Electrochemical properties of Li2MnO3 nanocrystals synthesized using a hydrothermal method†
Meng Chengab,
Weiping Tang*c,
Yi Sunc and
Kongjun Zhu*a
aState Key Laboratory of Mechanics and Control of Mechanical Structures, College of Aerospace Engineering, Nanjing University of Aeronautics and Astronautics, Nanjing, 210016, P. R. China. E-mail: kjzhu@nuaa.edu.cn
bCollege of Materials Science and Engineering, Nanjing University of Aeronautics and Astronautics, Nanjing 210016, China
cShanghai Institute of Space Power-Sources, Shanghai 200000, China. E-mail: tangwp@sina.cn
First published on 11th August 2015
Abstract
In this article, we report the synthesis of Li2MnO3 nanocrystals using a hydrothermal process. XRD, SEM, and TEM analyses are performed, and the electrochemical properties of the resultant nanocrystals are investigated. Adding the oxidant KMnO4 influences the phase purity, size, and shape of the Li2MnO3 nanocrystals. An amount of KMnO4 that exceeds 6% of the total Mn source enhances the formation of the pure monoclinic Li2MnO3 phase. The effect of the amount of KMnO4 on Li2MnO3 grain size can be divided into three ranges. Li2MnO3 crystals with a size around 28.7 nm and plate morphology are obtained at less than 6% of KMnO4 content; those with a size of 28.7 nm to 9.8 nm and mixed morphology of plates and rods are obtained at 6% to 11% KMnO4 content, and those with a size around 9.8 nm and rod morphology are mainly obtained at KMnO4 content exceeding 11%. Discharge capacities increase with decreasing size of Li2MnO3 nanocrystals in a linear relationship. The voltage of the first charge shows a 4.55 V plateau for Li2MnO3 nanocrystals with 28.7 nm size, becoming 4.4 V with a decrease in size to 12.2 nm, and splitting into two plateaus at around 3.8 and 4.4 V with a further decrease in size to 9.8 nm. The XRD and XANES results of the Li2MnO3 electrodes obtained after charge/discharge experiments show that smaller sizes are more beneficial in maintaining a layered structure than larger nanocrystals.
Introduction
Rapid developments in electric and hybrid cars, as well as large power tools, in recent years have resulted in increased demand for higher energy and power density.1,2 Currently available cathode materials, such as LiMn2O4, LiNi1/3Co1/3Mn1/3O2, and LiFePO4, demonstrate specific capacities lower than 200 mA h g−1, severely limiting the energy density of lithium (Li) batteries.3,4 Given that Li-rich manganese (Mn)-based materials present high specific capacities (>250 mA h g−1), low raw material costs, and several other advantages, these compounds have attracted increasing research attention.5 Li2MnO3 is an important component of Li-rich material; thus, analyzing its electrochemical reaction mechanism is beneficial for structure change studies and composition design of Li-rich Mn-based solid solutions.Li2MnO3 can be written as Li(Li1/3Mn2/3)O2 and presents an α-NaFeO2 layered structure (monoclinic C2/m space group) similar to that of LiMO2. Li atoms occupy the 3a position of rock salt structures, while 1/3 Li and 2/3 Mn occupy the 3b position of transition metal layers, thereby forming a Li, Mn ordered super structure.6–8 Mn4+ ions in Li2MnO3 are electrochemically inactive; however, decreasing the particle size to sub-micrometer scale causes reversible electrochemical activation.9,10 The electrochemical activation of this material may be attributed to the mechanisms of oxidation (removal of oxygen to balance charges) and proton exchange.11–16 The electrochemical activation of Li2MnO3, which depend on the two mechanisms, is influenced by grain morphology and size, as well as structure. Grains that are smaller than submicrometer size show electrochemical activity, and their activation increases with decreasing size. Therefore, controlling the size and morphology of Li2MnO3 grains is the most important method in increasing electrochemical properties. The synthesis methods of controlling Li2MnO3 nanocrystals with particularly high crystallinity have been featured in several reports.
