fouling: more JR is close to 1, less the membrane is fouled.
Results and discussion
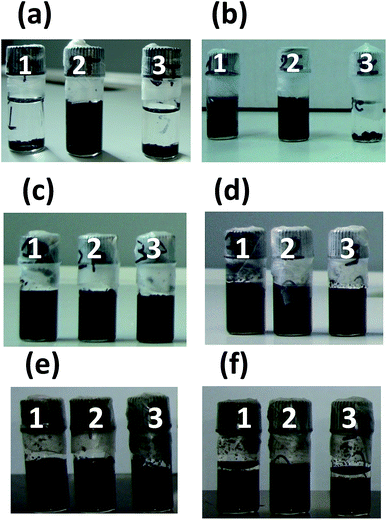
One of the main issues in the blending of nanoparticles (including CNTs) in composite membranes prepared by solution casting methods,31 is their poor dispersion in most of the solvents used for polymer solubilisation and, as a consequence, in the formed membranes.32 Therefore, the dispersion characteristics of the four different MWCNTs used in this work (pristine, purified, oxidized and thermally treated MWCNTs) are preliminarily tested in polar aprotic solvents in which the PVDF is soluble. The dispersions in DMA, DMF and NMP are visually inspected immediately after sonication and after leaving them in the quiescent state for one day. No appreciable variations of the dispersion degree are observed over this time. Uniform dispersion are obtained with all the three solvents in the case of the purified and oxidized MWCNTs (Fig. 2c and d). The presence of polar groups on the oxidized MWCNTs (OH, COOH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)21 reduces their tendency to aggregate through van der Waals interactions, while forming hydrogen bonds with the solvent, resulting in more homogeneous and stable dispersions. Also the purified sample contains some hydrophilic groups because of the presence of defects and vacancies in the graphitic structure susceptible to nucleophilic attack when in contact with the acid aqueous solution used for the purification step.21,33 On the contrary the thermally treated and pristine MWCNTs are dispersed in sufficient way only in DMF (Fig. 2a and b). Moreover, the thermally treated MWCNTs are more poorly dispersed than the pristine in DMA. The high temperature treatment eliminates most of the organic groups present as defects on the MWCNTs, resulting in a more inert material poorly dispersible in organic solvents and with a high tendency to aggregate. Among the three used solvents, the best performing in terms of MWCNTs dispersion is the DMF while the worst is NMP. In the case of the oxidized MWCNTs stable dispersion in DMF are observed after 3 months from the sonication (Fig. 2f). On the contrary after the same time a clear sedimentation of the MWCNTs is observed in the case of the purified MWCNTs (Fig. 2e). These observations well agree with the higher capacity of DMF to form hydrogen bonds34 (Table 2), and suggest close solubility parameters between MWCNTs and DMF, in agreement with literature data.35 Accordingly, DMF is selected as solvent for the preparation of the membranes. The four types of MWCNTs are used to prepare composite membranes following protocol A and fixing the carbon nanotubes loading at 1 wt% (casting solution composition corresponding to A-M-1 in Table 1). In agreement with the preliminary dispersion tests in organic solvent, membranes containing thermally treated MWCNTs show the poorest dispersion in the polymeric matrix (Fig. 3a), while the most homogeneous dispersion is obtained with the oxidized sample (Fig. 3d). An uniform black colour is observed in the case of the composite membranes containing oxidised MWCNTs, despite the low amount of additive in the casting solution. Pristine and purified MWCNTs display intermediate dispersion of the MWCNTs (Fig. 3b and c). For this reason, the oxidized MWCNTs are selected for further investigation. Composite membranes are prepared following both protocols, A and B, adding different amounts of the oxidised MWCNTs: 0.5, 1 and 2 wt%, with respect to the total mass of the formed membranes. The resulting membranes show an asymmetric structure with a denser skin layer and a porous structure underneath with finger-like macrovoids developing from the up surface towards the internal layers (SEM images in Fig. 4 and 5).
