Prospects for multitarget lipid-raft-coated silica beads: a remarkable online biomaterial for discovering multitarget antitumor lead compounds
Caleb Kesse Firempong
a,
Xia Cao
a,
Shanshan Tong
a,
Jiangnan Yu
ab and
Ximing Xu
*a
aDepartment of Pharmaceutics, School of Pharmacy, Centre for Nano Drug/Gene Delivery and Tissue Engineering, Jiangsu University, 301 Xuefu Rd, Zhenjiang, P. R. China. E-mail: xmxu@ujs.edu.cn; calebuse@yahoo.com; 841661898@qq.com; 877274194@qq.com; yjn@ujs.edu.cn; Fax: +86-511-8503845; Tel: +86-511-8503845
bSchool of Pharmacy, China Pharmaceutical University, Nanjing, P. R. China
First published on 28th May 2015
Abstract
A recently discovered receptor-rich lipid raft, which is linked to numerous transmembrane signal transduction pathways, has emerged as a medically significant biomaterial for screening antitumor agents. Compounds that interact with the biomaterial might potentially inhibit cancer cell growth, and thus be valuable as antitumor agents. Two standard anticancer drugs, lestaurtinib and gefitinib, interacted with the tropomyosin-related tyrosine kinase A (TrkA) receptor-rich lipid raft. The fact that they are both well known inhibitors of TrkA receptor strengthened the observed linkage. There is now a considerable interest in developing other related biomaterials to serve a similar purpose, and more importantly, support the concept of multitarget drug discovery. It is expected that new anticancer drugs strategically act on multiple pathways to achieve optimal therapeutic efficacies and decreased stimulation of acquired resistance. However, the current conventional approaches such as chemical screening systems and in silico methods cannot fully satisfy the increasing demand for the therapeutic agents. It has therefore become imperative to explore other alternatives to increase the number of clinically important antitumor agents. Here, we report the prospect of establishing lipid raft biomaterial with well endowed multiple cancer-related receptors for screening antitumor leads that affect multiple pathways. This review also examines receptor–ligand interactions in the hunt for novel antitumor agents and the associated key receptors.
1. Introduction
Growing evidence that several effective drugs produce their actions via interactions with multiple targets is boosting the development of research areas that can challenge the reductionist approach.1 Consistent with this, the concepts of drug repurposing,2 polypharmacology,3 chemogenomics,4 phenotypic screening and high-throughput in vivo testing of mixture-based libraries5 in an integrated manner have provided meaningful alternatives to the current paradigm of drug discovery. Thus, a shift from a single target to the more preferred multiple target approach. Although these platforms have provided clinically important multitarget lead compounds, their significant outputs are woefully inadequate in meeting the ever increasing demand for therapeutic drugs. As a result, there is an urgent need to investigate other promising approaches that could accelerate progress in this domain. To this end, establishing a biological model to effectively screen multitarget agents is a high priority for a successful anticancer drug discovery strategy.Previous report on monotarget lipid raft biomaterial strongly signalled the possibility of developing a multitarget model for identifying antitumor components which can act on multiple pathways. The biological interactions between receptor-rich biomaterial and the ligands, associated thermodynamics and effective applications of the model was extensively investigated.6 The presence of overexpressed TrkA receptors on lipid raft silica beads (LRSB) was confirmed via immunofluorescence bioassay. The receptor specificity was also validated by TrkA target (lestaurtinib and gefitinib) and non-TrkA target (gemcitabine) standard anticancer drugs (Fig. 1). The chromatograms of target drugs exhibited longer retention time as compared to non-target drugs. Likewise, bioactive LRSB isolate (ether fraction) exhibited similar trend as the target drugs, and significantly inhibited cancer cell growth. However, these active components failed to exert cytotoxic activities in the presence of TrkA inhibitors (K252a and primary antibody). These findings support the interactions between effective components and sufficiently expressed TrkA receptor in the lipid raft.
In spite of comprehensive studies on well-known cancer target receptors such as epidermal growth factor receptor (EGFR), vascular epidermal growth factor receptor (VEGFR) and Fas receptor (FasR), there is still very limited reporting regarding their bioscreening activities for potential antitumor agents.6,7 So far the best known studies have been limited to cell membrane chromatography (CMC) with highly expressed receptors like EGFR and VEGFR. The medical benefits of employing these receptors for affinity screening cannot be overemphasized in the quest for effective chemotherapeutic drugs. Fortunately, the first TrkA receptor-rich lipid raft from U251 glioma cancer cells has set the stage for obtaining other lipid rafts with multiple receptors from related cancer cells. Several bioscreening systems including immobilized DNA chromatography,8 immobilized plasma protein chromatography,9 immobilized liposome chromatography10 and immobilized biomembrane chromatography11 have been developed to detect interactions between a chemical and its target protein.12 The yet to be established multitarget lipid raft belongs to this class of screening systems with the advantage of having enriched multiple receptors. The multitarget biomaterial can provide different sites for bioactive compounds, and more importantly, isolate compounds capable of acting on multiple pathways.
The desired multitarget lipid raft can overcome most of the problems associated with multitarget drug discovery, particularly longer processing time, high cost implications, low “hit” rates, and most significantly, the lack of correlation between in vitro and in vivo data. Technically, the feasibility of the proposed lipid raft biomaterial has been verified via its monotarget analogue, and it can be seen as an extension of the existing model.6 Why does a multitarget lipid raft present such unique opportunities? Perhaps, it can offer a more realistic biological system with enriched diverse cancer-related receptors for bioactive compounds to locate and dock with. More importantly, it can isolate lead compounds from complex mixtures, especially natural products that could affect multiple pathways. The system might also provide experimentally verified data and enhance the search for multitarget agents. With the development of multitarget LRSB, the effective online pharmacological studies and rapid screens for multitarget agents could be easily accessible. However, it must be noted that present reports describe the exploratory stages, and there is a need for more comprehensive studies to demonstrate the clinical value of this novel technology.