In most reports, Li2MnO3 synthesis involves calcination (solid-state reaction and annealing of precursors from soft chemistry methods) to increase crystallinity, but this route renders controlling grain size and morphology difficult.17–20 Thus, several researchers have focused on hydrothermal synthesis. The hydrothermal treatment of transition metal hydroxides and δ-MnO2 gives rise to nanosized Li2MnO3 and Li2MnO3–LiMO2 (M = Fe, Ni, Co, etc.) plates, wires, and particles.21–26 Taking α and δ MnO2 as hydrothermal precursors, Baek obtained hierarchically assembled 2D nanoplates and 0D nanoparticles, respectively, wherein the samples with larger surface area of 113 m2 g−1 delivered larger discharge capacity of 280 mA h g−1.25 In Huang's report Li2MnO3 and Li2MnO3–LiMnO2 were obtained by a one-step hydrothermal process; the Li2MnO3 and LiMnO2 content varied with the employed amount of the oxidant.26 However, studies on the synthesis of Li2MnO3 nanocrystals with continuous change in size have not been reported thus far.
The particle size of Li2MnO3 also influences the structure change in the charge/discharge process. Numerous studies have examined the atomic rearrangement of Li2MnO3 and Li2MnO3–LiMO2 (M = Ni, Co) during activation, which is accompanied by changes in the Mn oxidation state and structural transformations to LiMnO2 and LiMn2O4.28–33 Wang observed the atomic rearrangement directly in Li2MnO3 particles and LiMnO2 domains by STEM after electrochemical delithiation. In his study, Mn atoms were mobile and partially migrated to the Li layer.29 Amalraj et al. found that the voltage plateau of 30 nm Li2MnO3 is 0.23 V lower than that of the micron-sized samples.30 Boulineau et al. reported that Li deintercalation caused irreversible transformation to the spinel phase at the edge of the bulk material.32 The size of Li2MnO3 crystal hardly influences the structural change during charge/discharge process; however, rare reports studied the influence regularity of size change of Li2MnO3 nanocrystals on structure change.
The current article reports the preparation of ultrafine Li2MnO3 nanopowders with high crystallinity through a hydrothermal process. Introduction of KMnO4 as a second oxidation agent allows for continuous size control of Li2MnO3 nanocrystals. Furthermore, the effect of grain size change on electrochemical properties is analyzed.
Experimental
Preparation of Li2MnO3nanocrystals
Li2MnO3 nanocrystals were prepared by hydrothermal method. MnSO4, (NH4)2S2O8, LiOH, and KMnO4 were purchased from Xilong Chemical Co. Ltd MnSO4 (3 mmol) and (NH4)2S2O8 (2.4 mmol) were dissolved at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in 10 mL of deionized water in a Teflon liner to obtain a mixed solution. Afterward, 20 mL of LiOH (3 mol L−1) was trickled into the solution, which was stirred for 30 min to yield a δ-MnO2 precursor precipitate.25 The Teflon liner was transferred to an autoclave and maintained at 200 °C for 24 h. The as-prepared powders were filtered, washed, and dried at 80 °C for 24 h. To produce an oxidizing atmosphere during the precipitation and formation reactions, a determined amount of KMnO4 was used as a raw material to substitute for MnSO4. KMnO4 was added at ratios of 0%, 3%, 6%, 7%, 9%, 11%, 14%, 16% and 20% relative to the total amount of the Mn source. The obtained samples were referred to as S0, S3, S6, S7, S9, S11, S14, S16 and S20, respectively, according to the percentage of added KMnO4.
1 in 10 mL of deionized water in a Teflon liner to obtain a mixed solution. Afterward, 20 mL of LiOH (3 mol L−1) was trickled into the solution, which was stirred for 30 min to yield a δ-MnO2 precursor precipitate.25 The Teflon liner was transferred to an autoclave and maintained at 200 °C for 24 h. The as-prepared powders were filtered, washed, and dried at 80 °C for 24 h. To produce an oxidizing atmosphere during the precipitation and formation reactions, a determined amount of KMnO4 was used as a raw material to substitute for MnSO4. KMnO4 was added at ratios of 0%, 3%, 6%, 7%, 9%, 11%, 14%, 16% and 20% relative to the total amount of the Mn source. The obtained samples were referred to as S0, S3, S6, S7, S9, S11, S14, S16 and S20, respectively, according to the percentage of added KMnO4.