O)21 reduces their tendency to aggregate through van der Waals interactions, while forming hydrogen bonds with the solvent, resulting in more homogeneous and stable dispersions. Also the purified sample contains some hydrophilic groups because of the presence of defects and vacancies in the graphitic structure susceptible to nucleophilic attack when in contact with the acid aqueous solution used for the purification step.21,33 On the contrary the thermally treated and pristine MWCNTs are dispersed in sufficient way only in DMF (Fig. 2a and b). Moreover, the thermally treated MWCNTs are more poorly dispersed than the pristine in DMA. The high temperature treatment eliminates most of the organic groups present as defects on the MWCNTs, resulting in a more inert material poorly dispersible in organic solvents and with a high tendency to aggregate. Among the three used solvents, the best performing in terms of MWCNTs dispersion is the DMF while the worst is NMP. In the case of the oxidized MWCNTs stable dispersion in DMF are observed after 3 months from the sonication (Fig. 2f). On the contrary after the same time a clear sedimentation of the MWCNTs is observed in the case of the purified MWCNTs (Fig. 2e). These observations well agree with the higher capacity of DMF to form hydrogen bonds34 (Table 2), and suggest close solubility parameters between MWCNTs and DMF, in agreement with literature data.35 Accordingly, DMF is selected as solvent for the preparation of the membranes. The four types of MWCNTs are used to prepare composite membranes following protocol A and fixing the carbon nanotubes loading at 1 wt% (casting solution composition corresponding to A-M-1 in Table 1). In agreement with the preliminary dispersion tests in organic solvent, membranes containing thermally treated MWCNTs show the poorest dispersion in the polymeric matrix (Fig. 3a), while the most homogeneous dispersion is obtained with the oxidized sample (Fig. 3d). An uniform black colour is observed in the case of the composite membranes containing oxidised MWCNTs, despite the low amount of additive in the casting solution. Pristine and purified MWCNTs display intermediate dispersion of the MWCNTs (Fig. 3b and c). For this reason, the oxidized MWCNTs are selected for further investigation. Composite membranes are prepared following both protocols, A and B, adding different amounts of the oxidised MWCNTs: 0.5, 1 and 2 wt%, with respect to the total mass of the formed membranes. The resulting membranes show an asymmetric structure with a denser skin layer and a porous structure underneath with finger-like macrovoids developing from the up surface towards the internal layers (SEM images in Fig. 4 and 5).
| Solvent | Solubility parameters [J1/2 cm−3/2] | |||
|---|---|---|---|---|
| δ | δd | δp | δh | |
| DMA | 22.1–22.8 | 16.8 | 11.5 | 10.2 |
| DMF | 24.9 | 17.4 | 13.7 | 11.3 |
| NMP | 22.9 | 17.9 | 12.3 | 7.2 |
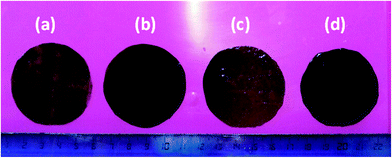 | ||
| Fig. 3 Images of the composite membranes prepared by protocol A containing: thermally treated (a), pristine (b), purified (c) and oxidized MWCNTs (d). The loading of MWCNTs in the membrane was 1 wt%. | ||
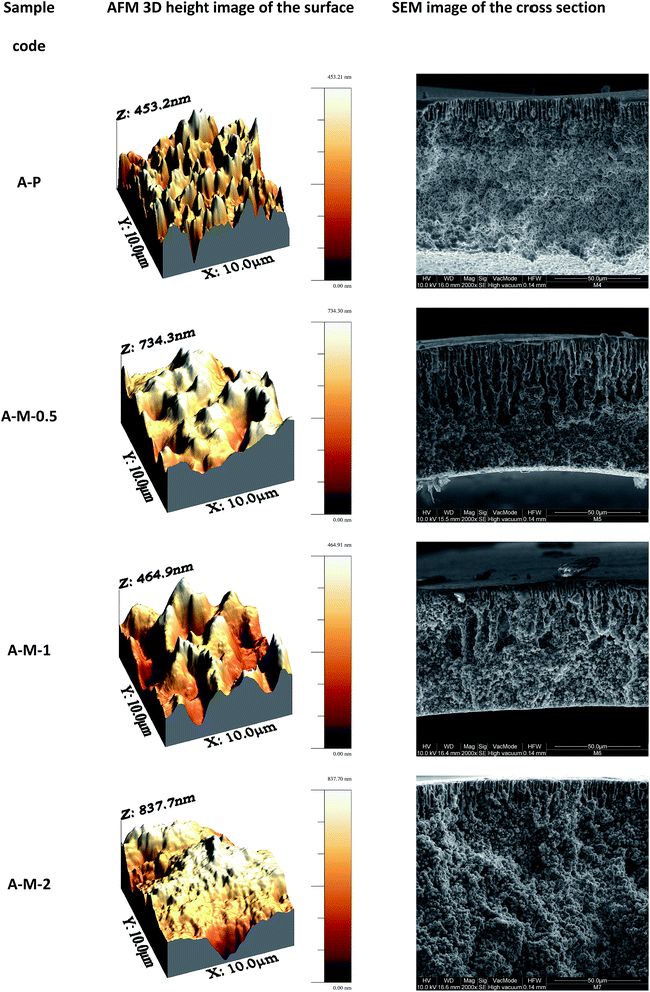 | ||
| Fig. 4 AFM images of the up surface and SEM micrographs of the cross sections of the polymeric and composite membranes prepared by protocol A. | ||
 | ||
| Fig. 5 AFM images of the up surface and SEM micrographs of the cross sections of the polymeric and composite membranes prepared by protocol B. | ||
