Solid-state thermal conversion of a nanoporous metal–organic framework to a nonporous coordination polymer†
Fatemeh Shahangi Shirazi and
Kamran Akhbari*
School of Chemistry, College of Science, University of Tehran, P.O. Box 14155-6455, Tehran, Islamic Republic of Iran. E-mail: akhbari.k@khayam.ut.ac.ir; Fax: +98 21 66495291; Tel: +98 21 61113734
First published on 3rd June 2015
Abstract
A nanoporous metal–organic framework of Zn(BDC)(4,4′-Bipy)0.5·(DMF)(H2O)0.5 (1·DMF·H2O; H2BDC = 1,4-benzenedicarboxylic acid, 4,4′-Bipy = 4,4′-bipyridine, DMF = N,N-dimethylformamide) which was also recognized as MOF-508 and has a pillared 2D square-grid net, was synthesized under reflux conditions. By thermal treatment of 1·DMF·H2O at 350 °C, it converts to a nonporous three-dimensional coordination polymer of Zn(BDC)(4,4′-Bipy) (2). During the solid-state structural transformation of 1·DMF·H2O to 2, a Zn(BDC) (3) unit was removed. Thus we have a mixture of compounds 2 and 3 at 350 °C. In addition, 1·DMF·H2O and a mixture of 2 and 3 powders with microrod morphologies, were used for the preparation of ZnO nanomaterials. With calcination of the host framework of 1·DMF·H2O, ZnO nanoprticles can be fabricated. The same process with a mixture of 2 and 3 results in the formation of agglomerated ZnO nanoparticles with similar morphology to the initial precursor.
Introduction
Metal–organic frameworks (MOFs) provide a unique opportunity for developing advanced functional materials.1 In particular, nanoporous metal–organic frameworks have received extensive attention because of their potential applications such as in gas storage,2 separation processes,3 drug delivery,4 sensor technology,5 heterogeneous catalysis,6 hosts for metal colloids or nanoparticles,7 luminescence,8 non-linear optics (NLO),9 magnetism,10 and recently, useful heat transformation including cooling applications11 through reversible water de- and adsorption. On the other hand, the solid-state structural transformation is one of the interesting topics in the crystal engineering field of metal–organic frameworks (MOFs) and coordination polymers (CPs).12 Solid-state transformation of MOFs is typically associated with the loss, addition, substitution, and/or modification of building components of them. This process including the expansions of the metal coordination numbers, thermal dissociation/association, condensation, rearrangement of bonds or the removal or exchange of solvents.13 J-P. Zhang, et al. were reported temperature- or guest-induced structural transformations of a nanoporous MOF,14 however, reports on structural transformation of porous MOFs to nonporous coordination polymers are very sparse.15 Zn(BDC)(4,4′-Bipy)0.5 (MOF-508; H2BDC = 1,4-benzenedicarboxylic acid, 4,4′-Bipy = 4,4′-bipyridine) for the first time was recognized by J. T. Hupp at 2005.16 This nanoporous MOF was also reported by K. Kim at this year.17 This MOF has primitive cubic framework which composed of paddle-wheel {Zn2(COO)4} units, which are bridged by the BDC2− ligands to form a distorted 2D square grid. The 2D square grids are pillared by 4,4′-Bipy molecules, to form a 3D framework. Two of the 3D frameworks interpenetrate in MOF-508a (1·DMF·H2O), reducing the pore size. Thus, a dense doubly interpenetrating 3D framework is formed with pores that can be tuned by double interpenetration to have 1D channels of approximately 4.0 × 4.0 Å in cross section. They found that the guest-free phase MOF-508b (1) reveals that the framework retains the overall connectivity, but the {Zn2(COO)4} paddle-wheel clusters are significantly distorted, and the 4,4′-Bipy linkers are bent.18 B. Chen et al. used this microporous MOF for highly selective GC separation of alkanes.18 M. Kondo et al. presented a method to selectively synthesize crystal polymorphs of Zn(BDC)(4,4′-Bipy)0.5, as a pillared 2D Kagomé nets by simply changing the crystallization temperature. The framework of this polymorph with 2D Kagomé nets has a larger pore window (7.7 × 4.9 Å along the a axis and 5.2 × 14 Å along the c axis).19 In this work we wish to report solid-state structural transformation of a nanoporous MOF of Zn(BDC)(4,4′-Bipy)0.5·(DMF)(H2O)0.5 (1·DMF·H2O) with the pillared 2D square-grid net to a nonporous coordination polymer of Zn(BDC)(4,4′-Bipy) (2) upon thermal treatment at 350 °C.Results and discussion