Characterization of the Li2MnO3 products
The XPS Mn 3s region of the precursor was performed, using X-ray photoelectron spectroscopy (ESCALAB250Xi). XRD measurements were carried out using a Rigaku D/max-2600 PC with Cu Kα radiation (λ = 0.15406 nm). Grain sizes (D) were calculated by Scherrer formula (D = Kλ/B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, K, B and θ were Scherrer constant, full width at half maximum and the incident angle). Particle morphologies were probed by SEM (S-4800) and TEM (JEM-2100F). Raman spectroscopy (InVia-Reflex) was carried out to analyze the microstructure of Li2MnO3 samples. Specific surface areas were measured using a specific surface area and pore size analyzer (ASAP 2020 M). Grain sizes (D) were calculated by specific surface areas (D = 6/BET × ρ, ρ was the theoretical density of a unit cell). Mn K-edge X-ray absorption spectra were obtained using a BL14W1 beamline at the Shanghai Synchrotron Radiation Facility.
θ, K, B and θ were Scherrer constant, full width at half maximum and the incident angle). Particle morphologies were probed by SEM (S-4800) and TEM (JEM-2100F). Raman spectroscopy (InVia-Reflex) was carried out to analyze the microstructure of Li2MnO3 samples. Specific surface areas were measured using a specific surface area and pore size analyzer (ASAP 2020 M). Grain sizes (D) were calculated by specific surface areas (D = 6/BET × ρ, ρ was the theoretical density of a unit cell). Mn K-edge X-ray absorption spectra were obtained using a BL14W1 beamline at the Shanghai Synchrotron Radiation Facility.
Electrochemical measurement
The cathode was prepared by thoroughly mixing the active material, super P carbon black, and a polyvinylidene fluoride binder at a weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Coin-type battery cells containing a cathode, a Li metal anode, and a microporous polyethylene separator were prepared in an argon-filled glove-box. Electrochemical measurements were conducted with button-type cells using 1.2 M LiPF6 with ethyl methyl carbonate/ethylene carbonate (7
1. Coin-type battery cells containing a cathode, a Li metal anode, and a microporous polyethylene separator were prepared in an argon-filled glove-box. Electrochemical measurements were conducted with button-type cells using 1.2 M LiPF6 with ethyl methyl carbonate/ethylene carbonate (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, vol%) as electrolyte. The coin-type cells were tested at a constant current density of 20 mA g−1 using a Land battery test system. CV curves were performed by Princeton 273A (0.1 mV s−1, 2–4.8 V).
3, vol%) as electrolyte. The coin-type cells were tested at a constant current density of 20 mA g−1 using a Land battery test system. CV curves were performed by Princeton 273A (0.1 mV s−1, 2–4.8 V).
Results and discussion
Formation of Li2MnO3 nanocrystals
The phase compositions of the products with varying KMnO4 contents were analyzed by XRD (Fig. 1). The XRD patterns of the samples showed a single Li2MnO3 phase when the KMnO4 content exceeded 6% (i.e., S6, S9, S11, and S20). Without KMnO4, a main phase of Li2MnO3 with minor O-LiMnO2 (JPCDS card no. 72-0411) was observed (S0). These results indicated that KMnO4 enhanced the formation of a single Li2MnO3 phase. The diffraction lines of Li2MnO3 were indexed to a monoclinic unit cell with a space group symmetry of C2/m (JPCDS card no. 84-1634). The diffraction lines showed decreased density and increased broadness with increasing KMnO4 content because of the decreases in the grain size of the Li2MnO3 products. | ||
| Fig. 1 XRD patterns of the obtained samples of (a) for S0, (b) for S6, (c) for S9, (d) for S11 and (e) for S20. | ||
(NH4)2S2O8 was used as an oxidant to synthesize the δ-MnO2 precursor at room temperature. Mn valence of the precursor was +3.57, calculated from the XPS Mn 3s region in Fig. S1 of the ESI.† 27 Mn3+ precipitate led to LiMnO2 in the hydrothermal process. The XRD patterns of C1–C4 samples (comparison experiment without (NH4)2S2O8) showed pure Li2MnO3 phase only when the average Mn valence exceeded +4 in Fig. S2.† KMnO4, which took effect in the hydrothermal process, raised average Mn valence of the precursor and produced a weak oxidizing environment. While average Mn valence of the precursor was close to tetravalence, LiMnO2 phase disappeared. A small amount of KMnO4 could achieve pure Li2MnO3; proper excess oxidant did not affect phase purification.