In the NIPS technique, when the cast liquid film is immersed in the coagulation bath, the homogeneous polymeric solution is initially demixed into two liquid phases because of the diffusive exchanges of the solvent and non-solvent.31,36,37 The phase with the higher polymer concentration forms the solid membrane while the phase with the lower polymer concentration gives the porous structure. The exchange of solvent and non-solvent during the demixing stage increases the concentration in the polymer rich phase surrounding the polymer lean phase. The polymer molecules may rearrange their structure until the solidification takes place. However, the rate of the precipitation slows down as the precipitation front moves from the interface with the coagulation bath (where the precipitant reaches earlier a threshold concentration for phase separation) towards the internal layers, resulting in an asymmetric structure.31,36,37 A net transport of the polymer perpendicularly to the surface is the result of the high gradient in chemical potential at the interface, resulting in a denser skin layer which hinders the precipitant inlet and solvent outlet to/from the bulk of the membrane.31,36,37 The final membrane morphology and microstructure is therefore strongly dependent from the local rate of the solvent/non-solvent diffusion that, in turns, is related to the chemical and physical properties of the systems involved and operative conditions (e.g. activity, viscosity and temperature). In the case of the semicrystalline PVDF, two different mechanisms govern the phase separation process: liquid–liquid demixing and solid–liquid demixing.38,39 The membranes prepared by protocol A are characterized by a more compact structure (less porous) with less elongated finger-like macrovoids than those prepared by protocol B (Fig. 4 and 5). Moreover, they present spherical PVDF crystallites formed by the liquid–solid demixing process (polymer crystallization) which precedes the liquid–liquid demixing. On the contrary, a cellular structure is observed in the case of protocol B because of the liquid–liquid demixing occurring at an earlier stage, due to the lower concentration of polymer (13 vs. 15 wt% in protocol B and A, respectively). Moreover, in the protocol B LiCl is used as pore forming additive.40 Because of its solubility both in the solvent (DMF) and in the coagulant (water), LiCl diffuses in the water bath and promotes the non-solvent influx, thus facilitating macropores formation. Despite all the samples are cast at the same initial height of the liquid film (350 μm), the final thickness of the membranes varied as a function of the composition (Table 3 and SEM images in Fig. 4 and 5). As expected, solutions containing a higher amount of polymer form thicker membranes (protocol A). Moreover, the addition of the MWCNTs up to 1 wt% heavily reduces the final thickness because of the slower phase separation process (delayed demixing due to the increased solution viscosity which reduces the solvents diffusion rates), providing more time to eliminate the solvent before polymer solidification (kinetic effect on the phase separation).13
| Sample code | Mean pore diameter [μm] | Total porosity [%] | Thickness [μm] | Roughness parameters [nm] | Water contact angles [°] | Water fluxa [L h−1 m−2] | Relative flux [—] | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ra | Rms | Rmax | SCA | ACA | RCA | ||||||
| a Water flux measured at 25 °C and TMP 5.7 bar for membranes A, and 1 bar for membranes B. | |||||||||||
| A-P | 0.047 | 72 | 91 | 54 | 68 | 453 | 95 | 101 | 68 | 38 | 0.56 |
| A-M-0.5 | 0.026 | 71 | 75 | 87 | 117 | 734 | 115 | 117 | 75 | 1.0 | 0.89 |
| A-M-1 | 0.032 | 71 | 74 | 70 | 86 | 465 | 103 | 108 | 70 | 20 | 0.86 |
| A-M-2 | 0.042 | 72 | 120 | 103 | 130 | 838 | 123 | 125 | 83 | 1.8 | 0.86 |
| B-P | 0.060 | 78 | 77 | 43 | 57 | 518 | 74 | 79 | 33 | 63 | 0.77 |
| B-M-05 | 0.047 | 82 | 55 | 39 | 54 | 455 | 81 | 84 | 47 | 27 | 0.86 |
| B-M-1 | 0.051 | 82 | 57 | 23 | 29 | 223 | 71 | 77 | 27 | 56 | 0.85 |