The reaction between benzene-1,4-dicarboxylic acid (H2BDC) 4,4′-bipyridine (4,4′-Bipy) and Zn(NO3)2·6H2O in DMF under reflux condition at 150 °C results in formation of white powder which was dried at room temperature (Scheme 1). As was mentioned earlier, two polymorphs were recognized for Zn(BDC)(4,4′-Bipy)0.5. Under reflux condition, we may obtain pillared 2D square-grid net or pillared 2D Kagomé net Zn(BDC)(4,4′-Bipy)0.5 (Fig. S1a in the ESI†). A comparison between the XRD patterns simulated from single crystal X-ray data of these two polymorphs (Fig. S2a and b in the ESI†) and that of the prepared powder (Fig. S2c in the ESI†), approved the formation of Zn(BDC)(4,4′-Bipy)0.5·(DMF)(H2O)0.5 (1·DMF·H2O) with pillared 2D square-grid network (Fig. 1a). It composed of paddle-wheel {Zn2(COO)4} unit, which are pillared by 4,4′-Bipy molecules (Fig. S1c in the ESI†). | ||
| Scheme 1 The produced material from the reaction of H2BDC, 4,4′-Bipy and Zn(NO3)2·6H2O in DMF under reflux condition and conversion of it to nonporous coordination polymer of 2. | ||
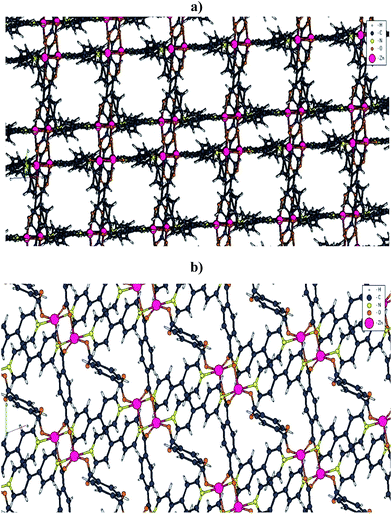 | ||
| Fig. 1 A fragment of (a) Zn(BDC)(4,4′-Bipy)0.5·(DMF)(H2O)0.5 (1·DMF·H2O) MOF with pillared 2D square-grid network and (b) nonporous three-dimensional coordination polymer of Zn(BDC)(4,4′-Bipy) (2). | ||
TGA data indicated that 1·DMF·H2O releases its guest molecules over the temperature range 25–145 °C to form the guest-free phase Zn(BDC)(4,4′-Bipy)0.5 (1), which is thermally stable to 360 °C.18 B. Chen et al. prepared single crystals of 1 by heating a sample of 1·DMF·H2O at 120 °C under vacuum for 24 h.18 By single-crystal X-ray crystallography, they showed that the guest-free phase (1) is significantly different from that of the as synthesized 1·DMF·H2O (Fig. S1c and d in the ESI†).18
In order to find out, what happens by thermal treatment of 1·DMF·H2O at higher temperatures, a sample of it was heated at 350 °C in a furnace and static atmosphere of air. The XRD pattern of the resulting powder (Fig. S2g in the ESI†) is completely different with the simulated XRD pattern from single crystal X-ray data of 1 (Fig. S2d and S3 in the ESI†). A broad search was done in literature about Zn(II) compounds with the same BDC2− and 4,4′-Bipy ligands. We found out that the XRD pattern of the sample which was heated at 350 °C (Fig. S2g in the ESI†) approximately matches with the simulated XRD pattern from single crystal X-ray data of Zn(BDC)(4,4′-Bipy) (2) (Fig. S2f in the ESI†). Zn(BDC)(4,4′-Bipy) (2) is a nonporous three-dimensional coordination polymer which for the first time was reported by J. Tao et al.20 In 2, Zn metal ion is bridged by BDC2− ligands to form a linear or zigzag coordination chain (Fig. S2e in the ESI†), and adjacent chains are further linked by BDC2− ligands to form two-dimensional rectangular [Zn(BDC)] sheet. Adjacent sheets are pillared by 4,4′-Bipy spacers into a three-dimensional coordination network through the covalently linking mode of …4,4′-Bipy–MII–4,4′-Bipy–MII…, which feature cuboidal [Zn16(BDC)8(4,4′-Bipy)8] structural units.20 Two fold interpenetration of the above three-dimensional coordination networks results in stable crystal structure of it (Fig. 1b).20 According to the following equation, during the conversion of 1·DMF·H2O to 2, a Zn(BDC) unit with a paddle-wheel structure should be removed (Scheme 1).