2. Prominent transmembrane activities of lipid rafts
Certain cues for cell growth, survival and other physiological processes are transmitted through specialized sub-domains in plasma membrane known as lipid rafts.13 The rafts contain a variety of proteins, e.g., caveolins, flotillins, GPI-linked proteins, G proteins, Src family kinases, EGF receptors, PDGF receptors, endothelin receptors, phosphotyrosine phosphatase, MAPK, protein kinase C and PI 3-kinase.14 They also have the potential to regulate signal transduction by activating or suppressing phosphorylation cascades.15 For instance, they can function as negative regulators of EGFR tyrosine phosphorylation,16 thus, upon ligand binding and receptor activation, the EGFR migrates out of the rafts.17 The membrane rafts might serve as a novel target in cancer therapy due to the ability to organize receptors and their downstream molecules. It has been reported that several growth factor signalling pathways depend on lipid raft. This is evident in the role of lipid rafts in the regulation of differentiation, apoptosis and cell migration, which is linked to invasiveness and metastasis.18 The study also produced certain synthetic and naturally occurring substances that are known to affect the lateral membrane organization in tumour cell growth which could provide potential or actual therapeutics. Even though most drugs bind to proteins and regulate their activities, some drugs act via a new therapeutic approach called membrane-lipid therapy (binding to lipids).19 Mollinedo and Gajate identified Fas/CD95 death receptor and lipid rafts as new targets for apoptosis-directed cancer therapy.20 Similarly, the cannabinoid CB1 receptor antagonist rimonabant (SR141716) inhibited human breast cancer cell proliferation via a lipid raft-mediated mechanism.21Many “homing devices”, including folate,22 transferrin23 and peptides,24 have been installed on liposomes or nanoparticles as a means of achieving effective receptor-target tumour therapy.25 Similarly, lipid rafts have been conjugated to certain drugs to impact lipophilicity, and simultaneously, a target moiety that can be recognized by a specific transporter/receptor in cell membrane was also tethered to the rafts. The findings indicated that both the lipid rafts and target moiety acted synergistically toward cellular uptake.26 Nanogel surfaces decorated with alginate or mannosylated alginate were used to target dendritic cell receptors. The uptake of nanogel was dependent on endosomal-based processes. However, inhibition of lipid raft activities impaired the uptake which suggested the involvement of more than one entry routes.27 In a related study, a drug delivery mechanism for target nanoparticles that utilizes direct cytoplasmic delivery into a target cell while avoiding endosomal incorporation was observed in lipophilic delivery. This phenomenon entails the direct delivery of lipophilic substances to cell membrane via lipid mixing, and subsequent intracellular trafficking via lipid raft dependent processes.28
Effective delivery of therapeutic genes to designated target cells and their action at the intracellular site are crucial requirements for successful gene therapy.29 Studies have suggested that lipid rafts of microvascular dermal endothelial cells are essential to Kaposi'sarcoma-associated herpes virus (KSHV) infection and gene expression. This could be due to their potential roles in the modulation of KSHV-induced PI3-K, RhoA-GTPase and Dia-2 signal molecules in post-binding and entry stages of infection.30 The data indicate a possible role for lipid rafts in gene delivery. DNA nanoparticles (DNPs) are non-viral gene vectors with excellent in vivo potential. Disruption of lipid rafts by depletion of membrane cholesterol significantly inhibits DNP transfection, while the inhibition of other endocytic pathways has little effect. These events reveal the active involvement of lipid rafts in transfection.31 Other reports have also shown that dendritic cell utilizes multiple endocytic routes for synthetic virus-like particle uptake which is dominated by lipid raft-mediated macropinocytosis.32 Currently, there is limited information on the application of receptor-rich lipid raft biomaterial for screening antitumor agents. However, recent developments have ignited a special interest in this biomaterial, as evidenced in reports of lipid raft bioscreening assays for antitumor components in complex natural mixtures.6
3. Screening of antitumor lead compounds
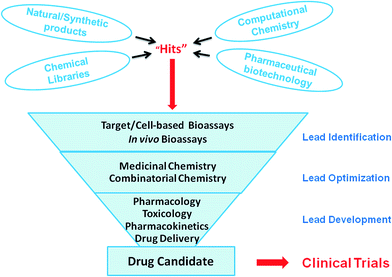
New antitumor agents of pharmaceutical importance, described as “hits”, could be discovered from different sources such as natural/synthetic products, computational chemistry, chemical libraries and pharmaceutical biotechnology (Fig. 2). The “hits” are pharmacologically, pharmacodynamically and pharmacokinetically enhanced via chemical or functional modification to obtain a lead compound. The leads usually have known structures with well documented mechanism of actions. They could serve as drug candidates that are safe for human clinical trials after optimization and developmental studies (Fig. 2).33 Generally, the initial routine bioscreening of any “hits” irrespective of their origin is based on cell/target assays using established cell lines to measure the cytotoxic activities (Fig. 2). Some well-established methods commonly employed in these assays include colony formation methods, crystal violet methods, tritium-labelled thymidine uptake methods, and MTT[3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] and WST-8[2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium] bioassays. Toxicity studies of active agents on normal cells are investigated using canine kidney (MDCK), mouse fibroblasts (L929), rhesus monkey kidney from Macaca mulatta cells (LLC-MK2) and human peripheral blood mononuclear cells.34 The agents or compounds that are found to be active and non-toxic in the in vitro models are tested for in vivo antitumor efficacy. In vivo models, in which tumours are induced, using NOD-SCID, Balb C57BL/6 and athymic mice, are widely employed for this kind of investigations.35 In fact, the entire process of screening for antitumor agents is characterized by low output, undue delays and high financial costs. This prevailing condition has accounted for several attempts to solve the problem.4. Lipid rafts with highly expressed cancer-related receptors
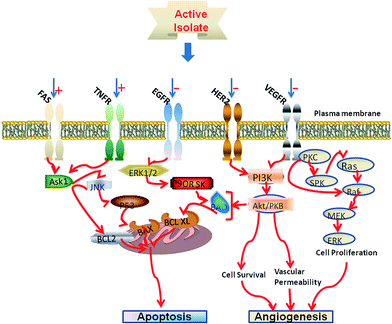
Several studies have reported overexpressed receptors on some cancer cells which are clearly absent in normal cells.36 The situation can be clinically explored to discover more antitumor agents with high therapeutic efficacies. As microenvironments in the exoplasmic leaflet of cell membrane, lipid rafts might contain cancer-related receptors. Despite their reduced receptor numbers as compared to cell membrane, the rafts still demonstrate high specificity and increased receptor densities, which could lead to increased output of bioactive compounds.6 Previous monotarget lipid raft, with abundant TrkA receptors, and the desired multitarget LRSB models, with prominent therapeutic receptors like EGFR, VEGFR and FasR can support the molecular basis of this clinically useful receptor–ligand screening bioassay.37 In Fig. 3, the bioactive isolate from lipid raft (containing multiple receptors – FasR, TNFR, VEGFR, EGFR and HER2) can interact with any of the associated receptors in its antitumor applications. A follow up study could easily ascertain the specific receptors involved in the receptor–ligand interactions. Most of these receptors are sufficiently expressed in predominant cancer cell lines (prostrate, breast and colon), and therefore, present a unique opportunity that can be therapeutically explored to provide more treatment options for patients.TrkA receptors which can be activated to engage molecules for signal transduction are highly embedded in lipid rafts.38 Therefore, TrkA-rich lipid raft could act as a linchpin from which a potent death signal could be launched to become a new promising anticancer target.20,39 The identification of EGFR as an oncogene has led to the development of anticancer therapeutics which is directed against EGFR (called ‘EGFR inhibitors’). Similarly, the overactivation of HER2 oncogene has been linked to the progression of certain aggressive cancers. Recently, this protein has become an important biomarker and therapeutic target for approximately 30% breast cancer patients.40 The dysregulation of TNFR pathways results in a wide range of pathological conditions, including cancer, autoimmune disease, inflammation and viral infection.41 This explains the importance of these proteins as potential drug targets.41c,42 The membrane-anchored Fas ligand on adjacent cell causes the oligomerization of Fas receptor molecules in death-inducing signalling complex (DISC).43 This event could be mimicked by the binding of agonistic lead compounds which might be unravelled by a Fas-based LRSB screening system. Additionally, the vascular endothelial growth factor (VEGF), one of the important elements in regulating angiogenesis, is now a key focus in antiangiogenic therapy.44 Thus, the VEGFR and its respective ligands, particularly VEGF, could play prominent roles in various cancer cells where they promote the growth of tumour blood vessels nurturing malignant cells.45
5. Receptors as tools for rapid isolation of antitumor agents
Antitumor drug discovery has become one of the most challenging and researched fields in cancer therapy due to the complex mechanism of tumour initiation and growth, as well as the difficulty in treating them.46 Numerous screening techniques, based on target receptors or enzymes, and virtual screening system, using computer-aided drug design, have been employed for investigating lead compounds or drugs.47 A target enzyme (DNA topoisomerase II) was employed to isolate antitumor agents from a mixture of compounds, and concurrently, the agents were analysed by high performance liquid chromatography/electrospray ionization-mass spectrometry (HPLC/ESI-MS).48 Two combined receptors (nicotinic acetylcholine and oestrogen receptors) were used in liquid chromatography for online pharmacological studies, particularly in the identification of lead candidates from complex biological or chemical mixtures.49 Similarly, other efficient screening methods like immobilized β2-adrenoceptor (β2-AR), immobilized human vitamin D3 receptor (hVDR) and TrkA receptor-rich lipid raft have been used for such purposes.6,50 Some target-based screening models for antitumor agents are presented in Table 1. Generally, these models are used to separate active compounds from complex natural products.| Molecular target | Screening model | Active compounds | Mixtures | Reference |
|---|---|---|---|---|
| a Abbreviations: A431, epidermoid carcinoma cell line; 17-AAG, 17-allylaminogeldanamycin; CMC, cell membrane chromatography; DNA, deoxyribonucleic acid; EGFR, epidermal growth factor receptor; HEK293, human embryonic kidney 293 cells; Hsp90, heat shock protein 90; Melan A, melanoma antigen; MU89, melanoma cell line; NA, non applicable; OBAA, 3-(4-octadecyl)benzoylacrylic acid; PMA, phorbol-12-myristate-13 acetate; TrkA, tropomyosin-related tyrosine kinase A; VEGFR-2, vascular endothelial growth factor receptor-2. | ||||
| β2-Adrenoceptor | Purified receptor | Amygdalin | Semen armeniacae amarum | 50a |
| DNA topoisomerase | Purified enzyme | Doxorubicin, daunorubicin | Doxorubicin, daunorubicin, pravastain | 48 |
| EGFR | HEK293/CMC | Asarinin | Radix et rhizoma asari | 56 |
| EGFR | HEK293/CMC | Vauquline, strychnine | Semen strychni | 7 |
| EGFR | HEK293/CMC | Resveratrol | Rhizoma polygoni cuspidati | 57 |
| EGFR | A431/CMC | Taspine, caulophine | Radix caulophylli | 58 |
| EGFR | A431/CMC | Oxymatrine, matrine | Radix sophorae flavescentis | 59 |
| Hsp90 | Progesterone receptor chaperone complex | Capsaicin | Chemical library | 60 |
| Melan A | MU89 | OBAA, flunarizine, 17-AAG, aphidicolin, damnacanthal, PMA, dantrolene, glyburide | Chemical library | 61 |
| TrkA receptor | Lipid raft | NA | Albizziae cortex, Galla chinensis | 6a and 62 |
| VEGFR-2 | HEK293/CMC | Mesaconitine, aconitine, hypaconitine | Aconitum carmichaeli debx | 54 |
Currently, most studies focus on receptor-rich membranes to provide a platform for direct screening of active compounds.51 These membranes usually have receptors, like EGFR and VEGR, which work in a form of bionic affinity chromatography. It is normally coupled to HPLC/MS to study receptor–ligand interactions. EGFR is closely associated with tumour cell proliferation, angiogenesis, tumour invasion, metastasis and inhibition of apoptosis.52 It is therefore a strategic target for screening tumour inhibitors. As a key receptor in the progression of tumour, VEGFR-2 has been an important target for antitumor therapeutic research.53 Some small molecules, such as sunitinib and sorafinib, acting against neovascularisation by blocking the signal transduction pathway of VEGFR-2 have shown potent clinical effects.54 In order to improve receptor–ligand interaction studies and existing drug screening strategies aimed at specific receptors, some biological affinity systems with better specificity have to be designed. Currently, artificial cell cultures overexpressing VEGFR-2 on cell surface, named HEK293/VEGFR cell lines, have been constructed in HEK293 engineered cell line.55 The established HEK293/VEGFR-based CMC was considered as a model that could underline the performance of specific interactions between VEGFR-2 and its ligands.
6. Receptor-rich lipid rafts as an online biomaterial for screening antitumor agents
The online challenges in screening antitumor agents have always been: (1) designing limited bioscreening systems, (2) difficulty in reducing costs, and (3) lack of reconciling in vitro and in vivo data.63 Over the years, CMC models have been able to address some of the problems. The techniques have resulted in successful isolation of numerous bioactive components from complex samples that interact with membrane receptors. The dynamic simulation of the action of drug in vivo by the CMC presents a direct screening technique for active compounds.37a,59,64,65 But the CMC is plagued with several issues regarding its selectivity, specificity, stability and service life span. These drawbacks are due to the use of homogenized cell membrane which contains receptors at relatively low densities and existing as different varieties.37a,64 The situation has impeded further development of the CMC, and therefore, other alternatives are urgently required.For the first time, a novel TrkA receptor-rich lipid raft for identifying antitumor agents has been established (Fig. 1). As a promising technology with inherent high selectivity and specificity, the monotarget lipid raft offers an efficient approach for online isolation and analysis of active compounds. Previous reports have revealed that the biomaterial was stable and able to withstand extreme conditions.6b Fluorescent images of the biomaterial showed no obvious changes in intensity before and after packing the column. Most importantly, no fluorescence attenuation was observed in the lipid raft even after 30 days of continuous use. Further treatment with different mobile phases showed no effect on fluorescent intensity. In short, the long-lasting stability was a great improvement on the cell membrane-immobilized stationary phase (only 3 days or just 1 week under continuous usage).66 Significantly, the lipid raft biomaterial provided unique retention behaviours for easy identification of active agents (Fig. 1).
Currently, there is only one type of receptor-rich lipid raft biomaterial, and therefore, this concept can be extended to other related cells to develop more antitumor screening tools. It is possible to discover other lipid rafts with abundant multiple receptors to provide different targets for screening multitarget agents. Studies on the monotarget model have now paved way for scientists to explore the possibility of designing a multitarget analogue of this biomaterial. Technically, both the mono- and multi-target models are likely to operate on the same principle with the latter having the benefit of providing multiple interaction sites.
7. Prospect for multiple receptor-based lipid raft biomaterial in cancer therapy
Multitarget agents have been increasingly explored67 for enhanced therapeutic efficacies, improved safety profiles, and reduced resistance,68 while limiting unwanted cross-reactivities via optimization of target selectivity.69 Some clinically successful multitarget anticancer drugs include the anticancer kinase inhibitors sunitinib against PDGFR and VEGFR, dasatinib against Abl and Src, and lapatinib against EGFR and HER2.70 However, these drugs are administered to few patients and the need to develop additional drugs could be a life-saving. For years, studies aiming at multitarget antitumor drug discovery have not been very successful, and the situation continues to greatly limit the number of validated leads. The desired multitarget biomaterial could significantly increase the true hit rate which will give rise to large number of therapeutic multitarget agents. Table 2 reveals some reported antitumor agents that act on multiple receptors with related cancer target diseases. The number of such agents could be remarkably increased if the current drug discovery protocols are improved and additional ones provided. The multitarget biomaterial will not only provide bioscreening, but also serve as a tool for understanding molecular-level actions associated with cancers. Comparatively, the specificity, stability and life span of the lipid raft will stand out among numerous antitumor bioscreening systems.6b,66 Hence, employing the biomaterial as routine mass screening system for potential antitumor agents will increase the number of leads for further development.| Agent | Targeted disease | Multitarget action | Reference |
|---|---|---|---|
| a Abbreviations: AHR, aryl hydrocarbon receptor; AR, androgen receptor; CSF1R, colony stimulating factor 1 receptor; CXCR-4, C-X-C chemokine receptor type 4; DR-4/5, death receptor-4/5; EGFR, epidermal growth factor receptor; EPCR, endothelial protein C receptor; ER-α, oestrogen receptor-alpha; FasR, Fas receptor; FGFR-1, fibroblast growth factor receptor 1; HER-2, human epidermal growth factor receptor 2; H2R, histamine (2) receptor; HUVEC, human umbilical vein endothelial cells IL-8-R, interleukin-8-receptor; IR, integrin receptor; KIT, KIT receptor; LDL-R, low density lipoprotein–receptor; RET, RET receptor; VEGFR-1/2, vascular endothelial growth factor receptor-1/2. | |||
| ABT-869 | Solid malignancies, acute myeloid leukemia | VEGFR-2, CSF1R | 37c and 71 |
| AMG-706 | Thyroid cancers, non-small-cell lung cancer | VEGFR-2, KIT | 37c and 72 |
| Berberine | Breast cancer, prostate cancer, cervical cancer | HER-2, AR, FasR | 73 |
| Celasterol | Prostate cancer, non-small-cell lung cancer, breast cancer, human glioma | AR, FasR, DR-4/5, VEGFR-2 | 74 |
| Curcumin | Colon cancer, pancreatic cancer | ER-α, FasR, IR, EPCR, H2R, HER-2, EGFR, DR-5, IL-8-R, CXCR-4, LDLR, AHR, ITR, AR | 75 |
| Indirubin/indirubin-3′-monoxime | Prostate cancer, myeloid leukaemia | VEGFR-2, FGFR-1 | 76 |
| Lapatinib | Breast cancer | EGFR, ERBB-2 | 37c and 77 |
| Norcantharidin | Colorectal carcinoma, colon cancer, hepatoma cells | FasR, VEGFR-2, DR-5 | 78 |
| Quercetin | Pancreatic cancer, breast cancer | EGFR, ER-α | 79 |
| Resveratrol | Prostate cancer, breast cancer | AR, ER-α | 80 |
| Sunitinib | Gastrointestinal stromal tumor, renal cell carcinoma | VEGFR-2, KIT | 37c and 81 |
| Wogonin | Breast cancer, HUVEC | EGFR, ER-α, VEGFR-1 | 82 |
| ZD-6474 | Non-small-cell lung cancer, small-cell lung cancer, myeloma | EGFR, VEGFR-2, RET | 37c and 83 |
Establishing a multitarget biomaterial at this crucial moment could be very timely. Fig. 1 shows the successful development of monotarget lipid raft for screening antitumor agents. The as yet undeveloped multitarget analogue can be seen as an improved version of the previous system. The compatibility of the proposed biomaterial to existing structures will be unquestionable as witnessed in the monotarget model. The biomaterial can also be easily constructed from a range of prominent receptor-rich cancer cell lines. Structure–activity relationship (SAR) studies can be facilitated since the system can support large scale bioscreening investigations. Preliminary information on lead compounds for different target receptors and even possible mechanistic actions prior to employing confirmatory assays could be ascertained. Most significantly, the multitarget LRSB can provide multiple sites for lead antitumor compounds which might affect multiple pathways. Now comprehensive studies in the application of lipid raft as screening biomaterial are in the exploratory stages, and additional work needs to be done to help generate such systems that will eventually enhance the number of multitarget agents employed in combating cancers.
8. Challenges and future considerations in lipid raft screening applications
Generally, the lipid raft screening system can suffer from problems relating to the search for multitarget antitumor agents. But the retrieval rate of the lead compounds could be highly enhanced with shortened processes, and even very strong correlation between in vitro and in vivo data. The capacity to effectively develop and identify sufficiently expressed receptors in cancer cells can be problematic. The use of appropriate technology to rapidly culture target cells within a reasonable time could also be a daunting task with some cost implications. Optimization of lipid raft extraction and technical modification of the biomaterial to improve the life span cannot be ignored. To further build therapeutically useful models, we need to have an in-depth molecular knowledge about the target receptors and also consider the role of co-receptors in modulating these activities. Establishing a new lipid raft model capable of screening multitarget antitumor agents will be characterized by high failures, but it is worth the effort.More studies should be directed at developing receptor-rich multitarget chemical screening systems. In that regard, understanding the mechanistic actions of these cancer-related receptors will not only become academically prudent, but also could lead to the development of highly specific drug inhibitors for clinical applications. It may also be necessary to modify existing chemical screening approaches from single to multiple target-oriented standard operating systems. As a necessity, knowledge on the whole receptor-based screening system including ligands, stationary and mobile phases, and other accessory components requires a scientific upgrade. The efficient activities of the proposed multitarget model could also be realised by incorporating existing multitarget drug information on the current and future discovery. Now the scientific community has at its disposal a monotarget analogue of lipid raft biomaterial which is still in its infancy. In the opinion of these authors, there is a high probability of developing a multitarget analogue of the biomaterial for effective isolation of multitarget antitumor agents. The monotarget model was constructed from U251 cells; however, other cancer cells with sufficiently expressed receptors can also be employed. The final outcome could be clinically very important.
9. Conclusion
TrkA receptor-rich lipid raft biomaterial has been successfully employed to screen antitumor compounds which dramatically inhibited the growth of cancer cells. Comprehensive studies on this monotarget biomaterial in relation to its specificity, sensitivity, stability, life span and thermodynamic properties have been promising. The biomaterial has been easily adaptable to existing HPLC protocols for sample analysis. Most importantly, this monotarget model has paved way for the establishment of multitarget analogue which will operate on the same principle. This report therefore presents a promising would-be multitarget biomaterial for fast screening of antitumor lead components with the potential to interact with multiple targets.10. Conflict of interest
No potential conflicts of interest.Acknowledgements
We appreciate the support by National Natural Science Foundation of China (30973677, 81373371), Doctoral Fund of Ministry of Education of China (20113227110012) and Special Funds (JHB2012-37, GY2012049, GY2013055, GY2011028) in Zhenjiang, Jiangsu Province for all projects related to lipid raft biomaterial.References
- J. L. Medina-Franco, M. A. Giulianotti, G. S. Welmaker and R. A. Houghten, Shifting from the single to the multitarget paradigm in drug discovery, Drug Discovery Today, 2013, 18(9), 495–501 CrossRef PubMed.
- J. Aubé, Drug repurposing and the medicinal chemist, ACS Med. Chem. Lett., 2012, 3(6), 442–444 CrossRef PubMed.
- G. V. Paolini, R. H. Shapland, W. P. van Hoorn, J. S. Mason and A. L. Hopkins, Global mapping of pharmacological space, Nat. Biotechnol., 2006, 24(7), 805–815 CrossRef CAS PubMed.
- E. Jacoby, Computational chemogenomics, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2011, 1(1), 57–67 CrossRef CAS PubMed.
- (a) R. A. Houghten, C. T. Dooley and J. R. Appel, In vitro and direct in vivo testing of mixture-based combinatorial libraries for the identification of highly active and specific opiate ligands, AAPS J., 2006, 8(2), E371–E382 Search PubMed; (b) F. I. Carroll and R. A. Houghten, From rapid in vitro screening to rapid in vivo screening in the drug discovery process, Neuropsychopharmacology, 2009, 34(1), 251–252 CrossRef CAS PubMed.
- (a) S. Tong, M. Fu, X. Cao, C. K. Firempong, C. Yi, Q. Zhen, H. Zhong, J. Yu and X. Xu, Lipid Raft Stationary Phase Chromatography for Screening Anti-tumor Components from Galla chinensis, Chromatographia, 2014, 77, 419–429 CrossRef CAS; (b) S. Tong, C. Sun, X. Cao, Q. Zheng, H. Zhang, C. K. Firempong, Y. Feng, Y. Yang, J. Yu and X. Xu, Development and thermodynamic evaluation of novel lipid raft stationary phase chromatography for screening potential antitumor agents, Biomed. Chromatogr., 2014, 28, 1615–1623 CrossRef CAS PubMed.
- M. Sun, Y. Guo, B. Dai, C. Wang and L. He, High-expression EGFR/cell membrane chromatography-online-high-performance liquid chromatography/mass spectrometry: rapid screening of EGFR antagonists from Semen Strychni, Rapid Commun. Mass Spectrom., 2012, 26(17), 2027–2032 CrossRef CAS PubMed.
- X. Su, L. Hu, L. Kong, X. Lei and H. Zou, Affinity chromatography with immobilized DNA stationary phase for biological fingerprinting analysis of traditional Chinese medicines, J. Chromatogr. A, 2007, 1154(1), 132–137 CrossRef CAS PubMed.
- (a) T. A. Noctor, I. W. Wainer and D. S. Hage, Allosteric and competitive displacement of drugs from human serum albumin by octanoic acid, as revealed by high-performance liquid affinity chromatography, on a human serum albumin-based stationary phase, J. Chromatogr. B: Biomed. Sci. Appl., 1992, 577(2), 305–315 CrossRef CAS; (b) K. Vuignier, D. Guillarme, J.-L. Veuthey, P.-A. Carrupt and J. Schappler, High performance affinity chromatography (HPAC) as a high-throughput screening tool in drug discovery to study drug–plasma protein interactions, J. Pharm. Biomed. Anal., 2013, 74, 205–212 CrossRef CAS PubMed.
- C. Zhang, J. Li, L. Xu and Z.-G. Shi, Fast immobilized liposome chromatography based on penetrable silica microspheres for screening and analysis of permeable compounds, J. Chromatogr. A, 2012, 1233, 78–84 CrossRef CAS PubMed.
- (a) F. Beigi and P. Lundahl, Immobilized biomembrane chromatography of highly lipophilic drugs, J. Chromatogr. A, 1999, 852(1), 313–317 CrossRef CAS; (b) R. Moaddel, H. K. Musyimi, M. Sanghvi, C. Bashore, C. R. Frazier, M. Khadeer, P. Bhatia and I. W. Wainer, Synthesis and characterization of a cellular membrane affinity chromatography column containing histamine 1 and P2Y1 receptors: A multiple G-protein coupled receptor column, J. Pharm. Biomed. Anal., 2010, 52(3), 416–419 CrossRef CAS PubMed; (c) K. Habicht, C. Frazier, N. Singh, R. Shimmo, I. Wainer and R. Moaddel, The synthesis and characterization of a nuclear membrane affinity chromatography column for the study of human breast cancer resistant protein (BCRP) using nuclear membranes obtained from the LN-229 cells, J. Pharm. Biomed. Anal., 2013, 72, 159 CrossRef CAS PubMed.
- (a) L. Silverman, R. Campbell and J. R. Broach, New assay technologies for high-throughput screening, Curr. Opin. Chem. Biol., 1998, 2(3), 397–403 CrossRef CAS; (b) B. R. Stockwell, Exploring biology with small organic molecules, Nature, 2004, 432(7019), 846–854 CrossRef CAS PubMed; (c) C. Lipinski and A. Hopkins, Navigating chemical space for biology and medicine, Nature, 2004, 432(7019), 855–861 CrossRef CAS PubMed.
- (a) L. J. Pike, Lipid rafts bringing order to chaos, J. Lipid Res., 2003, 44(4), 655–667 CrossRef CAS PubMed; (b) B. van Deurs, K. Roepstorff, A. M. Hommelgaard and K. Sandvig, Caveolae: anchored, multifunctional platforms in the lipid ocean, Trends Cell Biol., 2003, 13(2), 92–100 CrossRef CAS.
- (a) D. A. Brown and J. K. Rose, Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface, Cell, 1992, 68(3), 533–544 CrossRef CAS; (b) P. Liu, Y. Ying, Y.-G. Ko and R. G. Anderson, Localization of platelet-derived growth factor-stimulated phosphorylation cascade to caveolae, J. Biol. Chem., 1996, 271(17), 10299–10303 CrossRef CAS PubMed; (c) C. Mineo, G. L. James, E. J. Smart and R. G. Anderson, Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane, J. Biol. Chem., 1996, 271(20), 11930–11935 CrossRef CAS PubMed; (d) M. Sargiacomo, M. Sudol, Z. Tang and M. P. Lisanti, Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells, J. Cell Biol., 1993, 122(4), 789–807 CrossRef CAS; (e) M. Chun, U. K. Liyanage, M. P. Lisanti and H. F. Lodish, Signal transduction of a G protein-coupled receptor in caveolae: colocalization of endothelin and its receptor with caveolin, Proc. Natl. Acad. Sci. U. S. A., 1994, 91(24), 11728–11732 CrossRef CAS; (f) A. Gorodinsky and D. A. Harris, Glycolipid-anchored proteins in neuroblastoma cells form detergent-resistant complexes without caveolin, J. Cell Biol., 1995, 129(3), 619–627 CrossRef CAS; (g) P. E. Bickel, P. E. Scherer, J. E. Schnitzer, P. Oh, M. P. Lisanti and H. F. Lodish, Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins, J. Biol. Chem., 1997, 272(21), 13793–13802 CrossRef CAS PubMed.
- M. Edidin, Membrane cholesterol, protein phosphorylation, and lipid rafts, Sci. Signaling, 2001, 2001(67), pe1 CrossRef CAS PubMed.
- (a) K. Roepstorff, P. Thomsen, K. Sandvig and B. van Deurs, Sequestration of epidermal growth factor receptors in non-caveolar lipid rafts inhibits ligand binding, J. Biol. Chem., 2002, 277(21), 18954–18960 CrossRef CAS PubMed; (b) M. Takebayashi, T. Hayashi and T. P. Su, σ-1 Receptors potentiate epidermal growth factor signaling towards neuritogenesis in PC12 cells: Potential relation to lipid raft reconstitution, Synapse, 2004, 53(2), 90–103 CrossRef CAS PubMed.
- (a) A. Abulrob, S. Giuseppin, M. F. Andrade, A. McDermid, M. Moreno and D. Stanimirovic, Interactions of EGFR and caveolin-1 in human glioblastoma cells: evidence that tyrosine phosphorylation regulates EGFR association with caveolae, Oncogene, 2004, 23(41), 6967–6979 CrossRef CAS PubMed; (b) C. Mineo, G. N. Gill and R. G. Anderson, Regulated migration of epidermal growth factor receptor from caveolae, J. Biol. Chem., 1999, 274(43), 30636–30643 CrossRef CAS PubMed.
- A. Hryniewicz-Jankowska, K. Augoff, A. Biernatowska, J. Podkalicka and A. F. Sikorski, Membrane rafts as a novel target in cancer therapy, Biochim. Biophys. Acta, Rev. Cancer, 2014, 1845(2), 155–165 CrossRef CAS PubMed.
- P. V. Escribá, Membrane-lipid therapy: a new approach in molecular medicine, Trends Mol. Med., 2006, 12(1), 34–43 CrossRef PubMed.
- F. Mollinedo and C. Gajate, Fas/CD95 death receptor and lipid rafts: new targets for apoptosis-directed cancer therapy, Drug Resist. Updates, 2006, 9(1), 51–73 CrossRef CAS PubMed.
- D. Sarnataro, S. Pisanti, A. Santoro, P. Gazzerro, A. M. Malfitano, C. Laezza and M. Bifulco, The cannabinoid CB1 receptor antagonist rimonabant (SR141716) inhibits human breast cancer cell proliferation through a lipid raft-mediated mechanism, Mol. Pharmacol., 2006, 70(4), 1298–1306 CrossRef CAS PubMed.
- A. Gabizon, D. Tzemach, J. Gorin, L. Mak, Y. Amitay, H. Shmeeda and S. Zalipsky, Improved therapeutic activity of folate-targeted liposomal doxorubicin in folate receptor-expressing tumor models, Cancer Chemother. Pharmacol., 2010, 66(1), 43–52 CrossRef CAS PubMed.
- S.-J. Chiu, S. Liu, D. Perrotti, G. Marcucci and R. J. Lee, Efficient delivery of a Bcl-2-specific antisense oligodeoxyribonucleotide (G3139) via transferrin receptor-targeted liposomes, J. Controlled Release, 2006, 112(2), 199–207 CrossRef CAS PubMed.
- H. D. Han, L. S. Mangala, J. W. Lee, M. M. Shahzad, H. S. Kim, D. Shen, E. J. Nam, E. M. Mora, R. L. Stone and C. Lu, Targeted gene silencing using RGD-labeled chitosan nanoparticles, Clin. Cancer Res., 2010, 16(15), 3910–3922 CrossRef CAS PubMed.
- B. Yu, H. C. Tai, W. Xue, L. J. Lee and R. J. Lee, Receptor-targeted nanocarriers for therapeutic delivery to cancer, Mol. Membr. Biol., 2010, 27(7), 286–298 CrossRef CAS PubMed.
- A. D. Vadlapudi, R. K. Vadlapatla, D. Kwatra, R. Earla, S. K. Samanta, D. Pal and A. K. Mitra, Targeted lipid based drug conjugates: a novel strategy for drug delivery, Int. J. Pharm., 2012, 434(1), 315–324 CrossRef CAS PubMed.
- L. Thomann-Harwood, P. Kaeuper, N. Rossi, P. Milona, B. Herrmann and K. McCullough, Nanogel vaccines targeting dendritic cells: contributions of the surface decoration and vaccine cargo on cell targeting and activation, J. Controlled Release, 2013, 166(2), 95–105 CrossRef CAS PubMed.
- K. C. Partlow, G. M. Lanza and S. A. Wickline, Exploiting lipid raft transport with membrane targeted nanoparticles: a strategy for cytosolic drug delivery, Biomaterials, 2008, 29(23), 3367–3375 CrossRef CAS PubMed.
- I. A. Khalil, K. Kogure, H. Akita and H. Harashima, Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery, Pharmacol. Rev., 2006, 58(1), 32–45 CrossRef CAS PubMed.
- H. Raghu, N. Sharma-Walia, M. V. Veettil, S. Sadagopan, A. Caballero, R. Sivakumar, L. Varga, V. Bottero and B. Chandran, Lipid rafts of primary endothelial cells are essential for Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8-induced phosphatidylinositol 3-kinase and RhoA-GTPases critical for microtubule dynamics and nuclear delivery of viral DNA but dispensable for binding and entry, J. Virol., 2007, 81(15), 7941–7959 CrossRef CAS PubMed.
- X. Chen, S. Shank, P. B. Davis and A. G. Ziady, Nucleolin-mediated cellular trafficking of DNA nanoparticle is lipid raft and microtubule dependent and can be modulated by glucocorticoid, Mol. Ther., 2011, 19(1), 93–102 CrossRef CAS PubMed.
- R. Sharma, A. Ghasparian, J. A. Robinson and K. C. McCullough, Synthetic virus-like particles target dendritic cell lipid rafts for rapid endocytosis primarily but not exclusively by macropinocytosis, PLoS One, 2012, 7(8), e43248 CAS.
- M.-H. Pang, Y. Kim, K. W. Jung, S. Cho and D. H. Lee, A series of case studies: practical methodology for identifying antinociceptive multi-target drugs, Drug Discovery Today, 2012, 17(9), 425–434 CrossRef CAS PubMed.
- (a) I. Sanchez, J. Calderon, B. Ruiz, J. Tellez, L. Calzada and J. Taboada, In vitro cytotoxicity of flavonoids against MK2 and C6 tumour cells, Phytother. Res., 2001, 15(4), 290–293 CrossRef CAS PubMed; (b) M. Franco-Molina, R. Gomez-Flores, P. Tamez-Guerra, R. Tamez-Guerra, L. Castillo-Leon and C. Rodríguez-Padilla, In vitro immunopotentiating properties and tumour cell toxicity induced by Lophophora williamsii (peyote) cactus methanolic extract, Phytother. Res., 2003, 17(9), 1076–1081 CrossRef CAS PubMed; (c) G. Mena-Rejon, E. Caamal-Fuentes, Z. Cantillo-Ciau, R. Cedillo-Rivera, J. Flores-Guido and R. Moo-Puc, In vitro cytotoxic activity of nine plants used in Mayan traditional medicine, J. Ethnopharmacol., 2009, 121(3), 462–465 CrossRef CAS PubMed.
- A. J. Alonso-Castro, M. L. Villarreal, L. A. Salazar-Olivo, M. Gomez-Sanchez, F. Dominguez and A. Garcia-Carranca, Mexican medicinal plants used for cancer treatment: pharmacological, phytochemical and ethnobotanical studies, J. Ethnopharmacol., 2011, 133(3), 945–972 CrossRef CAS PubMed.
- G. M. Brodeur, J. E. Minturn, R. Ho, A. M. Simpson, R. Iyer, C. R. Varela, J. E. Light, V. Kolla and A. E. Evans, Trk receptor expression and inhibition in neuroblastomas, Clin. Cancer Res., 2009, 15(10), 3244–3250 CrossRef CAS PubMed.
- (a) J. HOU, Evaluation of drug-muscarinic receptor affinities using cell membrane chromatography and radioligand binding assay in guinea pig jejunum membrane, Acta Pharmacol. Sin., 2005, 26(1), 113–116 CrossRef PubMed; (b) S. Sadagopan, N. Sharma-Walia, M. V. Veettil, H. Raghu, R. Sivakumar, V. Bottero and B. Chandran, Kaposi's sarcoma-associated herpesvirus induces sustained NF-κB activation during de novo infection of primary human dermal microvascular endothelial cells that is essential for viral gene expression, J. Virol., 2007, 81(8), 3949–3968 CrossRef CAS PubMed; (c) S. Sathornsumetee and D. A. Reardon, Targeting multiple kinases in glioblastoma multiforme, 2009 Search PubMed.
- (a) L. J. Pike, Lipid rafts, J. Lipid Res., 2003, 44(4), 655–667 CrossRef CAS PubMed; (b) L. Zhuang, J. Kim, R. M. Adam, K. R. Solomon and M. R. Freeman, Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts, J. Clin. Invest., 2005, 115(4), 959–968 CrossRef CAS.
- S. K. Patra, Dissecting lipid raft facilitated cell signaling pathways in cancer, Biochim. Biophys. Acta, 2008, 1785(2), 182–206 CAS.
- Z. Mitri, T. Constantine and R. O'Regan, The HER2 receptor in breast cancer: pathophysiology, clinical use, and new advances in therapy, Chemother. Res. Pract., 2012, 2012, 743193 Search PubMed.
- (a) T. Hehlgans and K. Pfeffer, The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: players, rules and the games, Immunology, 2005, 115(1), 1–20 CrossRef CAS PubMed; (b) I. A. Clark, How TNF was recognized as a key mechanism of disease, Cytokine Growth Factor Rev., 2007, 18(3), 335–343 CrossRef CAS PubMed; (c) M. G. Tansey and D. E. Szymkowski, The TNF superfamily in 2009: new pathways, new indications, and new drugs, Drug Discovery Today, 2009, 14(23), 1082–1088 CrossRef CAS PubMed.
- I. S. Grewal, Overview of TNF superfamily: a chest full of potential therapeutic targets, in Therapeutic Targets of the TNF Superfamily, Springer, 2009, pp. 1–7 Search PubMed.
- L. Wang, J. K. Yang, V. Kabaleeswaran, A. J. Rice, A. C. Cruz, A. Y. Park, Q. Yin, E. Damko, S. B. Jang and S. Raunser, The Fas-FADD death domain complex structure reveals the basis of DISC assembly and disease mutations, Nat. Struct. Mol. Biol., 2010, 17(11), 1324–1329 CAS.
- Z. F. Yi, S. G. Cho, H. Zhao, Y. Y. Wu, J. Luo, D. Li, T. Yi, X. Xu, Z. Wu and M. Liu, A novel peptide from human apolipoprotein (a) inhibits angiogenesis and tumor growth by targeting c-Src phosphorylation in VEGF-induced human umbilical endothelial cells, Int. J. Cancer, 2009, 124(4), 843–852 CrossRef CAS PubMed.
- N. Ferrara, Vascular endothelial growth factor: basic science and clinical progress, Endocr. Rev., 2004, 25(4), 581–611 CrossRef CAS PubMed.
- C. J. Sherr, Principles of tumor suppression, Cell, 2004, 116(2), 235–246 CrossRef CAS.
- (a) S. F. Kingsmore, I. E. Lindquist, J. Mudge, D. D. Gessler and W. D. Beavis, Genome-wide association studies: progress and potential for drug discovery and development, Nat. Rev. Drug Discovery, 2008, 7(3), 221–230 CrossRef CAS PubMed; (b) R. P. Araujo, L. A. Liotta and E. F. Petricoin, Proteins, drug targets and the mechanisms they control: the simple truth about complex networks, Nat. Rev. Drug Discovery, 2007, 6(11), 871–880 CrossRef CAS PubMed.
- Y. Pan, H. Zhang and Y. Chen, New approach for screening anti-tumor compounds, Chin. Sci. Bull., 2003, 48(7), 630–633 CrossRef CAS.
- R. Moaddel, L. Lu, M. Baynham and I. W. Wainer, Immobilized receptor-and transporter-based liquid chromatographic phases for on-line pharmacological and biochemical studies: a mini-review, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2002, 768(1), 41–53 CrossRef CAS.
- (a) X. Zheng, X. Zhao, R. Yang, S. Wang, Y. Wei and J. Zheng, β2-Adrenoceptor affinity chromatography and its application in the screening of the active compounds from Semen Armeniacae Amarum, Chin. Sci. Bull., 2008, 53(6), 842–847 CrossRef CAS; (b) M. A. Arai, E. Kobatake, T. Koyano, T. Kowithayakorn, S. Kato and M. Ishibashi, A method for the rapid discovery of naturally occurring products by proteins immobilized on magnetic beads and reverse affinity chromatography, Chem.–Asian J., 2009, 4(12), 1802–1808 CrossRef CAS PubMed.
- (a) M.-J. Liang, L.-C. He and G.-D. Yang, Screening, analysis and in vitro vasodilatation of effective components from Ligusticum Chuanxiong, Life Sci., 2005, 78(2), 128–133 CrossRef CAS PubMed; (b) X. Hou, S. Wang, J. Hou and L. He, Establishment of A431 cell membrane chromatography-RPLC method for screening target components from Radix Caulophylli, J. Sep. Sci., 2011, 34(5), 508–513 CrossRef CAS PubMed.
- J. Richard, C. Sainsbury, G. Needham, J. Farndon, A. Malcolm and A. Harris, Epidermal-growth-factor receptor status as predictor of early recurrence of and death from breast cancer, Lancet, 1987, 329(8547), 1398–1402 CrossRef.
- (a) R. S. Kerbel, Tumor angiogenesis, N. Engl. J. Med., 2008, 358(19), 2039–2049 CrossRef CAS PubMed; (b) D. S. Krause and R. A. Van Etten, Tyrosine kinases as targets for cancer therapy, N. Engl. J. Med., 2005, 353(2), 172–187 CrossRef CAS PubMed; (c) J. Zhang, P. L. Yang and N. S. Gray, Targeting cancer with small molecule kinase inhibitors, Nat. Rev. Cancer, 2009, 9(1), 28–39 CrossRef CAS PubMed.
- M. Li, S. Wang, Y. Zhang and L. He, An online coupled cell membrane chromatography with LC/MS method for screening compounds from Aconitum carmichaeli Debx. acting on VEGFR-2, J. Pharm. Biomed. Anal., 2010, 53(4), 1063–1069 CrossRef CAS PubMed.
- L. Wang, J. Ren, M. Sun and S. Wang, A combined cell membrane chromatography and online HPLC/MS method for screening compounds from Radix Caulophylli acting on the human α 1A-adrenoceptor, J. Pharm. Biomed. Anal., 2010, 51(5), 1032–1036 CrossRef CAS PubMed.
- S. Han, J. Huang, J. Hou and S. Wang, Screening epidermal growth factor receptor antagonists from Radix et Rhizoma Asari by two-dimensional liquid chromatography, J. Sep. Sci., 2014, 37(13), 1525–1532 CrossRef CAS PubMed.
- M. Sun, Y.-M. Zhang, J. Zhang, S.-C. Wang and L.-C. He, A high expression EGFR/cell membrane chromatography and online high performance liquid chromatography/mass spectrometry method for screening EGFR antagonists from Rhizoma Polygoni Cuspidati, Acta Pharm. Sin. B, 2011, 1(2), 115–120 CrossRef CAS PubMed.
- M. Sun, J. Ren, H. Du, Y. Zhang, J. Zhang, S. Wang and L. He, A combined A431 cell membrane chromatography and online high performance liquid chromatography/mass spectrometry method for screening compounds from total alkaloid of Radix Caulophylli acting on the human EGFR, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2010, 878(28), 2712–2718 CrossRef CAS PubMed.
- S. Wang, M. Sun, Y. Zhang, H. Du and L. He, A new A 431/cell membrane chromatography and online high performance liquid chromatography/mass spectrometry method for screening epidermal growth factor receptor antagonists from Radix sophorae flavescentis, J. Chromatogr. A, 2010, 1217(32), 5246–5252 CrossRef CAS PubMed.
- C. A. Patwardhan, E. Alfa, S. Lu and A. Chadli, Progesterone Receptor Chaperone Complex–Based High-Throughput Screening Assay Identification of Capsaicin as an Inhibitor of the Hsp90 Machine, J. Biomol. Screening, 2014, 20, 223–229 CrossRef PubMed.
- T. J. Haggerty, I. S. Dunn, L. B. Rose, E. E. Newton and J. T. Kurnick, A screening assay to identify agents that enhance T-cell recognition of human melanomas, Assay Drug Dev. Technol., 2012, 10(2), 187–201 CrossRef CAS PubMed.
- S. Tong, C. Sun, X. Cao, Q. Zheng, H. Zhang, C. K. Firempong, Y. Feng, Y. Yang, J. Yu and X. Xu, Development and thermodynamic evaluation of novel lipid raft stationary phase chromatography for screening potential antitumor agents, Biomed. Chromatogr., 2014, 28(12), 1615–1623 CrossRef CAS PubMed.
- (a) D. F. Horrobin, Modern biomedical research: an internally self-consistent universe with little contact with medical reality?, Nat. Rev. Drug Discovery, 2003, 2(2), 151–154 CrossRef CAS PubMed; (b) H. Kubinyi, Drug research: myths, hype and reality, Nat. Rev. Drug Discovery, 2003, 2(8), 665–668 CrossRef CAS PubMed.
- Y. Bing-Xiang, H. Jin, Y. Guang-De, Z. Li-mei and H. Lang-Chong, Comparison of determination of drug–muscarinic receptor affinity by cell-membrane chromatography and by radioligand-binding assay with the cerebrum membrane of the rat, Chromatographia, 2005, 61(7–8), 381–384 Search PubMed.
- L. He, G. Yang and X. Geng, Enzymatic activity and chromatographic characteristics of the cell membrane immobilized on silica surface, Chin. Sci. Bull., 1999, 44(9), 826–831 CrossRef CAS.
- X. Hou, M. Zhou, Q. Jiang, S. Wang and L. He, A vascular smooth muscle/cell membrane chromatography–offline-gas chromatography/mass spectrometry method for recognition, separation and identification of active components from traditional Chinese medicines, J. Chromatogr. A, 2009, 1216(42), 7081–7087 CrossRef CAS PubMed.
- (a) K. S. Smalley, N. K. Haass, P. A. Brafford, M. Lioni, K. T. Flaherty and M. Herlyn, Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases, Mol. Cancer Ther., 2006, 5(5), 1136–1144 CrossRef CAS PubMed; (b) N. V. Sergina, M. Rausch, D. Wang, J. Blair, B. Hann, K. M. Shokat and M. M. Moasser, Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3, Nature, 2007, 445(7126), 437–441 CrossRef CAS PubMed.
- B. A. Larder, S. D. Kemp and P. R. Harrigan, Potential mechanism for sustained antiretroviral efficacy of AZT-3TC combination therapy, Science, 1995, 269(5224), 696–699 CAS.
- X. Zhang, A. Crespo and A. Fernández, Turning promiscuous kinase inhibitors into safer drugs, Trends Biotechnol., 2008, 26(6), 295–301 CrossRef CAS PubMed.
- (a) M. Krug and A. Hilgeroth, Recent advances in the development of multi-kinase inhibitors, Mini-Rev. Med. Chem., 2008, 8(13), 1312–1327 CrossRef CAS; (b) G. L. Adrian, V. Marcel, B. G. Robert and T. Richard, A comparison of physicochemical property profiles of marketed oral drugs and orally bioavailable anti-cancer protein kinase inhibitors in clinical development, Curr. Top. Med. Chem., 2007, 7(14), 1408–1422 CrossRef.
- (a) C.-I. Wong, T.-S. Koh, R. Soo, S. Hartono, C.-H. Thng, E. McKeegan, W.-P. Yong, C.-S. Chen, S.-C. Lee and J. Wong, Phase I and biomarker study of ABT-869, a multiple receptor tyrosine kinase inhibitor, in patients with refractory solid malignancies, J. Clin. Oncol., 2009, 27(28), 4718–4726 CrossRef CAS PubMed; (b) D. B. Shankar, J. Li, P. Tapang, J. O. McCall, L. J. Pease, Y. Dai, R.-Q. Wei, D. H. Albert, J. J. Bouska and D. J. Osterling, ABT-869, a multitargeted receptor tyrosine kinase inhibitor: inhibition of FLT3 phosphorylation and signaling in acute myeloid leukemia, Blood, 2007, 109(8), 3400–3408 CrossRef CAS PubMed.