| B-M-2 | 0.028 | 81 | 73 | 29 | 37 | 272 | 71 | 71 | 23 | 30 | 0.87 |
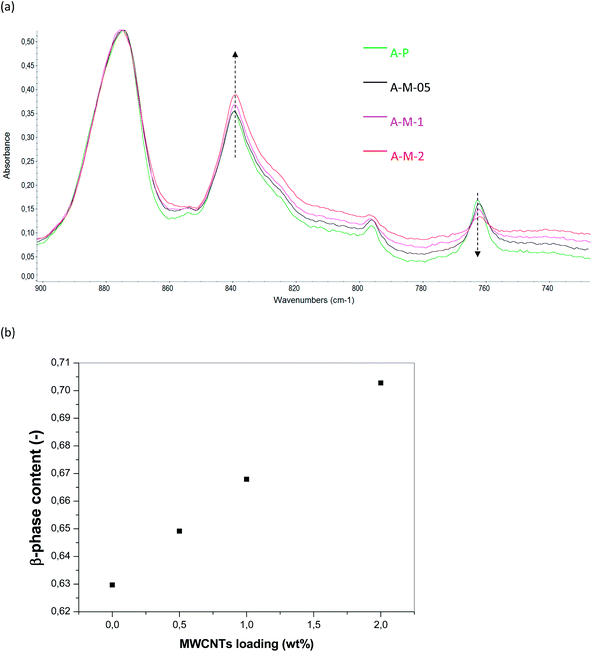
However, a further increase of the MWCNTs content (up to 2 wt%) results in thicker membranes because of the increased miscibility gap induced by the MWCNTs, which work as non-solvent for the polymer (thermodynamic effect on the phase separation, i.e. less water is required to induce phase separation).13 In the presence of MWCNTs up to 1 wt%, macrovoids are more extended than in the case of the polymeric samples; this means that they have more time to coalesce before polymer solidification (Fig. 4 and 5). On the contrary, less developed macrovoids are formed as a result of a shorter coalescence time with 2 wt% MWCNTs. For what concerns surface topography, it is possible to appreciate in the bottom surface of the samples prepared by protocol A a spherulitic morphology (Fig. 6b). Smoother down surface are observed in the case of membranes prepared by protocol B (Fig. 6d), as liquid–liquid demixing dominates over solid–liquid demixing. From SEM images the up surfaces appear dense and substantially similar for both protocols (Fig. 6a and b). On the contrary, AFM analyses highlighted significant effects of the membrane preparation conditions on the roughness of the up surface (AFM images in Fig. 4, 5 and Table 3). The polymeric membranes prepared by protocol A show higher surface roughness than samples prepared by protocol B. Moreover, the presence of the MWCNTs in the protocol A further increase the surface roughness. An opposite effect is observed in the case of protocol B, i.e., MWCNTs decrease roughness. These differences can be explained considering that the hydrophilic oxygen-containing groups grafted on the MWCNTs tend to migrate toward the interface with the coagulation bath to reduce the interfacial energy during phase separation. The result is an increasing surface roughness because of the rigid cylindrical structure of the MWCNTs. A competitive migration of the LiCl particles toward the surface occurs in the case of the membrane prepared by protocol B, resulting in a smoother surface. The MWCNTs diffusivity in the casting solution is expected to decreases with the increasing of the MWCNTs loading because of their more severe aggregation by van deer Waals interactions. The membranes prepared by protocol A are characterized by a higher water contact angle, as a consequence of their higher roughness, in agreement with the Cassie–Baxter model which predicts that a hydrophobic surface can be changed to superhydrophobic with an increase in roughness.41–43 As a consequence, composite membranes prepared by protocol A have a relevant potential for application in membrane contactors, where hydrophobic, waterproof surfaces, are required. On the contrary, the membranes prepared by protocol B are less rough and more hydrophilic, resulting as potential candidates for low-fouling applications in liquid phase separations, where fouling can be mitigated by increasing the surface hydrophilicity. Another key aspect to be considered in order to correlate the effect of membrane preparation conditions with the surface wettability, is their influence on the crystalline state of the polymer. The PVDF polymer used in the present work (Solef 6010) is in the α-phase, as revealed by FT-IR spectra showing the typical bands of this phase (763, 795, 854, 975, and 1384 cm−1; Fig. 7a). However, the crystalline composition of the polymer changes during the membrane fabrication procedure, which includes a solubilisation step at 50 °C and successive phase separation. In the case of membranes prepared by protocol A (Fig. 7b), FT-IR spectra display the signals of two different phases: α-phase and β-phase (840, 1172 and 1273 cm−1).