In order to confide this happening, we synthesized [Zn2(BDC)2(H2O)2·(DMF)2]n (3·2H2O·2DMF) MOF and prepared the activated sample of Zn(BDC) (3) (see ESI†). The extra peak at 2θ = 15° (Fig. S2g in the ESI†) which does not observed in simulated pattern of 2, completely matches with that exists in Fig. S2e,† approved that conversion of Zn(BDC)(4,4′-Bipy)0.5·(DMF)(H2O)0.5 (1·DMF·H2O) to Zn(BDC)(4,4′-Bipy) (2) is accompanied with removal of Zn(BDC) (3). In TGA analysis (Fig. S4 in the ESI†), mass loss calculations of compounds 2 and 3 mixture showed that in the first stage between 290–410 °C, removal of BDC2− from compound Zn(BDC) (3) occurred with mass loss of 27.5% (calcd 26.67%). The second stage which begin at 410 °C up to 525 °C, attributed to decomposition process of compound 2 and formation of ZnO. It should be mentioned that these results also approved the J. Tao et al. results on no chemical decomposition observed for complex 2 up to 403 °C.20 Thus this conversion is one of the rare instance solid-state structural conversion of porous MOF to nanoporous coordination polymer which also indicated that activation process of MOFs and preparation guest free samples of them should be carried out carefully.
The “before and after” structures provide insight into the mechanism and the driving force for these solid-state framework rearrangements. Fig. 2 shows the changes in Zn(II) coordination sphere during this solid-state structural transformation. In addition to removal of one Zn(BDC) (3) unit (with paddle-wheel structure), during this transformation one Zn–O coordination bond of two bidentate bridging carboxylate groups are breaking and two new Zn–N coordination bonds from 4,4′-Bipy ligand are forming (Fig. 2). Thus the Zn(II) ions in 1 and 2 have coordination number of five with ZnO4N and ZnO3N2 coordination spheres, respectively.
 | ||
| Fig. 2 Schematic representation of changes in Zn(II) coordination sphere during the solid-state structural transformation of 1·DMF·H2O (left) to 2 (right) upon thermal treatment at 350 °C. | ||
TGA data showed that there was no chemical decomposition up to 403 °C for complex 2. Two fold interpenetration of the three-dimensional coordination networks in 2 and its nonporosity result in highly stable crystal structure.20 Thus the pore destruction in 1·DMF·H2O during the solid-state structural conversion of nanoporous framework to nonporous coordination polymer and formation of 2 with higher thermal stability than 1 are two driving force during this conversion.
