Bioactive Ti alloy with hydrophilicity, antibacterial activity and cytocompatibility
Vinod
Prabu†
a,
P.
Karthick†
a,
Archana
Rajendran
a,
Duraipandy
Natarajan
b,
M. S.
Kiran
b and
Deepak K.
Pattanayak
*a
aCSIR-Central Electrochemical Research Institute, Karaikudi, Tamil Nadu 630006, India. E-mail: deepak@cecri.res.in; Tel: +91-4565-241397
bCSIR-Central Leather Research Institute, Chennai, Tamil Nadu 600020, India
First published on 29th May 2015
Abstract
Titanium–6 aluminium–4 vanadium (Ti64) alloy was modified to a hydrophilic, cytocompatible, antibacterial and bioactive surface via a simple cost effective chemical treatment method. A fine porous network structure of sodium hydrogen titanate (SHT) was formed on the Ti64 alloy surface by using sodium hydroxide treatment. The incorporated Na+ ions are replaced by Ag+ ions by a subsequent silver nitrate treatment. Contact angle measurement indicated that the silver containing Ti64 alloy surface is hydrophilic at lower silver concentration. The antibacterial study of the thus prepared sample against Staphylococcus aureus confirmed the bacterial resistance of the Ti64 alloy. As evident from the AAS result, the sustained release of Ag into the culture medium results in antibacterial activity. Cytocompatibility studies on MG63 cell lines showed above 80% cell viability and also good cell attachment. This confirmed the nontoxic behavior of the present optimized silver concentration on the Ti64 surface for MG63 cells. In vitro bioactivity of the silver containing Ti64 sample in simulated body fluid showed bone-like apatite formation and the apatite-forming ability is not affected by Ag concentration or by heat treatment. Taken together, this surface modification study adds further information to our knowledge on the development of a bioactive Ti64 alloy with hydrophilicity, antibacterial activity and biocompatibility that may have considerable potential application as orthopedic and dental implants.
1. Introduction
Biomaterials are a class of materials that interact with biological systems and help to replace any damaged tissues or organs of the body.1,2 Materials such as metals, ceramics, polymers or their combinations are used for the fabrication of man-made biomaterials. Among them, metallic biomaterials are very often useful in orthopedic devices such as artificial hip joints, knee joints, spine, bone plates and dental devices.3 The metals used in this respect are stainless steel, cobalt–chromium alloys, titanium and its alloys. Among them, Ti and its alloys have good biocompatibility, better mechanical properties and high corrosion resistance.4 Apart from the above properties, Ti and its alloys have excellent fatigue and wear resistance and comparable modulus of elasticity to that of human bone.5Although, Ti and its alloys are biocompatible, new bone formation directly in contact with the implant takes very long time.6 This necessitates the researcher to develop bioactive Ti and Ti alloys through various surface modifications that can enhance the osteointegration. These surface modifications can be achieved by mechanical methods such as machining,7–11 polishing,12 grinding and blasting13–16 or chemical methods such as chemical treatment, CVD, electrochemical and biochemical etc.7 or by physical modification methods like thermal spraying and physical vapor deposition.
Among various well known chemical modification approaches, acid treatment,17–19 alkali treatment,20–24 hydrogen peroxide treatment,25–27 heat treatment28 and passivation treatment29 are well documented in the literature. Although bioactivity could be enhanced by the above methods, colonization of the medical device with bacteria may lead to infection.30 Despite significant advances in microbiology, this is still one of the major problems to all medical devices, which may lead to severe pain and secondary surgery depending on the location and type of device.30,31
Thus, it could be visualized that Ti and Ti alloys have potential to come out as a biocompatible, bioactive and an antibacterial biomaterial if proper surface chemical treatment is provided prior to the implantation. Although bioactivity could be achieved by various chemical treatment methods, antibacterial property coupled with bioactivity to the implant surface is still a challenge.27 Several strategies are reported for attributing antibacterial behavior to the material, they include coatings loaded with antibiotics,32–34 coatings containing non antibiotic organic antimicrobial agents,35 inorganic antimicrobial agents,36–39 biofunctionalization with antibacterial bioactive polymers.40 However, the antimicrobial agent may have adverse effect on human cells due to its low biocompatibility and cytotoxicity.41 Hence there is a pressing need to have an antimicrobial agent that should show both low cytotoxicity as well as good antibacterial activity.
Recently literature reports the surface modification approaches to provide bioactivity coupled with antibacterial activity on Ti and Ti alloy.42–45 This includes antibacterial activity of nanostructured silver titanate against methicillin resistant Staphylococcus aureus.42 However, they have not discussed the bioactivity and cytocompatibility of thus developed silver titanate thin film. Since silver is cytotoxic at higher concentrations, cytocompatibility study of silver substituted samples are inevitable. On the other hand, the titanate–silver nanoparticle–titanate sandwich structure developed on Ti surface with an antibacterial activity and cytocompatibility is reported by Ren et al.43 Similar experiment is reported on silver nanoparticle filled nanotube layer on Ti surface.44 Silver incorporation on Ti and Ti64 alloy surface after hydrogen peroxide treatment for increased surface properties have also been proposed.27,45 Only limited amount of apatite were observed on such surfaces even after 15 days soaking in simulated body fluid (SBF).45 In the present investigation, in vitro bioactivity, antibacterial activity and cell compatibility of silver incorporated Ti64 alloy with respect to the silver concentration was systematically examined.