 | ||
| Fig. 6 Up and down surfaces of the composite membranes prepared with 1 wt% of oxidized MWCNTs by protocol A (a: up and b: down) and by protocol B (c: up and d: down). | ||
 | ||
| Fig. 7 FT-IR-ATR spectra of the PVDF polymer (a), polymeric membrane prepared by protocol A (b), and by protocol B (c). | ||
Membranes prepared by protocol B (Fig. 7c), present only the β-phase peaks and the formation of the most apolar phase of the PVDF, i.e. the α-phase, is prevented because of the presence of LiCl in the casting solutions which favours the formation of the most polar phase by polar interactions with the polymer. These results are in agreement with previous works reporting that solvents with larger dipole moment tended to form polar β phase of PVDF.27,44 The higher content of the most polar phase induces a higher wettability of the of the PVDF membranes prepared by protocol B than those prepared by protocol A (contact angles in Table 3). The β-phase content (F(β)) in the membrane series A is quantified using the following equation:45,46
The preparation protocol has a relevant effect on porous membrane microstructure. Protocol B gives membranes with higher mean pore diameter and total porosity in comparison with protocol A (Table 3). In general, the presence of the MWCNTs in the casting solution reduces the mean pore size of the composites with respect to the polymeric samples. MWCNTs distribution is investigated by a combination of TEM and SEM spectroscopy. TEM images highlight in the dense part of the membrane a fine distribution of the MWCNTs with diameter between 10–30 nm surrounded by the polymer matrix (Fig. 9e). The interactions between the MWCNTs and the polymer (hydrogen bonds and electron donor–electron acceptor interactions) reduce the tendency of the carbon nanotubes to agglomerate by π–π attractions. Moreover, it is interesting to note that some MWCNTs form a bridge through the pores. It is possible to speculate that this bridging effect is more relevant in the skin layer, where the pores are smaller, influencing in relevant way the transport through the asymmetric composite membranes. MWCNTs with an apparent larger diameter (30–100 nm) are observed in the macropores of the membranes (Fig. 9b–e). The formation of this larger MWCNTs is attributed to the adhesion of a thin polymer layer around the MWCNTs, confirming the good affinity between PVDF and MWCNTs. Several MWCNTs wrapped around the polymer spherulites and/or other MWCNTs are present along the whole membrane cross section. They are firmly entrapped into the polymer structure as confirmed by the absence leaching out from the membrane during water filtration test, even after long time storage in water (>1 year). Increasing the loading of the carbon nanotubes in the casting solution it is possible to appreciate an increase of their content in the membrane samples by TEM images (Fig. 10). However, membranes prepared with higher loading of MWCNTs (2 wt%) are characterized by a poor dispersion of the additive with the formation of several aggregates of MWCNTs.
 | ||
| Fig. 10 TEM images of the longitudinal section of the composite membranes prepared at different loading of MWCNTs by protocol A and protocol B. | ||
Micro Raman spectroscopy contributed to further characterize the composite membranes. Raman spectra of the oxidized MWCNTs show two characteristic bands at 1345 cm−1 and 1579 cm−1, indicated respectively as D-band and G-band33,34 (Fig. 11a). Moreover, a weak shoulder of the G-band is visible at 1613 cm−1, corresponding to the D′ band. The D and D′ bands are usually attributed to the presence of amorphous or disordered phase in the carbon nanotubes (e.g. topological perturbations by non-hexagonal rings, functional groups, impurities and vacancies susceptible to nucleophilic attack).47,48 The G-band corresponds to the in plane tangential stretching of the carbon–carbon bonds in the graphene sheets. The oxidized MWCNTs are characterized by a lower ratio of the intensity of the G band to the D band, in comparison to the purified MWCNTs (IG/ID are respectively 1.08 vs. 1.22). The oxidative functionalization of the MWCNTs causes etching of the graphitic sheets, resulting in MWCNTs with a large number of defect sites where the oxygen-containing functionalities are present. However, the presence of these groups assures a good dispersion of the MWCNTs in polar solvents. Micro Raman analyses are carried out in several points of the up surface of the composite membranes. In all spectra it is possible to clearly distinguish both the D and G bands of the MWCNTs, but they are found at higher Raman shift values compared to the oxidized MWCNTs, because of the electron donor–acceptor interactions between the fluorine atoms of the polymer and the π-system of the carbon nanotubes. Moreover, micro Raman analyses reveal zones with more intense signals of the MWCNTs in comparison to those of the polymer, and zones where they are less intense (Fig. 11b and c, respectively). The former appear at the optical microscope as darker spots distributer on a more clear surface, and correspond probably to membrane pores (with a macrovoid developing just beneath) in which the MWCNTs have an higher local concentration. The clearer zones are related to the dense part of the membrane, composed principally by the polymer. The polymeric and mixed matrix membranes are characterized by pure water permeation test (Table 3) and rejection test using a Blue Dextran (MW 2000 kDa) as model of organic foulant (Fig. 12). The composite membranes containing MWCNTs have higher rejection with respect to polymeric membranes, but lower flux. MWCNTs are present both in the porous and solid part of the membrane providing higher selective pathways for solvent transport but increasing also the mass transport resistance. The water transport through the hydrophobic internal channel of a CNTs is known to occur in fast way as a result of the slippage of water on hydrophobic surfaces (strong