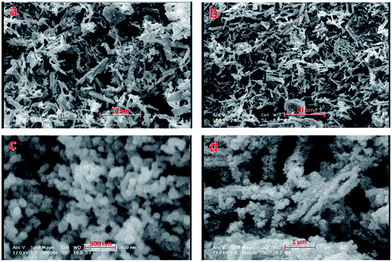
The conversion of the MOF into metal oxide nanoparticles has been used as a new strategy for preparing nanoscale functional entities.21 In this method, the secondary building units of MOF that are mostly composed of metal oxide clusters in angstrom scale were transformed into metal oxide nanomaterials by thermal treatments. Search in the case of fabrication nanomaterials from metal–organic frameworks, as new precursors, indicates that nanomaterials such as ZnO nanoparticles from [Zn2(btec)(DMF)2]n, (btec = 1,2,4,5-benzenetetracarboxylate, DMF = N,N-dimethylformamide),22 Zn2(1,4-bdc)2(dabco), (1,4-bdc = 1,4-benzenedicarboxylate, dabco = 1,4-diazabicyclo[2.2.2]octane),23 TiO2 nanoparticles from MIL-125 and MIL-125-NH2,24 CuO nanostructures from {[Cu2(BDC-NH2)2(dabco)]DMF·3H2O,25 and Co3O4 nanoparticles from Co3(NDC)3(DMF)4, (NDC = 2,6-naphthalene-dicarboxylate; DMF = N,N′-dimethyl formamide)26 were prepared. In addition to metal oxide nanomaterials, metal–organic frameworks also can be used for preparation of porous carbons. For example, L. Zhang, et al. were reported the thermal decomposition of MOF-5 to ZnO which was covered by amorphous carbon, resulting in the C/ZnO nanoparticles. The removal of ZnO could generate the mesoporous amorphous carbon with a large surface area of 1844 m2 g−1.27 In another work, nanoporous carbon particles with different particle sizes are synthesized by simple carbonization of monodispersed zeolitic imidazolate framework-8 (ZIF-8) crystals.28 Nanoporous carbon is prepared by direct carbonization of Al-based porous coordination polymers (Al-PCP), too.29 Metal oxides are used in several applications such as microelectronic circuits, sensors, fuel cells, anticorrosion coatings and catalysts. Among the large family of metal oxides, ZnO is probably the most widely used inorganic oxide in advanced materials and industrial chemicals such as electronic materials, sunscreen cosmetics, pigments and food additives.30 Thus in order to preparation of zinc oxide nanostructures and consideration the role of the guest free solvent molecules and porosity on formation of different morphologies of zinc oxide nanostructures, calcination of 1·DMF·H2O and mixture of 2 and 3 powders with microrod morphologies (Fig. 3a and b respectively) were done at 550 °C in static atmosphere of air. The XRD patterns of residues obtained from calcination process (Fig. S5 in the ESI†) are in agreement with the typical wurtzite structure of ZnO (hexagonal phase, space group P63mc, with lattice constants a = 3.24982(9) Å, c = 5.20661 Å, Z = 2, JCPDS no. 36-1451).
Fig. 3c shows the SEM images of ZnO nanoparticles obtained from calcination of the 1·DMF·H2O host framework. The corresponding particle size distribution histogram (Fig. S6a in the ESI†) showed that these nanoparticles have diameters between 50–100 nm, but the frequency (number of nanoparticles in each size distribution) of nanoparticles with 80–90 nm diameter are higher than others. If we consider the SEM images of ZnO nanostructures, which obtained from calcination mixture of 2 and 3 powders (Fig. 3d), it is obvious that, very different morphologies compared with that obtained from calcination of 1·DMF·H2O is fabricated. In fact, calcination mixture of 2 and 3 microrods with interpenetrated nonporous network (in 2) and no guest solvent molecules (in 3 MOF channels) results in removal of the organic parts of 2, 3 and formation of agglomerated ZnO nanoparticles with similar morphology to initial precursor (Fig. 3b) and with particle size distribution of 40–100 nm (Fig. 5b in the ESI†), which is smaller than that observed from 1·DMF·H2O (Fig. 5a in the ESI†), but the tendency of the ZnO nanoparticles to aggregate is increased. In addition, the frequencies of nanoparticles with size distribution of 70–80 nm are higher than others (Fig. S6b in the ESI†). Results of our work indicate that probably fully solvated MOFs such as 1·DMF·H2O can be used for preparation ZnO nanoparticles. In fact the role of organic ligands and free guest solvent molecules in 1·DMF·H2O MOF is similar to the role of polymeric stabilizers in formation nanoparticles. With removal of the guest solvent molecules from the channels of the host framework and conversion of the porous MOF to nonporous coordination polymer, the tendency of nanoparticles to agglomerate, due to large specific surface area as well as high surface energy, was increased. Finally with regard to various applications of ZnO nanomaterials such as light-emitting diodes,31 photodetectors,32 photodiodes,33 gas sensors,34 and dye-sensitized solar cells (DSSCs),35 it seems that preparation of ZnO nanomaterials from their MOFs is one of the simple and effective methods which may be applied for preparation of them.