- (a) S. I. Sherman, Early clinical studies of novel therapies for thyroid cancers, Endocrinol. Metab. Clin. North Am., 2008, 37(2), 511–524 CrossRef CAS PubMed; (b) C. B. Lee and M. A. Socinski, Vascular endothelial growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer: a review of recent clinical trials, Rev. Recent Clin. Trials, 2007, 2(2), 117–120 CrossRef CAS.
- (a) H.-P. Kuo, T.-C. Chuang, M.-H. Yeh, S.-C. Hsu, T.-D. Way, P.-Y. Chen, S.-S. Wang, Y.-H. Chang, M.-C. Kao and J.-Y. Liu, Growth suppression of HER2-overexpressing breast cancer cells by berberine via modulation of the HER2/PI3K/Akt signaling pathway, J. Agric. Food Chem., 2011, 59(15), 8216–8224 CrossRef CAS PubMed; (b) J. Li, B. Cao, X. Liu, X. Fu, Z. Xiong, L. Chen, O. Sartor, Y. Dong and H. Zhang, Berberine suppresses androgen receptor signaling in prostate cancer, Mol. Cancer Ther., 2011, 10(8), 1346–1356 CrossRef CAS PubMed; (c) B. Lu, M. Hu and J. Peng, Cytotoxicity of berberine on human cervical carcinoma HeLa cells through mitochondria, death receptor and MAPK pathways, and in-silico drug-target prediction, Toxicol. In Vitro, 2010, 24(6), 1482–1490 CrossRef CAS PubMed.
- (a) H. Yang, D. Chen, Q. C. Cui, X. Yuan and Q. P. Dou, Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine,” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice, Cancer Res., 2006, 66(9), 4758–4765 CrossRef CAS PubMed; (b) H. Mou, Y. Zheng, P. Zhao, H. Bao, W. Fang and N. Xu, Celastrol induces apoptosis in non-small-cell lung cancer A549 cells through activation of mitochondria-and Fas/FasL-mediated pathways, Toxicol. In Vitro, 2011, 25(5), 1027–1032 CrossRef CAS PubMed; (c) B. Sung, B. Park, V. R. Yadav and B. B. Aggarwal, Celastrol, a triterpene, enhances TRAIL-induced apoptosis through the down-regulation of cell survival proteins and up-regulation of death receptors, J. Biol. Chem., 2010, 285(15), 11498–11507 CrossRef CAS PubMed; (d) Y. Huang, Y. Zhou, Y. Fan and D. Zhou, Celastrol inhibits the growth of human glioma xenografts in nude mice through suppressing VEGFR expression, Cancer Lett., 2008, 264(1), 101–106 CrossRef CAS PubMed.
- (a) A. Goel, A. B. Kunnumakkara and B. B. Aggarwal, Curcumin as “Curecumin”: from kitchen to clinic, Biochem. Pharmacol., 2008, 75(4), 787–809 CrossRef CAS PubMed; (b) P. Anand, C. Sundaram, S. Jhurani, A. B. Kunnumakkara and B. B. Aggarwal, Curcumin and cancer: an “old-age” disease with an “age-old” solution, Cancer Lett., 2008, 267(1), 133–164 CrossRef CAS PubMed.
- (a) X. Zhang, Y. Song, Y. Wu, Y. Dong, L. Lai, J. Zhang, B. Lu, F. Dai, L. He and M. Liu, Indirubin inhibits tumor growth by antitumor angiogenesis via blocking VEGFR2-mediated JAK/STAT3 signaling in endothelial cell, Int. J. Cancer, 2011, 129(10), 2502–2511 CrossRef CAS PubMed; (b) Y. Zhen, V. Sørensen, Y. Jin, Z. Suo and A. Więdłocha, Indirubin-3′-monoxime inhibits autophosphorylation of FGFR1 and stimulates ERK1/2 activity via p38 MAPK, Oncogene, 2007, 26(44), 6372–6385 CrossRef CAS PubMed.
- J. Schwartz, Current combination chemotherapy regimens for metastatic breast cancer, Am. J. Health-Syst. Pharm., 2009, 66(23 suppl. 6), S3–S8 CrossRef CAS PubMed.
- (a) F. Peng, Y.-Q. Wei, L. Tian, L. Yang, X. Zhao, Y. Lu, Y.-Q. Mao, B. Kan, S. Lei and G.-S. Wang, Induction of apoptosis by norcantharidin in human colorectal carcinoma cell lines: involvement of the CD95 receptor/ligand, J. Cancer Res. Clin. Oncol., 2002, 128(4), 223–230 CrossRef CAS PubMed; (b) L. Zhang, Q. Ji, X. Liu, X. Chen, Z. Chen, Y. Qiu, J. Sun, J. Cai, H. Zhu and Q. Li, Norcantharidin inhibits tumor angiogenesis via blocking VEGFR2/MEK/ERK signaling pathways, Cancer Sci., 2013, 104(5), 604–610 CrossRef CAS PubMed; (c) C.-H. Yeh, Y.-Y. Yang, Y.-F. Huang, K.-C. Chow and M.-F. Chen, Induction of apoptosis in human Hep3B hepatoma cells by norcantharidin through a p53 independent pathway via TRAIL/DR5 signal transduction, Chin. J. Integr. Med., 2012, 18, 676–682 CrossRef CAS PubMed.
- (a) L.-T. Lee, Y.-T. Huang, J.-J. Hwang, P. Lee, F.-C. Ke, M. P. Nair, C. Kanadaswam and M.-T. Lee, Blockade of the epidermal growth factor receptor tyrosine kinase activity by quercetin and luteolin leads to growth inhibition and apoptosis of pancreatic tumor cells, Anticancer Res., 2001, 22(3), 1615–1627 Search PubMed; (b) P. Miodini, L. Fioravanti, G. Di Fronzo and V. Cappelletti, The two phyto-oestrogens genistein and quercetin exert different effects on oestrogen receptor function, Br. J. Cancer, 1999, 80(8), 1150 CrossRef CAS PubMed.
- (a) S. H. Mitchell, W. Zhu and C. Y. Young, Resveratrol inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells, Cancer Res., 1999, 59(23), 5892–5895 CAS; (b) E. Pozo-Guisado, M. J. Lorenzo-Benayas and P. M. Fernández-Salguero, Resveratrol modulates the phosphoinositide 3-kinase pathway through an estrogen receptor α-dependent mechanism: Relevance in cell proliferation, Int. J. Cancer, 2004, 109(2), 167–173 CrossRef CAS PubMed.
- (a) G. D. Demetri, A. T. van Oosterom, C. R. Garrett, M. E. Blackstein, M. H. Shah, J. Verweij, G. McArthur, I. R. Judson, M. C. Heinrich and J. A. Morgan, Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial, Lancet, 2006, 368(9544), 1329–1338 CrossRef CAS; (b) R. J. Motzer, T. E. Hutson, P. Tomczak, M. D. Michaelson, R. M. Bukowski, O. Rixe, S. Oudard, S. Negrier, C. Szczylik and S. T. Kim, Sunitinib versus interferon alfa in metastatic renal-cell carcinoma, N. Engl. J. Med., 2007, 356(2), 115–124 CrossRef PubMed.
- (a) H. Chung, Y. M. Jung, D. H. Shin, J. Y. Lee, M. Y. Oh, H. J. Kim, K. S. Jang, S. J. Jeon, K. H. Son and G. Kong, Anticancer effects of wogonin in both estrogen receptor-positive and-negative human breast cancer cell lines in vitro and in nude mice xenografts, Int. J. Cancer, 2008, 122(4), 816–822 CrossRef CAS PubMed; (b) C.-M. Lin, H. Chang, Y.-H. Chen, I.-H. Wu and J.-H. Chiu, Wogonin inhibits IL-6-induced angiogenesis via down-regulation of VEGF and VEGFR-1, not VEGFR-2, Planta Med., 2006, 72(14), 1305–1310 CrossRef CAS PubMed.
- D. Bates, ZD-6474. AstraZeneca, Curr. Opin. Invest. Drugs, 2003, 4(12), 1468–1472 CAS.
| This journal is © The Royal Society of Chemistry 2015 |