hydrogen-bonding between water molecules in the nanoconfined environment in which the water molecules tend to recede from nonpolar surfaces).49,50 Membranes made of vertically aligned CNTs have been reported to show high permeability enhancement (>10 for gases and >1000 for liquids) than predictions for the transport through the inner channel.51,52 However they are usually produced on small scale (from few μm2 to few mm2) by long and complex (i.e. expensive) fabrication processes. On the contrary the preparation of membranes containing CNTs by blending techniques, like in this work, are cheaper and easier to be scale-upped. Despite the CNTs are not aligned in the prepared membranes and, as a consequence, only a small portion can be opportunely oriented in order to have transport through the inner core, the CNTs can contribute to the transport of the permeating species, as well as, to the reduction of fouling phenomena, by interactions (attractive or repulsive) of the permeating species with their external graphitic surface. This hypothesis is supported by recent molecular dynamic simulation studies which show a strong curvature dependence of the interfacial friction of water at graphitic interfaces which increases with carbon nanotube radius increasing for water inside, while decreases for water outside, tending to reach the value of water confined between two graphene planes when CNT radii is ≥10 nm.53,54
It is also interesting to note that a similar dependence of the transport properties (analysed in terms of flux and rejection), from the loading of the MWCNTs is observed for both series of samples (Fig. 12). The data evidence that the intermediate loading of 1 wt% allowed to reach better performance. TEM analyses showed that the 1 wt% loading corresponds to a better distribution of the MWCNTs in the polymeric matrix than 0.5 wt% and 2 wt% (Fig. 10). The Blue Dextran solution flux of all the PVDF membranes is lower than the pure water flux, because of the higher viscosity. Moreover, concentration polarization and fouling phenomena induced the formation of a boundary layer between the membrane and bulk solution that provides additional resistance to mass transport. Membrane fouling during solution filtration is confirmed by the relative flux values lower than 1 (Table 3). The combined effect of surface roughness and crystalline phase composition on membrane wettability resulted in an increased fouling resistance of the polymeric membrane prepared by protocol B (B-P) in comparison to polymeric membranes prepared by protocol A (A-P). B-P sample is characterized by a lower surface roughness and higher percentage of the β-phase than A-P, and both factors reduce the fouling tendency of the membranes. In the case of the composite membranes prepared by protocol, A, the presence of the MWCNTs increased the content of the more hydrophilic β-phase, and this effect dominates the increased surface roughness in term of influence on relative flux that resulted higher than for the polymeric sample. In the case of the composite membranes prepared by protocol B, the presence of the MWCNTs reduced the surface roughness in comparison to B-P increasing the relative flux (only the β-phase is present in all the B-series samples). Finally the inclusion of the MWCNTs in polymeric matrix could contribute to reduce the fouling of both series of composite membranes by the formation of a new interface with the permeating solution in comparison with the polymeric samples, with a certain degree of water slippage at the graphitic surface.
Conclusions
The main goal of this work is to gain more insight into the role of physicochemical and morphological properties of PVDF membranes on the separation performance and fouling control.Novel porous composite membranes are prepared combining PVDF with oxidised MWCNTs following two different procedures (protocol A or B). The presence of oxygen-containing polar groups on oxidized MWCNTs, results in a good dispersion in the casting solution and, consequently, in the formed porous film, by the establishment of hydrogen bonds with the solvent and the polymer. Experimental results demonstrated that the membrane preparation conditions are heavily influential parameters on the morphology, crystalline phase and transport properties of the porous asymmetric membranes. The membranes prepared by protocol A (higher polymer concentration) are characterized by spherical PVDF crystallites formed by the liquid–solid demixing (i.e. polymer crystallization) which preceded the liquid–liquid demixing. On the contrary, a cellular structure is the result of the liquid–liquid demixing occurring at an earlier stage in the case of the protocol B (lower concentration of polymer and hydrophilic pore forming additive LiCl). The β-form of the PVDF crystallite (the most polar crystalline phase of the PVDF) is the dominant phase in all the membranes produced, but a relevant fraction of α-phase (the most apolar crystalline phase) is formed in the membrane obtained by protocol A. The crystalline composition of the PVDF membranes prepared by protocol A is influenced by the presence of the MWCNTs which favour the formation of the β-phase by polar interactions. Membranes prepared from casting solutions containing LiCl (protocol B) present only the β phase of the PVDF.
Also, the surface roughness is affected by the membrane formation parameters and MWCNTs loading, resulting in more rough surfaces in the case of the composite systems prepared by protocol A. A relationship between the surface roughness and crystalline composition with the membrane wettability is individuated: increasing surface roughness and the content of α-phase, more hydrophobic membrane are obtained; on the contrary, decreasing surface roughness and increasing the content of β-phase of PVDF, more hydrophilic membrane are formed.
The first type of membranes can be favoured using higher polymer concentration and avoiding the use of polar additives (protocol A).