2. Experimental
2.1 Preparation of samples
Ti64 alloy rod of diameter 15 mm was cut into circular samples of thickness 1 mm, and abraded with #400 SiC paper to remove the oxide layer formed on its surface. The polished samples were then washed in sequence with acetone, 2-propanol, and ultra-pure water, respectively for 15 min each in an ultrasonic cleaner. The cleaned samples were dried in an oven at 40 °C for overnight. The dried samples were immersed in 10 mL of 5 M NaOH (Sigma Aldrich, purity 98%) solution under shaking at 120 rpm at 60 °C for 24 h. After removal from sodium hydroxide solution, the samples were washed with ultra-pure water, to remove the weakly bonded Na+ ions. These samples were subsequently soaked in AgNO3 (Sigma Aldrich, purity 99.8%) solution of different concentrations ranging from 0.01–100 mM under shaking at 120 rpm at 40 °C for 24 h. Silver incorporated samples were then gently washed with ultra-pure water and allowed to dry in an oven. Some of the samples were heat treated at 600 °C for 1 h in air atmosphere and the rate of heating was maintained at 5 °C min−1. Table 1 shows the notations of various chemical and thermal treatments used in the present study.| Chemical treatments | Notations |
|---|---|
| Untreated Ti–6Al–4V alloy (as-polished) | Ti64 |
| Ti–6Al–4V alloy treated with 5 M NaOH solutions, 60 °C, 24 h | Ti64-N |
| Ti–6Al–4V alloy treated with 5 M NaOH solutions, 60 °C, 24 h and 0.01–100 mM AgNO3 solution, 40 °C, 24 h | Ti64-N-0.01-100Ag |
| Ti–6Al–4V alloy, heat treated at 600 °C, 1 h | Ti64-H |
| Ti–6Al–4V alloy treated with 5 M NaOH solutions, 60 °C, 24 h, heat treated at 600 °C, 1 h | Ti64-N-H |
| Ti–6Al–4V alloy treated with 5 M NaOH solutions, 60 °C, 24 h and 0.01–100 mM AgNO3 solution, 40 °C, 24 h, heat treated at 600 °C, 1 h | Ti64-N-0.01-100Ag-H |
2.2 Surface analysis of chemically treated Ti64 alloys
The surfaces of the Ti64 alloy subjected to various chemical treatments described above were observed using scanning electron microscope (SEM; TESCAN, Czech Republic). The chemical composition of surfaces of chemically treated Ti64 alloy were analyzed by energy dispersive X-ray spectroscopy analysis (EDX) attached to the SEM under an acceleration voltage of 15 kV. This analysis was carried out in three different location of each sample and averaged to know the amount of silver ions incorporated into the Ti64 alloy. In order to identify the surface functionalization, laser Raman spectroscopy (RENISHAW Co., UK) was used for Ti64-N-0.05 to 100Ag sample before and after heat treatment. For this measurement, He–Ne laser with a wavelength of 630 nm was used.X-ray diffraction (XRD) analysis was carried out to observe the differences in the crystallographic structure for the Ti64-N and Ti64-N-100Ag samples before and after heat treatment. XRD measurements were made on a Bruker D8 Advance diffractometer in Cu Kα radiation and detected using a Bruker Lynx Eye detector. XRD spectra were recorded in the range 10–60° in 2θ at a step size of 0.02° and a count time of 5![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) s. Chemical state and composition of Ag containing Ti64 alloy after heat treatment was investigated by X-ray photoelectron spectroscopy (XPS; Thermo V G Scientific, UK). All XPS data presented here were acquired using Al Kα line 1486.6 eV. The photoelectrons were collected at an electron take off angle of 45 °C. Peak positions were then calibrated with respect to the C1S peak at 284.5 eV. Silver release in SBF was measured by atomic absorption spectrophotometer (AAS; Varian Co., Australia). Surface morphology of Ti64 alloy subjected to chemical and heat treatment and soaked in SBF was similarly characterized by SEM and XRD.
s. Chemical state and composition of Ag containing Ti64 alloy after heat treatment was investigated by X-ray photoelectron spectroscopy (XPS; Thermo V G Scientific, UK). All XPS data presented here were acquired using Al Kα line 1486.6 eV. The photoelectrons were collected at an electron take off angle of 45 °C. Peak positions were then calibrated with respect to the C1S peak at 284.5 eV. Silver release in SBF was measured by atomic absorption spectrophotometer (AAS; Varian Co., Australia). Surface morphology of Ti64 alloy subjected to chemical and heat treatment and soaked in SBF was similarly characterized by SEM and XRD.
2.3 Contact angle study
Wetability (extent of hydrophilicity/hydrophobicity) of various chemically treated Ti64 surfaces was evaluated by contact angle measurement, VCA Optima instrument and VCA Optima XC software. Measurements were carried out under static (equilibrium) mode using distilled water as the test liquid. After proper cleaning the syringe of 100 µL capacity, one drop (5 µL) of distilled water is put on the sample surface and the water contact angle is recorded by an optical microscope connected with a camera. The measurements were carried out at three different places for each sample and the results were averaged.2.4 Antibacterial study
Zone of inhibition method was used to evaluate the antimicrobial properties of Ti64, Ti64-N, Ti64-N-0.01 to 100Ag samples. For antimicrobial activity study, the procedure reported earlier was followed.27 In brief, 200 mL of nutrient medium was prepared with sterile distilled water in a conical flask and then autoclaved at 121 °C for 15 min. Then at the hand bearable heat the sterilized nutrient medium was poured aseptically into the sterile petriplates and allowed it to solidify. After solidification, the plates were kept in an inverted position to avoid the formation of water droplets inside the plates. Lyophilized cells of Staphylococcus aureus were collected from Microbial Type Culture Collection and Gene Bank (MTCC), Dept of Biotechnology, Chandigarh, India. The vials were cut open and the lyophilized cells were aseptically transferred into the nutrient broth (0.5% NaCl, 0.15% yeast extract, 0.15% beef extract, 0.5% peptone) at room temperature and incubated at 37 °C for about 24 h. The overnight culture of Staphylococcus aureus were taken and the cells were swabbed on the nutrient agar plates using sterile cotton swab under sterile condition to avoid the over growth of cells and contamination. All the treated Ti64 alloy samples were aseptically placed on the inoculated plates using a sterile forceps and incubated at 37 °C for 24 h.2.5 Cytotoxicity study
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per mL) were seeded in a 24 well tissue culture multiplates containing Ti64-N-0.02 to 0.1Ag samples. The cells were maintained in DMEM medium in a CO2 incubator at 37 °C (5% CO2/95% atmosphere) for 48 h. Control groups include untreated cells and the sample alone with starting reagents. After 48 h of incubation, the cells were imaged using a Leica Phase contrast microscope. The culture medium was removed and the cells were washed with sterile PBS. The cells were then incubated for 3 h with 250 µL phosphate buffer saline (PBS) containing MTT (0.5 mg mL−1). After 3 h the PBS was removed and the formazan product formed was solubilised in 150 µL of DMSO. The absorbance was measured at 560 nm using BioRad microplate reader.47 The results given are average of three independent experiments.
000 cells per mL) were seeded in a 24 well tissue culture multiplates containing Ti64-N-0.02 to 0.1Ag samples. The cells were maintained in DMEM medium in a CO2 incubator at 37 °C (5% CO2/95% atmosphere) for 48 h. Control groups include untreated cells and the sample alone with starting reagents. After 48 h of incubation, the cells were imaged using a Leica Phase contrast microscope. The culture medium was removed and the cells were washed with sterile PBS. The cells were then incubated for 3 h with 250 µL phosphate buffer saline (PBS) containing MTT (0.5 mg mL−1). After 3 h the PBS was removed and the formazan product formed was solubilised in 150 µL of DMSO. The absorbance was measured at 560 nm using BioRad microplate reader.47 The results given are average of three independent experiments.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells (MG63) were seeded on sample surface without overflowing and incubated in a CO2 incubator at 37 °C, 5% CO2 and 95% air for 24 h. After incubation the cells on the sample surface were fixed with 4% w/w formaldehyde in PBS and incubated for 30 min at room temperature. Further the cells were dehydrated through a series of ethyl alcohol solution (from 50% to 100% v/v in distilled water), followed by a ratio of ethyl alcohol and hexamethyl disilazane solution at 3
000 cells (MG63) were seeded on sample surface without overflowing and incubated in a CO2 incubator at 37 °C, 5% CO2 and 95% air for 24 h. After incubation the cells on the sample surface were fixed with 4% w/w formaldehyde in PBS and incubated for 30 min at room temperature. Further the cells were dehydrated through a series of ethyl alcohol solution (from 50% to 100% v/v in distilled water), followed by a ratio of ethyl alcohol and hexamethyl disilazane solution at 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratios respectively. Sample was air dried and observed in SEM after gold sputtering.
3 ratios respectively. Sample was air dried and observed in SEM after gold sputtering.
2.6 Preparation of simulated body fluid (SBF)
SBF is an acellular solution with ion concentrations (in milli moles: Na+ 142.0, K+ 5.0, Mg2+ 1.5, Ca2+ 2.5, Cl− 147.8, HCO3− 4.2, HPO42− 1.0, SO42− 0.5) nearly equal to those of human blood plasma at 36.5 °C. The SBF was prepared as per Kokubo protocol by dissolving reagent-grade NaCl, NaHCO3, KCl, K2HPO4·3H2O, MgCl2·6H2O, CaCl2 and Na2SO4 (Sigma Aldrich, purity 99%) into ultra-pure water, and buffered at pH 7.4 with tris–hydroxymethylaminomethane ((CH2OH)3CNH2) and 1 M HCl (Sigma Aldrich, purity 99%).48 The SBF was kept in a refrigerator for 3 to 4 days prior to use.3. Results
3.1 Scanning electron microscopy and energy dispersive X-ray spectroscopy analysis
Fig. 1 show the SEM photographs of the surface of Ti64 alloy subjected to AgNO3 treatment of different concentrations after NaOH treatment compared to that of surface of untreated Ti64 alloy and that treated with only NaOH solution. The Ti64 alloy showed a smooth surface with uniform patterns on it, which is formed during polishing with #400 SiC paper while NaOH treated surface showed a fine porous network structure in nanometer scale. This fine porous network structure was retained by the subsequent AgNO3 solution of different concentrations. | ||
| Fig. 1 SEM images of Ti64 alloy after treatment with NaOH solution and then subsequently soaked in AgNO3 solutions at different magnifications. | ||
Fig. 2 show the EDX results of Ti64 alloy treated with AgNO3 solution under various concentrations followed by treatment in NaOH solution. It can be seen from Fig. 2 that the NaOH treatment incorporates about 2 at% of Na+ ions in to the Ti64 alloy. The amount of silver content increased with increasing AgNO3 concentration (about 2.2 at% for Ti64-N-100Ag sample) while the concentration of Na+ ions becomes almost negligible. This indicates that most of the Na+ ions are replaced by the Ag+ ions by the subsequent chemical substitution process. The amount of silver on NaOH treated Ti64 alloy samples soaked in AgNO3 solution up to 2 mM is depicted by dotted region in Fig. 2. Initially silver ion incorporation increased with increase in concentration of AgNO3 solution, and becomes almost saturated at a concentration of 10 mM.
 | ||
| Fig. 2 EDX results of Ti64 alloy after treatment with NaOH solution and then subsequently soaked in different concentrations of AgNO3 solution. | ||
3.2 Raman spectroscopy and X-ray diffraction study
Fig. 3(a) and (b) shows Raman spectra of the surfaces of Ti64 alloy after NaOH and NaOH–0.05 to 100Ag before and after heat treatment. The results showed that sodium hydrogen titanate (SHT) was formed on the surface of NaOH treated alloy sample, the spectrum of each specimen shows a set of five broad peaks at 276, 440, 660–670 and 906 cm−1, which are found to be in good agreement with earlier reports on hydrogen titanate (HT).51,52 The intrinsic hydrogen titanate (H2Ti3O7) bands at 276 and 906 cm−1 are due to the Ti–O–H bonding.53 Based on the studies of Ma et al.,54 the peak at 440 cm−1 is due to the Ti–O bending vibration involving six-coordinated titanium atoms and three-coordinated oxygen atoms. The peak at 660 cm−1, according to the studies of Kasuga et al.,55 is ascribed to the Ti–O–Ti vibration in [TiO6] octahedral layer in the titanate. On subsequent treatment with AgNO3 solution, the sodium ions in SHT are replaced by the silver ions from AgNO3 solution and form Ag+ containing hydrogen titanate, without any peak shift. On increasing the concentration of AgNO3 solution, amount of doped silver on Ti64 alloy surface also increases thereby the peak intensity get reduced.36 On subsequent heat treatment this SHT and HT layer were converted to sodium titanate (ST) and anatase phase, respectively as observed in Fig. 3(b). These results are in good agreement with our previous studies.27,52Fig. 3(c) shows the XRD spectra of Ti64-N and Ti64-N-100Ag samples before and after heat treatment. Apart from Ti peaks, small peak of ST was observed at 24.6° in 2 theta. On subsequent treatment with 100 mM AgNO3 solution and heat treatment, an anatase phase was observed along with silver peak (44° in 2 theta). This shows that during heat treatment Ag+ ions are transformed to metallic Ag.
3.3 X-ray photoelectron spectroscopy study
In order to understand the state of silver, XPS analysis of Ti64-N-0.05Ag-H sample was carried out and is shown in Fig. 4. The survey scan shows the presence of oxygen, titanium, silver, sodium and vanadium along with carbon peak. Narrow scan of Ag3d peak is shown as an inset. Peak corresponding to 367.2 eV (Ag+) indicates silver to be present as Ag2O. Additional broad peak at 368.1 is assigned to be Ag0 state. It is well known that silver on titania surface can easily oxidized in to silver oxide (Ag2O).27 | ||
| Fig. 4 XPS survey spectrum for Ti64 alloy subjected to NaOH–0.05Ag and heat treatment. The XPS spectra for Ag3d5/2 and Ag3d3/2 is shown as inset. | ||
3.4 Contact angle measurement
Fig. 5(a) shows the image of a water droplet when dropped on the surface of untreated Ti64 alloy and chemically treated samples. Distilled water contact with untreated Ti64 alloy at an angle of above 80° shows some amount of hydrophobicity which is evident in the Fig. 5(b). Comparing to Ti64 alloy, Ti64-N showed very low contact angle of 25°. The other images represent the samples treated with AgNO3 solution at different concentrations (0.1 to 100 mM) followed by NaOH treatment. Results showed that at lower concentration of silver (0.1 mM), the contact angle is 18° which is less than that of NaOH treated samples and the contact angle increases for increased amount of silver (140° for 100 mM).For an ideal biomaterial, it should have a higher wetability along with adequate antibacterial property because a material with better wetness will develop the tissue fragments and easily integrated with the biological system.56,57 But it is found in contact angle studies that wetability decreases with increased amount of silver on the surface. So, the amount of silver deposited on the surface of material needs to be optimized such that the material could have better wetability as well as good antibacterial behavior.
3.5 Antibacterial study
Fig. 6(a)–(c) shows the antibacterial study of Ti64 alloy and chemically treated samples against Staphylococcus aureus. Ti64 alloy and NaOH treated sample did not show any antibacterial activity as shown in Fig. 6(a). From Fig. 6(b) and (c) silver treated samples starting from 0.02–0.5 mM shows clear bacterial inhibition zone and the extent of zone formation was increased with increase in silver content. But, the admissible amount of silver ions into the human body is limited because excess silver ions might cause cytotoxicity.58–60 An attempt has been made to optimize the silver content in an implant material and to determine the minimal silver content, which would be sufficient to produce effective antibacterial effect. So, it could be observed that there was no inhibition zone in the case of samples treated in 0.01 mM Ag solution while a clear inhibition zone could be obtained for 0.02 mM and above AgNO3 treated specimens. The magnitude of the inhibition zone obtained has been depicted in Table 2.| Sample notations | Diameter of zone (including specimen diameter (1.5 cm)) (cm) | Area of inhibition zone (A = πd2/4) (cm2) |
|---|---|---|
| Ti64-N-0.02Ag | 1.60 | 2.0096 |
| Ti64-N-0.03Ag | 1.70 | 2.2687 |
| Ti64-N-0.04Ag | 1.80 | 2.5434 |
| Ti64-N-0.05Ag | 1.85 | 2.6867 |
| Ti64-N-0.1Ag | 1.90 | 2.8338 |
| Ti64-N-0.5Ag | 1.92 | 2.8938 |
3.6 Silver release study
Fig. 6(d) shows the concentration of silver ion released in SBF with respect to different immersion time. This study on the rate of release of silver ions, thus leading to anti-bacterial behavior was performed on Ti64 alloy treated with NaOH and 0.05 mM AgNO3 solution. Release rate of silver increases rapidly during the first 3 h after which the rate of release gets slower and becomes almost constant after 24 h.61,623.7 Cell culture study
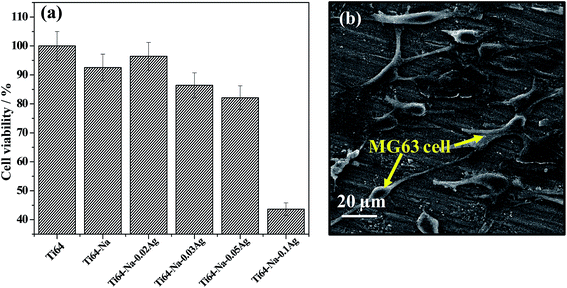
Thus prepared silver containing Ti64 samples were tested for its cytotoxicity using MTT assay. The cytocompatibility for various concentration of Ag ranging from 0.02 to 0.1 mM was evaluated and the results are presented in Fig. 7(a). The results suggested that above 90% cell viability for AgNO3 concentration of 0.02 mM, 86% cell viability for 0.03 mM and above 80% for 0.05 mM. However, it drastically decreased to 43% for 0.1 mM AgNO3 treated sample. The results indicated that the cell viability and biocompatibility was not affected at concentration ranging from 0.02 to 0.05 mM Ag indicating the concentration range would find application for biomedical interventions under diseased conditions.Fig. 7(b) shows MG63 cells adhered to Ti64-N-0.05Ag sample surface. Cells were uniformly adhered to the surface within 48 h of incubation and most of the cells showed random spreading. Cell attachment, spreading and further proliferation is directly related to surface characteristics of the material. The adherence of MG63 cells to the silver containing Ti64 surface indicates biocompatibility of the present surface and also expected to induce further proliferation of the cells.
3.8 In vitro bioactivity study in SBF
Fig. 8(a) show the SEM images of Ti64 alloy treated with NaOH and different concentration of AgNO3 and soaked in SBF for 7 days. Spherical globules were formed on all the NaOH and AgNO3 treated Ti64 alloy and there was no deposition formed on the untreated Ti64 alloy. Fig. 8(b) shows a typical XRD pattern of Ti64 alloy subjected to NaOH and AgNO3 treatment and soaked in SBF for 7 days. Ti64 alloy modified with NaOH and AgNO3 shows peak at 26 and 32° which confirms the formation of bone like apatite. The remaining peaks correspond to Ti in Ti64 alloy.63–65 This indicates that the chemical treatment enhances the apatite forming ability of Ti64 alloy in SBF.4. Discussions
Metals such as Ti and Ti alloys are used in orthopedic and dental devices for past several decades. However, strong bonding between implant and living bone is always a serious issue when implant needs to remain in the body for longer duration. In order to provide this strong bone-bonding ability in Ti and Ti alloys, either bioactive coating such as calcium phosphates65 or bioactive chemical treatment17 are developed. Ti and Ti alloys when subjected to NaOH treatment form a sodium titanate porous layer (Fig. 1) and this sodium titanate release Na+ ions into the SBF to form Ti–OH groups. This Ti–OH group accelerates the Ca2+ and PO43− ions deposition from SBF to form a bone-like apatite as evident in the SEM and XRD results (Fig. 8(a) and (b)). It has been reported that if a material form bone-like apatite on its surface in SBF it allows to deposit bone in the living body in the same manner and accelerate the bone integration. Both osteoconduction and osteoinduction abilities are well documented in the literature for various Ti and Ti–Zr–Nb–Ta alloys. However, Na+ ion release and its accumulation in the body may cause toxicity to the cells and cell death. In order to avoid such a situation, either water or dil. HCl treatment following NaOH treatment is reported by one of the author.52 However, NaOH or NaOH–H2O, dil. HCl treatments do not show any antibacterial activity and similar observations are also made in the present study (Fig. 6(a)–(c)).This indicates that thus manufactured orthopedic implant is susceptible to the bacterial infections during the course of surgery. This bacterial infection may lead to puss formation and ultimately the implant failure. In order to overcome this if an antibacterial agent can be impregnated into the porous network structure by chemical reaction; it can address the antibacterial behavior of the implant. Although, various organic compound coated over metallic implant to prevent bacterial infections are already reported, the present manufacturing process involves higher temperature that may degrade the activity of the organic compound.32–34 On the other hand, silver is a popularly known antibacterial agent and is being used in various applications.36,37
The mechanism of chemical reactions taking place on the surface of Ti64 alloy could be described as follows (considering the major composition of the alloy as Ti).
• NaOH treatment – Ti/TiO2 surface is attacked by hydroxyl ions to form sodium hydrogen titanate.7
• Ion exchange reaction – sodium ions in sodium hydrogen titanate is substituted by silver ions forming silver hydrogen titanate.
According to the EDX results (Fig. 2), the proportions of sodium and silver seem to be interrelated. The maximum amount of sodium observed is 1.91 at% which is found to decrease proportionally with the increase in silver concentration. Although the proportional amount of Na+ ion is replaced by silver, the amount of sodium present is always lower after ion exchange reaction because the sodium ions are easily washed away while rinsing with water. After a certain amount of incorporated silver, its content becomes saturated due to limited availability of sodium ions for exchange reaction.
The treated Ti64 alloy samples formed a layer of silver titanate on its surface as confirmed by EDX result (Fig. 2). These alloy samples show bactericidal through zone of inhibition tests in which the clear zones could be seen around the samples in the Staphylococcus aureus culture medium (Fig. 6(a)–(c)). Result showed that Ti64 alloy treated with a concentration above 0.02 mM AgNO3 has antibacterial effect.
In metallic form, silver is unreactive and cannot kill bacteria. To become bactericidal, silver must lose an electron and become positively charged silver ions (Ag+). Elemental silver ionizes more readily when exposed to an aqueous environment such as in body fluids in the present study. Thus, there is antibacterial effect only when there is a release of silver ions from the implants. The release of silver from the surface modified alloy sample is evident from the AAS studies as seen in Fig. 6(d). The released silver ions are highly reactive and affect multiple sites within bacterial cells, ultimately causing bacterial cell death. They bind to bacterial cell membranes, causing disruption of the bacterial cell wall and cell leakage. Silver ions incorporated into the cell disrupt cell function by binding to proteins and interfering with energy production, enzyme function and cell replication.59,62
The above mentioned activities lead to the following two major effects on the bacterial cell.59
• Due to the detrimental effect of the silver ions the DNA molecules become condensed and lose their abilities to replicate.
• Silver ions interact with the thiol groups in proteins, thus inducing their inactivity.27
Contact angle measurement is a study performed to analyze the degree of hydrophobicity or hydrophilicity of the material of desire. This contact angle study is performed to analyze the wetability of the surface modified alloy samples. This would give an idea about the wetting behavior of the material when applied as a biomedical implant. The contact angle for Ti64 alloy is high (80°) (Fig. 5(a) and (b)), which decreases gradually on treatment with NaOH solution (25°). Initially at lower atomic% of silver ions the wetness is found to be increased but with further increase of silver ions at the surface the material becomes hydrophobic (Fig. 5(b)) i.e. the wetness decreases.66 The present optimized silver concentration (Ti64-N-0.05Ag) is having a lower contact angle comparing to the Ti64 alloy and Ti64-N surface. Thus we were able to achieve a highly hydrophilic surface on Ti64 alloy by a simple chemical treatment with NaOH and AgNO3 solutions.
Further bioactivity study in SBF showed that Ti-64 alloy did not form bone like apatite. However, NaOH treated Ti64 alloy formed apatite and the apatite-forming ability did not decrease even Na+ ions are replaced by Ag+ ions (Fig. 8(a)). It is well known that Na+ ions release into the SBF to form negatively charged Ti–OH group that attracts the positively charged Ca2+ ions and then negatively charged PO43− ions to form the bone-like apatite layer. Similar mechanism can be expected in Ti64 alloy containing Ag+ ions. Interestingly, the bioactivity was not decreased even after heat treatment. It has been already reported that NaOH treatment forms porous SHT layer which has very low scratch resistance.52 However, on heat treatment around 600 °C, a TiO2 layer is formed below the titanate network structure that improves the bonding of the network through this TiO2 layer and increase the scratch resistance.67 This indicates heat treatment is highly essential to stabilize the porous network structure and surface of the implant subjected to such chemical and heat treatment will not be destroyed during the implantation.52,68
From the present study we observed that the release of Na+/Ag+ ions accelerates the apatite deposition on chemically treated Ti64 alloy. From the AAS result we can see that the amount of Ag+ ion released from 0.05 mM AgNO3 treated Ti64 surface, within a period of 24 h, is in the range of 4–5 ppm (Fig. 6(d)). The antibacterial activity of the same surface against Staphylococcus aureus is confirmed by the formation of inhibition zone (Fig. 6(c)). Although, Ag+ ion release shows antibacterial effect, excess Ag+ ion may cause toxicity to the cells.27,69–74 Therefore, it is essential to optimize the Ag+ ion on the Ti64 alloy surface. Present studies show that up to 0.05 mM AgNO3 samples has more than 80% cell viability. Above 0.05 mM, samples showed a decrease in cell viability in the culture medium (Fig. 7(a)), indicating that 0.05 mM AgNO3 is an optimum silver concentration which shows both antibacterial property as well as cytocompatibility. Thus bioactive Ti64 alloy coupled with antibacterial behavior and cell viability could be provided by such a simple chemical treatment approach and this method is expected to be useful in development of orthopedic and dental devices.
5. Conclusions
Silver ions could be successfully incorporated into the sodium titanate layer formed on Ti64 alloy by NaOH and subsequent AgNO3 treatment. The resultant silver titanate layer release Ag+ ions into the surrounding and showed the antibacterial activity against Staphylococcus aureus. Further, in vitro bioactivity of thus treated Ti64 alloy in simulated body fluid showed bone-like apatite formation within 7 days soaking and this apatite-forming ability is not affected by either silver content or by heat treatment. Cell viability study in MG63 cell line showed more than 80% cell viability indicating the non-toxic behavior of the samples. NaOH treatment forms hydrophilic surface and the hydrophilicity is not affected for the present optimum silver concentration. This surface modification study to provide bioactive Ti64 alloy coupled with antibacterial property is expected to have considerable potential in orthopedic and dental implants.Acknowledgements
DKP acknowledges Department of Science and Technology (DST), New Delhi, India (SERB; Fast track research grant no. SB/FTP/ETA-257/2012, GAP 11/13) and start up research grant OLP 0083 from CSIR-CECRI. AR acknowledges UGC for providing the fellowship to carry out the work. Support from Central Instrument Facility (CIF) staff Mr R. Ravishankar, Ms Nalini (SEM in charge) and Mr J. Kennedy (XPS in charge) is greatly appreciated. DKP acknowledges Prof. T. Matsushita, Chubu University, Japan for providing the samples.References
- D. Williams, Biomaterials, 2009, 30, 5897–5909 CrossRef CAS PubMed.
- D. M. Brunette, P. Tengvall, M. Textor and P. Thomsen, Titanium in medicine: material science, surface science, engineering, biological responses and medical applications, Springer, Germany, 2001 Search PubMed.
- T. Kokubo, Bioceramics and Their Clinical Applications, Wood head publishing, Sawston, UK, 2008, p. 784 Search PubMed.
- Metals for Biomedical Devices, ed. M. Niinomi, Wood head Publishing, UK, 2010 Search PubMed.
- K. Bordji, J. Y. Jouzeau, D. Mainard, E. Payan, P. Netter, K. T. Rie, T. Stucky and M. Hage-Ali, Biomaterials, 1996, 17, 929–940 CrossRef CAS.
- D. K. Pattanayak, A. Fukuda, T. Matsushita, M. Takemoto, S. Fujibayashi, K. Sasaki, N. Nishida, T. Nakamura and T. Kokubo, Acta Biomater., 2011, 7, 1398–1406 CrossRef CAS PubMed.
- X. Liu, P. K. Chu and C. Ding, Mater. Sci. Eng., R, 2004, 47, 49–121 CrossRef PubMed.
- P. J. Henry, Int. J. Oral Maxillofac. Implants, 1987, 2, 23–27 CAS.
- J. Lausmaa, B. Kasemo and H. Mattsson, Appl. Surf. Sci., 1990, 4, 133–146 CrossRef.
- D. S. Sutherland, P. D. Forshaw, G. C. Allen, I. T. Brown and K. R. Williams, Biomaterials, 1993, 14, 893–899 CrossRef CAS.
- J. P. Lucchini, J. L. Aurelle, M. Therin, K. Donath and W. Becker, Clin. Oral Implants Res., 1996, 7, 397–404 CAS.
- B. Hignett, T. C. Andrew, W. Downing, E. J. Duwell, J. Belanger and E. H. Tulinski, Surface cleaning, finishing and coating, Metals Handbook, ed. W. G. Wood, American Society for Metals, Metals Park, OH, 1987, vol. 5, pp.107–127 Search PubMed.
- D. Buser, T. Nydegger, T. Oxland, D. L. Cochran, R. K. Schenk, H. P. Hirt, D. Snetivy and L. P. Nolte, J. Biomed. Mater. Res., 1999, 45, 75–83 CrossRef CAS.
- M. Baleani, M. Viceconti and A. Toni, Artif. Organs, 2000, 24, 296–299 CrossRef CAS.
- I. Degasne, M. F. Basle, V. Demais, G. Hure, M. Lesourd, B. Grolleau, L. Mercier and D. Chappard, Calcif. Tissue Int., 1999, 64, 499–507 CrossRef CAS.
- H. B. Wen, J. G. Wolke, J. R. Wijn, Q. Liu, F. Z. Cui and K. De Groot, Biomaterials, 1997, 18, 1471–1478 CrossRef CAS.
- H. B. Wen, Q. Liu, J. R. Wijn and K. De Groot, J. Mater. Sci.: Mater. Med., 1998, 9, 121–128 CrossRef CAS.
- D. K. Pattanayak, S. Yamaguchi, T. Matsushita, T. Nakamura and T. Kokubo, J. R. Soc., Interface, 2012, 9, 2145–2155 CrossRef CAS PubMed.
- T. Kawai, M. Takemoto, S. Fujibayashi, H. Akiyama, M. Tanaka, S. Yamaguchi, D. K. Pattanayak, K. Doi, T. Matsushita, T. Nakamura, T. Kokubo and S. Matsuda, PLoS One, 2014, 9, e88366 Search PubMed.
- P. Tengvall and I. Lundstro, Clin. Mater., 1992, 9, 115–134 CrossRef CAS.
- D. K. Pattanayak, T. Matsushita, K. Doi, H. Takadama, T. Nakamura and T. Kokubo, Mater. Sci. Eng., C, 2009, 29, 1974–1978 CrossRef CAS PubMed.
- H. M. Kim, F. Miyaji, T. Kokubo and T. Nakamura, J. Biomed. Mater. Res., 1996, 32, 409–417 CrossRef CAS.
- L. Jonasova, F. A. Muller, A. Helebrant, J. Strnad and P. Greil, Biomaterials, 2002, 23, 3095–3101 CrossRef CAS.
- S. Nishiguchi, T. Nakamura, M. Kobayashi, H. M. Kim, F. Miyaji and T. Kokubo, Biomaterials, 1999, 20, 491–500 CrossRef CAS.
- C. Ohtsuki, H. Iida, S. Hayakawa and A. Osaka, J. Biomed. Mater. Res., 1997, 35, 39–47 CrossRef CAS.
- S. Kaneko, K. Tsuru, S. Hayakawa, S. Takemoto, C. Ohtsuki, T. Ozaki, H. Innoue and A. Osaka, Biomaterials, 2001, 22, 875–881 CrossRef CAS.
- A. Rajendran and D. K. Pattanayak, RSC Adv., 2014, 4, 61444–61455 RSC.
- X. Wang, S. Hayakawa, K. Tsuru and A. Osaka, J. Biomed. Mater. Res., 2001, 54, 172–178 CrossRef CAS.
- C. A. B. Nava-Ortiz, G. Burillo, A. Concheiro, E. Bucio, N. Matthijs, H. Nelis, T. Coenye and C. A. Lorenzo, Acta Biomater., 2010, 6, 1398–1404 CrossRef CAS PubMed.
- D. S. Jones, C. P. Lorimer, C. P. McCoy and S. P. Gorman, J. Biomed. Mater. Res., Part B, 2008, 85, 417–426 CrossRef PubMed.
- L. Zhao, P. K. Chu, Y. Zhang and Z. Wu, J. Biomed. Mater. Res., Part B, 2009, 91, 470–480 CrossRef PubMed.
- P. Edupungati Om, V. Antoci, S. B. King, B. Jose, C. S. Adams, J. Parvizi, I. M. Shapiro, A. R. Zeiger, N. J. Hickok and E. Wickstrom, Bioorg. Med. Chem. Lett., 2007, 17, 2692–2696 CrossRef PubMed.
- B. Jose, V. Antoci, A. R. Zeiger, E. Wickstrom and N. J. Hickok, Chem. Biol., 2005, 12, 1041–1048 CrossRef CAS PubMed.
- S. Radin, J. T. Campbell, P. Ducheyene and J. M. Cuckler, Biomaterials, 1997, 18, 777–782 CrossRef CAS.
- M. K. Narbat, J. Kindrachuk, D. Ke, H. Jenssen, R. E. W. Hancock and R. Wanga, Biomaterials, 2010, 31, 9519–9526 CrossRef PubMed.
- Z. Wang, Y. Sun, D. Z. Wang, H. Liu and R. I. Boughton, Int. J. Nanomed., 2013, 8, 2903–2916 Search PubMed.
- Y. Inoue, M. Uota, T. Torikai, T. Watari, I. Noda, T. Hotokebuchi and M. Yada, J. Biomed. Mater. Res., Part A, 2010, 92, 1171–1180 Search PubMed.
- K. Tamai, K. Kawate, I. Kawahara, Y. Takakura and K. Sakaki, J. Orthop. Sci., 2005, 14, 204–209 CrossRef PubMed.
- A. Rajendran, G. Vinoth, V. Shanthi, R. C. Barik and D. K. Pattanayak, Mater. Technol., 2014, 29, B26–B34 CrossRef CAS PubMed.
- J. S. Patel, S. V. Patel, N. P. Talpada and H. A. Patel, Angew. Makromol. Chem., 1999, 271, 24–27 CrossRef CAS.
- P. V. Asharani, M. G. L. Kah, M. P. Hande and S. Valiyaveettil, ACS Nano, 2009, 3, 279–290 CrossRef CAS PubMed.
- Y. Mitsunori, I. Yuko, N. Iwao, M. Tomohiro, T. Toshio, W. Takanori and H. Takao, J. Nanomater., 2013, 1–9 Search PubMed.
- N. Ren, R. Li, L. Chen, G. Wang, D. Liu, Y. Wang, L. Zheng, W. Tang, X. Yu, H. Jiang, H. Liu and N. Wu, J. Mater. Chem., 2012, 22, 19151–19160 RSC.
- W. Zheng, S. Yan, W. Dongzhou, L. Hong and B. I. Robert, Int. J. Nanomed., 2013, 8, 2903–2916 Search PubMed.
- S. Ferraris, A. Venturello, M. Miola, A. Cochis, L. Rimondini and S. Spriano, Appl. Surf. Sci., 2014, 311, 279–291 CrossRef CAS PubMed.
- D. Gerlier and N. Thomasset, J. Immunol. Methods, 1986, 94, 57–63 CrossRef CAS.
- W. Chrzanowski, N. E. A. Abou, D. A. Armitage, X. Zhao, J. C. Knowles and V. J. Salih, J. Biomed. Mater. Res., Part A, 2010, 93, 1596–1608 Search PubMed.
- T. Kokubo and H. Takadama, Biomaterials, 2006, 27, 2907–2915 CrossRef CAS PubMed.
- S. Yamaguchi, T. Matsushita and T. Kokubo, RSC Adv., 2013, 3, 11274–11282 RSC.
- J. W. Park, Y. J. Kim, J. H. Jang and H. Song, Clin. Oral Implants Res., 2010, 21, 1278–1287 CrossRef PubMed.
- T. Kizuki, H. Takadama, T. Matsushita, T. Nakamura and T. Kokubo, J. Mater. Sci.: Mater. Med., 2013, 24, 635–644 CrossRef CAS PubMed.
- D. K. Pattanayak, T. Kawai, T. Matsushita, H. Takadama, T. Nakamura and T. Kokubo, J. Mater. Sci.: Mater. Med., 2009, 20, 2401–2411 CrossRef CAS PubMed.
- G. V. Rodriguez, A. S. Obregon, S. L. M. Lozano and S. W. Lee, J. Mol. Catal. A: Chem., 2012, 353, 163–170 CrossRef PubMed.
- R. Ma, K. Fukuda, T. Sakaki, M. Osada and Y. Bando, J. Phys. Chem. B, 2005, 109, 6210–6214 CrossRef CAS PubMed.
- T. Kasuga, M. Hiramatsu, A. Hoson, T. Sekino and K. Niihara, Adv. Mater., 1999, 11, 1307–1311 CrossRef CAS.
- Q. Wang, Y. Zhang, K. Yang and L. Tan, Surf. Rev. Lett., 2009, 16, 775–779 CrossRef CAS.
- Y. J. Lim, Y. Oshida, C. J. Andres and M. T. Barco, Int. J. Oral Maxillofac. Implants, 2001, 16, 333–342 CAS.
- A. B. G. Lansdown, J. Wound Care, 2002, 11, 125–130 CrossRef CAS PubMed.
- Q. L. Feng, J. Wu, G. Q. Chen, F. Z. Cui, T. N. Kim and J. O. Kim, J. Biomed. Mater. Res., 2000, 52, 662–668 CrossRef CAS.
- M. H. Hermans, Adv. Skin Wound Care, 2007, 20, 166–173 CrossRef PubMed.
- X. Wu, L. Jidong, L. Wang, D. Huang, Y. Zuo and L. Yubao, Biomed. Mater., 2010, 5, 44–105 Search PubMed.
- M. Yoshinari, Y. Oda, T. Kato and K. Okuda, Biomaterials, 2001, 22, 2043–2048 CrossRef CAS.
- S. L. R. da Silva, L. O. Kerber, L. Amaral and C. A. Dos Santos, Surf. Coat. Technol., 1999, 116–119, 342–346 CrossRef CAS.
- G. B. De Souze, C. M. Leipienski, C. E. Foerster, N. K. Kuromoto, P. Soares and H. D. A. Ponte, J. Mech. Behav. Biomed. Mater., 2011, 4, 756–765 CrossRef PubMed.
- S. R. Paital and N. B. Dahotre, J. Mater. Sci. Eng. R, 2009, 66, 1–70 CrossRef PubMed.
- S. Kasraei and M. Azarsina, Braz. Oral. Res., 2012, 26, 505–510 CrossRef PubMed.
- S. Nishiguchi, T. Nakamura, M. Kobayashi, H. M. Kim, F. Miyaji and T. Kokubo, Biomaterials, 1999, 20, 491–500 CrossRef CAS.
- D. K. Pattanayak, S. Yamaguchi, T. Matsushita and T. Kokubo, J. Mater. Sci.: Mater. Med., 2011, 22, 1803–1812 CrossRef CAS PubMed.
- C. N. Kraft, M. Hansis, S. Arens, M. D. Menger and B. Vollmar, J. Biomed. Mater. Res., 2009, 49, 192–199 CrossRef.
- G. Gosheger, J. Hardes, H. Ahrens, A. Streitburger, H. Buerger, M. Erren, A. Gunsel, F. H. Kemper, W. Winkelmann and C. V. Eiff, Biomaterials, 2004, 25, 5547–5556 CrossRef CAS PubMed.
- H. Cao, X. Liu, F. Meng and P. K. Chu, Biomaterials, 2011, 32, 693–705 CrossRef CAS PubMed.
- Y. Chen, X. Zheng, Y. Xie, C. Ding, H. Ruan and C. Fan, J. Mater. Sci.: Mater. Med., 2008, 19, 3603–3609 CrossRef CAS PubMed.
- J. Liau, M. Anchun, Z. Zhu and Y. Quan, Int. J. Nanomed., 2010, 5, 337–342 Search PubMed.
- T. Kizuki, T. Matsushita and T. Kokubo, J. Mater. Sci.: Mater. Med., 2014, 25, 1737–1746 CrossRef CAS PubMed.
Footnote |
| † Both the authors have equal contribution to the paper. |
| This journal is © The Royal Society of Chemistry 2015 |