Conclusion
In summary, white powder of Zn(BDC)(4,4′-Bipy)0.5·(DMF)(H2O)0.5 (1·DMF·H2O) with pillared 2D square-grid net was prepared under reflux condition at 150 °C. It composed of paddle-wheel {Zn2(COO)4} unit, which are pillared by 4,4′-Bipy molecules. TGA data indicated that the guest-free phase Zn(BDC)(4,4′-Bipy)0.5 (1) is thermally stable to 360 °C. By thermal treatment of 1·DMF·H2O at 350 °C, it converts to Zn(BDC)(4,4′-Bipy) (2) which is a nonporous three-dimensional coordination polymer. During the solid-state structural transformation of 1·DMF·H2O to 2, a Zn(BDC) unit (3) with paddle-wheel structure was removed. Finally we have mixture of compounds 2 and 3 at 350 °C. This conversion is one of the rare instance solid-state structural conversion of porous MOF to nanoporous coordination polymer which also indicated that activation of MOFs and preparation guest free samples of them should be carried out carefully. Compound 2 which is a nonporous coordination polymer and has a two fold interpenetration of the three-dimensional coordination networks is stable up to 403 °C. Thus the pore destruction in 1·DMF·H2O during the solid-state structural conversion of nanoporous framework to nonporous coordination polymer and formation of 2 with higher thermal stability than 1 are two driving force during this conversion. In addition, 1·DMF·H2O and mixture of 2 and 3 powders with microrod morphologies, were used for preparation of ZnO nanomaterials. With calcination the host framework of 1·DMF·H2O, ZnO nanoprticles can be fabricated. The same process on mixture of 2 and 3 results in formation of agglomerated ZnO nanoparticles with similar morphology to initial precursor. The role of organic ligands and free guest solvent molecules in 1·DMF·H2O MOF is similar to the role of polymeric stabilizers in formation nanoparticles.Experimental
Materials and physical techniques
All reagents for the synthesis and analysis were commercially available and used as received. Microanalyses were carried out using a Heraeus CHN–O-rapid analyzer. Melting points were measured on an Electrothermal 9100 apparatus and are uncorrected. IR spectra were recorded using Nicolet 510P spectrophotometer. The molecular structure plot and simulated XRD powder pattern based on single crystal data were prepared using TOPOS and Mercury software. X-ray powder diffraction (XRD) measurements were performed using a Philips X'pert diffractometer with mono chromatized Cu-Kα radiation. The samples were characterized with a scanning electron microscope (Philips XL 30) with gold coating.Synthesis of Zn(BDC)(4,4′-Bipy)0.5·(DMF)(H2O)0.5 (1)
White powder of Zn(BDC)(4,4′-Bipy)0.5·(DMF)(H2O)0.5 (1) was synthesized by dissolving 5 mmol (0.831 g) benzene-1,4-dicarboxylic acid (H2BDC), 5 mmol (1.485 g) Zn(NO3)2·6H2O and 2.5 mmol (0.390 g) 4,4′-Bipy in 50 mL DMF. The resulting mixture was refluxing at 150 °C for 12 hours. 5 hours after beginning of the reflux reaction, white precipitate was formed. After filtering, the white precipitate was washed with DMF, and dried at room temperature for 2 days. d.p. = above 300 °C, yield: 1.354 g, 69.5% based on final product, IR (selected bands; in cm−1): 531 w, 631 m, 747 s, 826 s, 1018 w, 1065 w, 1094 w, 1216 w, 1389 vs., 1503 s, 1601 vs., 1676 vs. and 2600–3600 br. Anal. calc. for C16H16N2O5.5Zn: C, 49.31; H, 4.14; N, 7.19 found; C, 49.40; H, 4.10; N, 7.25%.Conversion of 1 to mixture of Zn(BDC)(4,4′-Bipy) (2) and Zn(BDC) (3) upon thermal treatment
White powder of Zn(BDC)(4,4′-Bipy)0.5·(DMF)(H2O)0.5 (1) were heated at 350 °C in a furnace and static atmosphere of air for 5 hours. d.p. = above 300 °C, IR (selected bands; in cm−1): 517 m, 631 m, 749 s, 826 m, 1014 w, 1045 w, 1072 w, 1105 w, 1154 w, 1221 m, 1396 vs., 1504 s and 1607 vs. Anal. calc. for C13H8NO4Zn: C, 50.76; H, 2.62; N, 4.55 found; C, 50.88; H, 2.55; N, 4.48%.Preparation ZnO nanostructures from compounds 1–3
In order to preparation of zinc oxide nanostructures, after well grinding of 1 and mixture of 2 and 3 for 15 minutes, calcination of well scattered powder of them as a very thin film were done at 550 °C in a furnace and static atmosphere of air for 3 hours.Acknowledgements
The authors would like to acknowledge the financial support of University of Tehran for this research under grant number 01/1/389845.Notes and references
- (a) J.-C. G. Bünzli and C. Piguet, Chem. Soc. Rev., 2005, 34, 1048 RSC; (b) S. Kitagawa, R. Kitaura and S.-I. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS PubMed.
- (a) N. L. Rosi, J. Eckert, M. Eddaoudi, D. T. Vodak, J. Kim, M. O'Keeffe and O. M. Yaghi, Science, 2003, 300, 1127 CrossRef CAS PubMed; (b) M. P. Suh, Y. E. Cheon and E. Y. Lee, Coord. Chem. Rev., 2008, 252, 1007 CrossRef CAS PubMed; (c) C. J. Kepert, Chem. Commun., 2006, 695 RSC; (d) M. Eddaoudi, J. Kim, D. Vodak, A. Sudik, J. Wachter, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4900 CrossRef CAS PubMed; (e) K. Akhbari and A. Morsali, Dalton Trans., 2013, 4786 RSC.
- S. Ma, D. Sun, M. Ambrogio, J. A. Fillinger, S. Parkin and H. C. Zhou, J. Am. Chem. Soc., 2007, 129, 1858 CrossRef CAS PubMed.
- (a) P. Horcajada, C. Serre, M. Vallet-Regí, M. Sebban, F. Taulelle and G. Férey, Angew. Chem., Int. Ed., 2006, 45, 5974 CrossRef CAS PubMed; (b) M. Vallet-Regi, F. Balas and D. Arcos, Angew. Chem., Int. Ed., 2007, 46, 7548 CrossRef CAS PubMed; (c) G. Férey, Chem. Soc. Rev., 2008, 37, 191 RSC.
- (a) G. J. Halder, C. J. Kepert, B. Moubaraki, K. S. Murray and J. D. Cashion, Science, 2002, 298, 1762 CrossRef CAS PubMed; (b) J. A. Real, E. Andres, M. C. Munoz, M. Julve, T. Granier, A. Bousseksou and F. Varret, Science, 1995, 268, 265 CAS.
- (a) A. Henschel, K. Gedrich, R. Kraehnert and S. Kaskel, Chem. Commun., 2008, 4192 RSC; (b) J. W. Han and C. L. Hill, J. Am. Chem. Soc., 2007, 129, 15094 CrossRef CAS PubMed; (c) P. Mahata, G. Madras and S. Natarajan, J. Phys. Chem. B, 2006, 110, 13759 CrossRef CAS PubMed.
- M. Müller, S. Hermes, K. Kähler, M. W. E. van den Berg, M. Muhler and R. A. Fischer, Chem. Mater., 2008, 20, 4576 CrossRef.
- H. A. Habib, J. Sanchiz and C. Janiak, Inorg. Chim. Acta, 2009, 362, 2452 CrossRef CAS PubMed.
- O. R. Evans and W. Lin, Acc. Chem. Res., 2002, 35, 511 CrossRef CAS PubMed.
- (a) H. A. Habib, J. Sanchiz and C. Janiak, Dalton Trans., 2008, 1734 RSC; (b) J. Larionova, Y. Guari, C. Sangregorio and C. Guérin, New J. Chem., 2009, 33, 1177 RSC.
- S. K. Henninger, H. A. Habib and C. Janiak, J. Am. Chem. Soc., 2009, 131, 2776 CrossRef CAS PubMed.
- (a) E. Barea, J. A. R. Navarro, J. M. Salas, N. Masciocchi, S. Galli and A. Sironi, J. Am. Chem. Soc., 2004, 126, 3014 CrossRef CAS PubMed; (b) K. Akhbari and A. Morsali, CrystEngComm, 2011, 13, 2047 RSC; (c) K. Akhbari and A. Morsali, Inorg. Chem., 2013, 52, 2787 CrossRef CAS PubMed; (d) K. Akhbari and A. Morsali, CrystEngComm, 2013, 15, 8915 RSC; (e) Y.-Q. Lan, H.-L. Jiang, S.-L. Li and Q. Xu, Inorg. Chem., 2012, 51, 7484 CrossRef CAS PubMed; (f) K. Fukuhara, S.-I. Noro, K. Sugimoto, T. Akutagawa, K. Kubo and T. Nakamura, Inorg. Chem., 2013, 52, 4229 CrossRef CAS PubMed; (g) D. L. Reger, A. Leitner, P. J. Pellechia and M. D. Smith, Inorg. Chem., 2014, 53, 9932 CrossRef CAS PubMed; (h) R. Miyake and M. Shionoya, Inorg. Chem., 2014, 53, 5717 CrossRef CAS PubMed; (i) A. K. Chaudhari, S. S. Nagarkar, B. Joarder, S. Mukherjee and S. K. Ghosh, Inorg. Chem., 2013, 52, 12784 CrossRef CAS PubMed.
- (a) T. Kuroda-Sowa, S. Q. Liu, Y. Yamazaki, M. Munakata, M. Maekawa, Y. Suenaga, H. Konaka and H. Nakagawa, Inorg. Chem., 2005, 44, 1686 CrossRef CAS PubMed; (b) C.-C. Wang, C.-C. Yang, C.-T. Yeh, K.-Y. Cheng, P.-C. Chang, M.-L. Ho, G.-H. Lee, W.-J. Shih and H.-S. Sheu, Inorg. Chem., 2011, 50, 597 CrossRef CAS PubMed; (c) S. Hou, Q.-K. Liu, J.-P. Ma and Y.-B. Dong, Inorg. Chem., 2013, 52, 3225 CrossRef CAS PubMed; (d) B. F. Abrahams, A. D. Dharma, M. J. Grannas, T. A. Hudson, H. E. Maynard-Casely, G. R. Oliver, R. Robson and K. F. White, Inorg. Chem., 2014, 53, 4956 CrossRef CAS PubMed; (e) D. L. Reger, A. Leitner, P. J. Pellechia and M. D. Smith, Inorg. Chem., 2014, 53, 9932 CrossRef CAS PubMed.
- J.-P. Zhang, Y.-Y. Lin, W.-X. Zhang and X.-M. Chen, J. Am. Chem. Soc., 2005, 127, 14162 CrossRef CAS PubMed.
- (a) S. S. Kaye, A. Dailly, O. M. Yaghi and J. R. Long, J. Am. Chem. Soc., 2007, 129, 14176 CrossRef CAS PubMed; (b) W. Kaneko, M. Ohba and S. Kitagawa, J. Am. Chem. Soc., 2007, 129, 13706 CrossRef CAS PubMed.
- B.-Q. Ma, K. L. Mulfort and J. T. Hupp, Inorg. Chem., 2005, 44, 4912 CrossRef CAS PubMed.
- H. Chun, D. N. Dybtsev, H. Kim and K. Kim, Chem.–Eur. J., 2005, 11, 3521 CrossRef CAS PubMed.
- B. Chen, C. Liang, J. Yang, D. S. Contreras, Y. L. Clancy, E. B. Lobkovsky, O. M. Yaghi and S. Dai, Angew. Chem., Int. Ed., 2006, 45, 1390 CrossRef CAS PubMed.
- M. Kondo, Y. Takashima, J. Seo, S. Kitagawa and S. Furukawa, CrystEngComm, 2010, 12, 2350 RSC.
- J. Tao, M.-L. Tong and X.-M. Chen, J. Chem. Soc., Dalton Trans., 2000, 3669 RSC.
- (a) M. Y. Masoomi and A. Morsali, Coord. Chem. Rev., 2012, 256, 2921 CrossRef CAS PubMed; (b) R. Das, P. Pachfule, R. Banerjee and P. Poddar, Nanoscale, 2012, 4, 591 RSC; (c) M. Moeinian and K. Akhbari, J. Solid State Chem., 2015, 225, 459 CrossRef CAS PubMed.
- F. Z. Karizi, V. Safarifard, S. K. Khani and A. Morsali, Ultrason. Sonochem., 2015, 23, 238 CrossRef CAS PubMed.
- K. Akhbari and A. Morsali, J. Coord. Chem., 2011, 64, 3521 CrossRef CAS PubMed.
- J. Hyuk Im, E. Kang, S. J. Yang, H. J. Park, J. Kim and C. R. Park, Bull. Korean Chem. Soc., 2014, 35, 2477 CrossRef.
- M. A. Alavi, A. Morsali, S. W. Joo and B.-K. Min, Ultrason. Sonochem., 2015, 22, 349 CrossRef CAS PubMed.
- B. Liu, X. Zhang, H. Shioyama, T. Mukai, T. Sakai and Q. Xu, J. Power Sources, 2010, 195, 857 CrossRef CAS PubMed.
- L. Zhang and Y. H. Hu, J. Phys. Chem. C, 2010, 114, 2566 CAS.
- N. L. Torad, M. Hu, Y. Kamachi, K. Takai, M. Imura, M. Naito and Y. Yamauchi, Chem. Commun., 2013, 49, 2521 RSC.
- M. Hu, J. Reboul, S. Furukawa, N. L. Torad, Q. Ji, P. Srinivasu, K. Ariga, S. Kitagawa and Y. Yamauchi, J. Am. Chem. Soc., 2012, 134, 2864 CrossRef CAS PubMed.
- T. Sawaki and Y. Aoyama, J. Am. Chem. Soc., 1999, 121, 4793 CrossRef CAS.
- N. Saito, H. Haneda, T. Sekiguchi, N. Ohashi, I. Sakaguchi and K. Koumoto, Adv. Mater., 2002, 14, 418 CrossRef CAS.
- S. Liang, H. Sheng, Y. Liu, Z. Hio, Y. Lu and H. Shen, J. Cryst. Growth, 2001, 225, 110 CrossRef CAS.
- J. Y. Lee, Y. S. Choi, J. H. Kim, M. O. Park and S. Im, Thin Solid Films, 2002, 403, 553 CrossRef.
- N. Golego, S. A. Studenikin and M. Cocivera, J. Electrochem. Soc., 2000, 147, 1592 CrossRef CAS PubMed.
- C. Bauer, G. Boschloo, E. Mukhtar and A. Hagfeldt, J. Phys. Chem. B, 2001, 105, 5585 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Other synthetic procedures, figures and XRD patterns are available. See DOI: 10.1039/c5ra08350h |
| This journal is © The Royal Society of Chemistry 2015 |