The second type of membranes can be favoured using more diluted casting solutions and hydrophilic additives (protocol B).
All the composite membranes had a lower fouling tendency in comparison with the reference polymeric samples. Moreover, at the intermediate loading (1 wt% of MWCNTs vs. 0.5 or 2 wt%) better performance in separation test (higher flux and rejection with lower fouling) are obtained because of the better dispersion of these nanostructured additives in the polymer matrix.
The membranes produced have remarkable potentialities for possible applications in liquid phase separations (protocol B) or in membrane contactors (protocol A).
Acknowledgements
The work was partially funded by the King Abdulaziz City for Science and Technology (KACST) (project: “CNT-RO and NF membranes for the treatment of aqueous solutions and desalination”). Solvay Solexis (Italy) is gratefully acknowledged for supplying the polymer PVDF Solef 6010.References
- A. Gorak and A. Stankiewicz, Annu. Rev. Chem. Biomol. Eng., 2011, 2, 431 CrossRef CAS PubMed.
- A. Lovinger, Poly(viylidene fluoride), in Developments in Crystalline Polymers-1, Applied Science Publishers, London, 1982 Search PubMed.
- F. Liu, N. A. Hashim, Y. Liu, M. R. Moghareh Abed and K. Li, J. Membr. Sci., 2011, 375, 1 CrossRef CAS PubMed.
- G.-D. Kang and Y.-M. Cao, J. Membr. Sci., 2014, 463, 145 CrossRef CAS PubMed.
- P. Martins, A. C. Lopes and S. Lanceros-Mendez, Prog. Polym. Sci., 2014, 39, 683 CrossRef CAS PubMed.
- M. Tao, F. Liu and L. Xue, J. Mater. Chem., 2012, 22, 9131 RSC.
- H. Jang, D.-H. Song, I.-C. Kim and Y.-N. Kwon, J. Appl. Polym. Sci., 2015, 132, 41712 CrossRef PubMed.
- A. Mansourizadeh and A. F. Ismail, Int. J. Greenhouse Gas Control, 2011, 5, 640 CrossRef CAS PubMed.
- J. Zhang, Z. Xu, M. Shan, B. Zhou, Y. Li, B. Li, J. Niu and X. Qian, J. Membr. Sci., 2013, 448, 81 CrossRef CAS PubMed.
- J. Ying and B. Deng, J. Membr. Sci., 2015, 479, 256 CrossRef PubMed.
- J. Zhang, Z. Xu, W. Mai, C. Min, B. Zhou, M. Shan, Y. Li, C. Yang, Z. Wang and X. Qian, J. Mater. Chem. A, 2013, 1, 3101 CAS.
- A. Sotto, J. Kim, J. M. Arsuaga, G. del Rosario, A. Martınez, D. Nam, P. Luise and B. Van der Bruggen, J. Mater. Chem. A, 2014, 2, 7054 CAS.
- E. Fontananova, J. C. Jansen, A. Cristiano, E. Curcio and E. Drioli, Desalination, 2006, 192, 190 CrossRef CAS PubMed.
- E. Fontananova, L. Donato, E. Drioli, L. Lopez, P. Favia and R. d'Agostino, Chem. Mater., 2006, 1, 1561 CrossRef.
- L. C. Lopez, M. G. Buonomenna, E. Fontananova, G. Iacoviello, E. Drioli, R. d'Agostino and P. Favia, Adv. Funct. Mater., 2006, 16, 1417 CrossRef CAS PubMed.
- L. Wu, J. Sun and F. Tong, RSC Adv., 2014, 4, 63989 RSC.
- J. Sun, L. Wu and F. Hu, RSC Adv., 2015, 5, 40753 RSC.
- G. Di Profio, E. Fontananova, E. Curcio and E. Drioli, Cryst. Growth Des., 2012, 12, 3749 CAS.
- J. Ying and B. Deng, J. Membr. Sci., 2015, 479, 256 CrossRef PubMed.
- Y. Zhu, F. Zhang, D. Wang, X. Feng Pei, W. Zhang and J. Jin, J. Mater. Chem. A, 2013, 1, 5758 CAS.
- V. Grosso, D. Vuono, M. A. Bahattab, G. Di Profio, E. Curcio, S. A. Al-Jilil, F. Alsubaie, M. Alfife, J. B. Nagy, E. Drioli and E. Fontananova, Sep. Purif. Technol., 2014, 132, 684 CrossRef CAS PubMed.
- A. Bottino, G. Capanelli and A. Comite, Desalination, 2002, 46, 35 CrossRef.
- T. L. S. Silva, S. Morales-Torres, J. L. Figueiredo and A. M. T. Silva, Desalination, 2015, 357, 233 CrossRef CAS PubMed.