The image in Fig. 2 illustrated the Raman spectra of the samples. The Raman spectrum of S6 exhibited four significant bands at 369, 435, 487, and 635 cm−1. These bands belonged to Mn–O bonding of Li2MnO3 and in agreement with previous reports.28,29 The bands were assigned to phonon vibrations, and the peak at 635 cm−1 was attributed to the A1g mode of Mn–O bonding. As the KMnO4 ratio increased, the A1g peak position remained essentially the same but showed broadening.
 | ||
| Fig. 2 Raman spectra of the obtained samples of (a) for S6, (b) for S9, (c) for S11, and (d) for S20. | ||
Morphology of the Li2MnO3 products
The morphologies of the Li2MnO3 products with different KMnO4 contents were observed by SEM and TEM (Fig. 3). The addition of KMnO4 resulted in decreased grain sizes and gradual transformation from plates to rods. S0 and S6 [Fig. 3(a) and (b)] revealed square and hexagonal nanoplates, respectively, with well-distributed particle sizes. The sharp morphologies showed high crystallinity. Nanorods and nanoplates coexisted in S9 [Fig. 3(c)]. Increasing the KMnO4 ratios to 11% and 20% (S11 and S20) resulted in further decreases in particle size, and the morphologies substantially transformed from nanoplates to nanorods [Fig. 3(d) and (e) and 3(f) and (g), respectively]. The addition of KMnO4 decreased the size of the products and affected their morphology. By contrast, the samples without (NH4)2S2O8 did not show similar change while the average Mn valence varied from 3.5 to 4.25 in Fig. S3.† | ||
| Fig. 3 SEM images of (a) for S0, (b) for S6, (c) for S9, (d) for S11 and (f) for S20, and TEM images of (e) for S11, (g) for S20. | ||
The orientation of Li2MnO3 products with different sharpness was observed by high-resolution TEM images (Fig. 4). S6 showed square crystals with sharp corners and edges, whereas S11 showed unclear edges and reductions in size. Ten fringe spacing of the two crystal faces of S6 of approximately 4.74 and 2.07 nm were observed, corresponding to the (001) and (022) planes of Li2MnO3, respectively. These planes were indexed to the [100] axis. Spacing of the clear crystal face around 0.47 nm were observed for S11, corresponding to the (001) plane, in Fig. 4(b). Thus, the c-axis lay along the thickness direction. S6 and S11 samples might experience different growth processes.
Specific surface areas and particle sizes
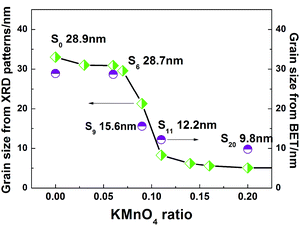
Table 1 summarized the morphologies, BET surface area, grain sizes derived from XRD Scherrer equation and BET calculation and the first discharge capacities of S6–S20 samples. Specific surface areas gradually increased from 80.8 m2 g−1 to 81.4 m2 g−1 for S6 with the addition of 6% KMnO4, and instantaneously to 150.2 m2 g−1 for S9 with the addition of 9% KMnO4. The specific surface areas maintained an approximate linear increase with increasing amount of added KMnO4 until 236.2 m2 g−1 for S20.| Sample | S0 | S6 | S9 | S11 | S20 |
| Morphology | Plate | Plate | Plate and rod | Plate and rod | Rod |
| Specific surface area (m2 g−1) | 80.8 | 81.4 | 150.2 | 191.5 | 236.2 |
| Grain sizes from BET (nm) | 28.9 | 28.7 | 15.6 | 12.2 | 9.8 |
| Grain sizes from XRD (nm) | 33 | 30 | 21 | 10 | 5 |
| Discharge capacity (mA h g−1) | 196 | 229 | 230 | 234 |
Grain sizes calculated from the (001) plane of the XRD patterns by Scherrer formula and from BET surface areas are shown in Fig. 5. The results obtained from these two methods were fairly similar. Changes in size with the amount of added KMnO4 could be classified as follows: KMnO4 ≤ 6%, 6% < KMnO4 ≤ 11%, and KMnO4 > 11%. Particle sizes displayed minor changes at KMnO4 contents of ≤6% and ≥11% areas, whereas significant decreases were observed at KMnO4 contents of 6% < KMnO4 < 11%. Grain sizes of around 28.7 nm with only nanoplate shape were obtained at KMnO4 ≤ 6%, whereas those around 9.8 nm with mainly nanorods were presented at KMnO4 > 11%. KMnO4 addition evidently resulted in continuous decreases in size between 28.7 and 12.2 nm at 6% < KMnO4 ≤ 11%. These results indicated that growth mechanism varied among these samples. The growth mechanism of Li2MnO3 nanocrystals might be oriented attachment.33 The amount of crystal nucleus controlled changes in grain size, morphology, and orientation. KMnO4, as a Mn source for Li2MnO3, accelerated the dissolution of the intermediate phase and enhanced amount of crystal nucleus of the samples. KMnO4 contents between 6% and 11% enabled rapid increases in the amount of crystal nucleus of the samples. At KMnO4 ≥ 11%, the amount of crystal nucleus reached a certain limit, such that grain size hardly changed thereafter.
In conclusion, adding the amount of KMnO4 raised average Mn valence of the precursor (be close to tetravalence) and introduced a weak oxidizing environment. Thus trivalent Mn compound disappeared. O2 decomposed from KMnO4, accelerated the dissolution of the intermediate phase and enhanced amount of crystal nucleus, which led to the decreasing grain sizes and the morphology change from nanoplates to nanorods. Increasing average Mn valence of precursor gave rise to the morphology transformation from nanoplates to nanorods and larger discharge capacities. Meanwhile (NH4)2S2O8 was significant for the δ-MnO2 precursor, without which grain sizes could not be controlled (shown in Fig. S3†).
Effects of particle size on the electrochemical properties of Li2MnO3 nanocrystals
The image in Fig. 6(a) demonstrated the first charge/discharge voltage profiles of S6, S9, S11, and S20 at a constant current density of 20 mA g−1. The first discharge capacities were 196, 229, 230, and 234 mA h g−1 for S6, S9, S11, and S20, respectively. S6, with a size of 28.7 nm, showed a relatively low capacity of 196 mA h g−1. Capacity considerably increased to 229 mA h g−1 for S9 with 15.6 nm. Then, the capacities showed small change for S11 and S20, with 12.2 and 9.8 nm, respectively. The capacities showed a linear change with the change in grain size in Fig. 6(b). First-cycle coulombic efficiencies of S6–S20 samples were 57.5%, 69.1%, 62.3% and 62.2% respectively, indicating approximate 40% Li+ could not insert into the original position. Minor improvement of the initial coulombic efficiencies was related to the grain size decreases. Meanwhile, decreasing grain sizes led to larger initial discharge capacities and lower capacity retentions after 50 cycles in Fig. S5.† Electrodes charged to 4.3 V, 4.6 V and 4.8 V and discharged to 3 V and 2 V were selected for analyzing the phase transformation in the first cycle. | ||
| Fig. 6 First charge and discharge curves at a rate of 20 mA g−1 (a), the relation between grain size and discharge capacity of the S6, S9, S11 and S20 samples (b). | ||
The downtrend of voltage plateaus were relevant with the decreasing grain sizes. To determine the voltage plateaus, corresponding dQ/dV curves were calculated, which were displayed in Fig. 7. Li2O extraction peaks shifted to lower voltages with decreasing grain sizes. S6, S9, and S11 showed oxidation peaks at 4.55, 4.4, and 4.4 V respectively, corresponding to Li2O extraction from Li2MnO3. By contrast, S20 demonstrated two peaks at 3.8 and 4.4 V. The new peak at 3.8 V might result from Li extraction of the outer structure in Li2MnO3 crystals. Stacking faults on the surface could provide electrochemical reaction points and facilitate Li extraction from outer structures.30,35 Reorganization of the outer structure subsequently occurred because of oxygen evolution, followed by Li deintercalation of inner Li2MnO3. In addition, the evident peaks at 4.7 V to 4.8 V might be ascribed to interfacial reactions. It might result in the poor cycle performance in the samples with smaller grain sizes in Fig. S5.† The discharge curves of S6, S9, S11, and S20 showed reduction peaks at 3.05 V, which were identified as Mn3+–Mn2+ reactions.
 | ||
| Fig. 7 dQ/dV of charge and discharge curves of the samples of (a) for S6, (b) for S9, (c) for S11, and (d) for S20. | ||
The charge and discharge curves of the second cycle demonstrated minor difference with each other in Fig. S4.† Corresponding dQ/dV curves were similar in all samples. The redox peaks appeared at around 4 V in the second cycle for all samples, being identified as Mn4+–Mn3+ reactions of the spinel phase. In all samples, the redox peaks at 3 V shifted to lower values, implying the voltage drop. Furthermore, the samples with smaller particle size demonstrated less voltage fade in the 3 V regime.
Evidently, larger surface area brought about lower voltage plateaus. Samples with more defects exhibited higher energies and decreased the potential barrier for Li2O extraction, thereby resulting in voltage plateau drops.34 The strong redox peak located at 4.55 V corresponded to 28.7 nm Li2MnO3 nanocrystals. Samples with 15.6 nm to 12.2 nm size showed voltage plateaus at 4.4 V. Two oxidation peaks at 3.8 and 4.4 V appeared in the 9.8 nm sample. The above conclusion was mainly consistent with the CV curves in Fig. S6 of the ESI.†
Effects of size on structural changes in the Li2MnO3 nanocrystals during charging/discharging
Representative S6 and S11 electrodes were obtained to analyze structural evolution using a series of XRD profiles. These electrodes were initially charged and discharged at different potentials, and results were shown in Fig. 8(a) and (b). The diffraction peaks of S6 and S11 at various voltages matched with monoclinic Li2MnO3 (C2/m) well. Charging S6 and S11 to 4.6 V led to the gradual disappearance of superlattice peaks around 2θ = 22°. About 67% Li ions were removed from Li2MnO3, and no other phases appeared at the 4.6 V charge state of S11 samples. LiMnO2 formed with further charging from 4.6 V to 4.8 V. According to the obtained dQ/dV curves, strong interfacial reactions were observed during charging from 4.6 V to 4.8 V in S11. During this process, LiMnO2 was formed because of interfacial reactions and electrolyte erosion. The spinel phase generated during the initial discharge. The (440) plane of the spinel phase was observed at 3 and 2 V of the first discharge. | ||
| Fig. 8 XRD patterns of the samples of S6 (a) and S11 (b), magnified (−202) peak of S6 and S11 (c), and the change of FWHM(−202) (d) after the initial discharge. | ||
The image in Fig. 8(c) showed the fitted peak near 45°. Here, the (440) spinel peaks [I(sp440)] of the S6 electrodes were stronger than those of S11 electrodes. This characteristic indicates fewer spinel phases in S11 electrodes, which might be related to particle size. Li extraction and reorganization at low potential prevented phase transformation in S11.
In both S6 and S11, initial charging to 4.8 V led to a broad (001) peak and a sharp (−202) peak, which gradually recovered after discharge. The full width at half-maximum (FWHM) of the (−202) peak [FWHM(−202)] was shown at various points of initial charging and discharging [Fig. 8(d)]. This phenomenon might involve microstress during Li deintercalation. Stress of Li removal engendered the migration of transition metal ions into the Li layers and the formation of LiMn2O4.31 However, whether microstress affected phase transformation in the cycles required further study.
The image in Fig. 9 showed the Mn K-edge XANES spectra of S6 and S11. Absorption peaks at 6560 and 6575 eV were assigned to 1s to 3d (teg) and 1s to 3d (eg) transitions, respectively. The edges of pristine S6 and S11 electrodes only partially overlapped, although their absorption peaks were both at 6561 eV. The intermediate bump at 6552 eV that was observed in S6 disappeared in S11 samples, being described as the disordered arrangement of MnO6 octahedra in this sample, which was due to stacking defects.20 Higher peak intensities near the edges of S11 indicated that Mn was in a high-energy state. Therefore, more structure distortion and stacking faults were found in S11 samples. Charging S6 and S11 to 4.8 V caused the edges shifting toward low energies, implying evident Mn3+ compound formation. According to the XRD patterns, the result indicated that only minor Mn4+ amounts were transformed into LiMnO2 after charging to 4.8 V in both S6 and S11 samples.
Conclusions
In this study, pure Li2MnO3 nanocrystals are synthesized via hydrothermal method. By adding KMnO4 as oxidation in the reaction system, pure Li2MnO3 nanocrystals with controlled size and morphology are obtained. The particle sizes remarkably influence the electrochemical performances of the nanocrystals. The discharge capacities decrease with decreasing size of Li2MnO3 nanocrystals in a linear relationship. The plateau voltage of the first charge decreases from 4.5 V to 4.4 V with the decrease in Li2MnO3 nanocrystals from 28.7 nm to 12.2 nm and splits to two plateaus around 3.8 and 4.4 V for nanocrystals of 9.8 nm size. Li2MnO3 crystals with smaller sizes are more beneficial for maintaining a layered structure than larger nanocrystals.Acknowledgements
This work was supported by the National Nature Science Foundation of China (NSFC No. 51372114), the Research Fund of State Key Laboratory of Mechanics and Control of Mechanical Structures (Nanjing University of Aeronautics and astronautics) (Grant No. 0514Y01), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).Notes and references
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359 CrossRef CAS PubMed.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652 CrossRef CAS PubMed.
- V. Etacheri, R. Marom, R. Elazari, G. Salitra and D. Aurbach, Energy Environ. Sci., 2011, 4, 3243 CAS.
- H. J. Yu and H. S. Zhou, J. Phys. Chem. Lett., 2013, 4, 1268 CrossRef CAS.
- M. M. Thackeray, S. H. Kang, C. S. Johnson, J. T. Vaughey, R. Benedek and S. A. Hackney, J. Mater. Chem., 2007, 30, 3053 Search PubMed.
- Y. Koyama, N. Yabuuchi, I. Tanaka, H. Adachi and T. J. Ozhuku, J. Electrochem. Soc., 2005, 152, A1434 CrossRef PubMed.
- A. Boulineau, L. Croguennec, C. Delmas and F. Weill, Chem. Mater., 2009, 21, 4216 CrossRef CAS.
- J. Bareno, C. H. Lei, J. G. Wen, S. H. Kang, I. Petrov and D. P. Abraham, Adv. Mater., 2010, 22, 1122 CrossRef CAS PubMed.
- C. S. Johnson, S. D. Korte, J. T. Vaughey, M. M. Thacheray, T. E. Bofinger, Y. Shao-Horn and S. A. Hachney, J. Power Sources, 1999, 81, 491 CrossRef.
- S. H. Park, Y. Sato, J. K. Kim and Y. S. Lee, Mater. Chem. Phys., 2007, 102, 225 CrossRef CAS PubMed.
- Z. Lu, Z. Chen and J. R. Dahn, Chem. Mater., 2003, 15, 3214 CrossRef CAS.
- J. Jiang, K. W. Eberman, L. J. Krause and J. R. Dahn, J. Electrochem. Soc., 2005, 152, A1874 CrossRef CAS PubMed.
- A. R. Armstrong, M. Holzapfel, P. Novák, C. S. Johnson, S. H. Kang, M. M. Thackeray and B. G. Bruce, J. Am. Chem. Soc., 2006, 128, 8694 CrossRef CAS PubMed.
- A. D. Robertson and P. G. Bruce, Chem. Commun., 2002, 2790 RSC.
- A. R. Armstrong, A. D. Robertson and P. G. Bruce, J. Power Sources, 2005, 146, 275 CrossRef CAS PubMed.
- W. Tang, H. Kanoh, X. Yang and K. Ooi, Chem. Mater., 2000, 12, 3291 Search PubMed.
- J. K. Ngala, S. Alia, A. Dobley, V. M. B. Crisostomo and S. L. Suib, Chem. Mater., 2007, 19, 229 CrossRef CAS.
- G. Jain, J. Yang, M. Balasubramanian and J. J. Xu, Chem. Mater., 2005, 17, 3850 CrossRef CAS.
- P. Lanz, C. Villevieille and P. Novák, Electrochim. Acta, 2013, 109, 426 CrossRef CAS PubMed.
- G. Z. Wei, X. Lu, F. S. Ke, L. Huang, J. T. Li, Z. X. Wang, Z. Y. Zhou and S. G. Sun, Adv. Mater., 2010, 22, 4364 CrossRef CAS PubMed.
- J. Cho, Y. Kim and M. G. Kim, J. Phys. Chem. C, 2007, 111, 3192 CAS.
- Y. Lee, M. G. Kim and J. Cho, Nano Lett., 2008, 8, 957 CrossRef CAS PubMed.
- A. Choi, K. Palanisamy, Y. Kim, J. Yoon, J. H. Park, S. W. Lee, W. S. Yoon and K. B. Kim, J. Alloys Compd., 2014, 591, 356 CrossRef CAS PubMed.
- M. Tabuchi, Y. Nabeshima, T. Takeuchi, K. Tatsumi, J. Imaizumi and Y. Nitta, J. Power Sources, 2010, 195, 834 CrossRef CAS PubMed.
- J. Y. Baek, H. W. Ha, I. Y. Kim and S. J. Hwang, J. Phys. Chem. C, 2009, 113, 17392 CAS.
- X. Huang, Q. Zhang, H. Chang, J. Gan, H. Yue and Y. Yang, J. Electrochem. Soc., 2009, 153, 162 CrossRef PubMed.
- M. G. Verde, H. Liu, K. J. Carroll, L. Baggetto, G. M. Veith and Y. S. Meng, ACS Appl. Mater. Interfaces, 2014, 6, 18868 CAS.
- W. Fang, H. Kanoh, K. Ooi and Y. Wang, J. Mater. Sci. Lett., 2000, 19, 1361 CrossRef.
- R. Wang, X. Q. He, L. H. He, F. W. Wang, R. J. Xiao, L. Gu, H. Li and L. Q. Chen, Adv. Eng. Mater., 2013, 3, 1358 CAS.
- F. Amalraj, D. Sharon, M. Talianker, C. M. Julien, L. Burlaka, R. Lavi, E. Zhecheva, B. Markovsky, E. Zinigrad, D. Kovacheva, R. Stoyanova and D. Aurbach, Electrochim. Acta, 2013, 97, 259 CrossRef PubMed.
- F. Amalraj, L. Burlaka, C. M. Julien, A. Mauger, D. Kovacheva, M. Talianker, B. Markovsky and D. Aurbach, Electrochim. Acta, 2014, 123, 395 CrossRef PubMed.
- A. Boulineau, L. Simonin, J. F. Colin, E. Canévet, L. Daniel and S. Patoux, Chem. Mater., 2012, 24, 3558 CrossRef CAS.
- R. L. Penn and J. F. Banfield, Science, 1998, 281, 969 CrossRef CAS.
- Y. Okamoto, J. Electrochem. Soc., 2012, 159, A152 CrossRef CAS PubMed.
- D. Y. W. Yu, K. Yanagida, Y. Kato and H. Nakamura, J. Electrochem. Soc., 2009, 156, A417 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra08387g |
| This journal is © The Royal Society of Chemistry 2015 |