- J. C. C. Ferreira, T. S. Monteiro, A. C. Lopes, C. M. Costa, M. M. Silva, A. V. Machado and S. Lanceros-Mendez, J. Non-Cryst. Solids, 2015, 412, 16 CrossRef CAS PubMed.
- P. Martins, A. C. Lopes and S. Lanceros-Mendez, Prog. Polym. Sci., 2014, 39, 683 CrossRef CAS PubMed.
- S. Lanceros-Méndez, J. F. Mano, A. M. Costa and V. H. Schmidt, J. Macromol. Sci., Part B: Phys., 2001, 40, 517 CrossRef.
- Y. Xiang, L. Xue, J. Shen, H. Lin and F. Liu, J. Appl. Polym. Sci., 2014, 131, 41065 CrossRef PubMed.
- http://www.solvayplastics.com.
- I. Horcas, R. Fernandez, J. M. Gomez-Rodriguez, J. Colchero, J. Gomez-Herrero and A. M. Baro, Rev. Sci. Instrum., 2007, 78, 1 CrossRef PubMed.
- http://www.cheaptubes.com/MWNTs.htm.
- M. Mulder, in Basic principles of membrane technology, Kluwer Academic, 1996 Search PubMed.
- M. M. Khan, V. Filiz, G. Bengtson, S. Shishatskiy, M. Rahman and V. Abetz, Nanoscale Res. Lett., 2012, 7, 504 CrossRef PubMed.
- P. G. Collins, Defects and disorder in carbon nanotubes, in Oxford Handbook of Nanoscience and Technology: Frontiers and Advances, Oxford Univ. Press, Oxford, 2009 Search PubMed.
- D. W. Van Krevelen, in Properties of Polymers, Their correlation with chemical structure, Their numerical estimation and prediction from additive group contributions, Elsevier, Amsterdam, 1990 Search PubMed.
- L. Kunsil, H. J. Lim, S. J. Yang, Y. S. Kim and C. R. Park, RSC Adv., 2013, 3, 4814 RSC.
- K. Kimmerle and H. Strathmann, Desalination, 1990, 79, 283 CrossRef CAS.
- A. K. Hołda and I. F. J. Vankelecom, J. Appl. Polym. Sci., 2015, 132, 42130 CrossRef PubMed.
- D.-J. Lin, C.-L. Lin and S.-Y. Guo, Macromolecules, 2012, 45, 8824 CrossRef CAS.
- M. Sharma, G. Madras and S. Bose, J. Mater. Chem. A, 2015, 3, 5991 CAS.
- A. Bottino, G. Capanelli and A. Turturro, Desalination, 1988, 68, 167 CrossRef CAS.
- R. N. J. Wenzel, Ind. Eng. Chem., 1936, 28, 988 CrossRef CAS.
- A. Cassie and S. Baxter, Trans. Faraday Soc., 1944, 40, 546 RSC.
- H. Y. C. Erbil and E. Cansoy, Langmuir, 2009, 25, 14135 CrossRef CAS PubMed.
- M.-M. Tao, F. Liu, B.-R. Ma and L.-X. Xue, Desalination, 2013, 316, 137 CrossRef CAS PubMed.
- P. Martins, A. C. Lopes and S. Lanceros-Mendez, Prog. Polym. Sci., 2014, 39, 683 CrossRef CAS PubMed.
- R. Gregorio and M. Cestari, J. Polym. Sci., Part B: Polym. Phys., 1994, 32, 859 CrossRef CAS PubMed.
- V. Datsyuk, M. Kalyva, K. Papagelis, J. Parthenios, D. Tasis, A. Siokou, I. Kallitsis and C. Galiotis, Carbon, 2008, 46, 833 CrossRef CAS PubMed.
- M. S. Dresselhaus, G. Dresselhaus, R. Saito and A. Jorio, Phys. Rep., 2005, 409, 47 CrossRef PubMed.
- G. Hummer, J. C. Rasaiah and J. P. Noworyta, Nature, 2001, 414, 188 CrossRef CAS PubMed.
- H. Verweij, M. C. Schillo and J. Li, Small, 2007, 3, 1996 CrossRef CAS PubMed.
- M. S. Mauter, M. Elimelech and C. O. Osuji, ACS Nano, 2010, 4, 6651 CrossRef CAS PubMed.
- M. Majumder, N. Chopra and J. H. Bruce, ACS Nano, 2011, 5, 3867 CrossRef CAS PubMed.
- K. Falk, F. Sedlmeier, L. Joly, R. R. Netz and L. Bocquet, Nano Lett., 2010, 10, 4067 CrossRef CAS PubMed.
- K. Falk, F. Sedlmeier, L. Joly, R. R. Netz and L. Bocquet, Langmuir, 2012, 28, 14261 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |