A review of upgrading heavy oils with supercritical fluids
Ting Yan
a,
Jie Xu
a,
Litao Wang
b,
Yindong Liu
b,
Chun Yang
c and
Tao Fang
*a
aDepartment of Chemical Engineering, Xi’an Jiaotong University, China
bProject Office of Heavy to Light Conversion, Petrochemical Research Institute, China
cThe First Affiliated Hospital of Xi’an Jiaotong University, Xi’an Jiaotong University, China
First published on 7th August 2015
Abstract
Due to the decrease in light crude oil and the ever-increasing demand for the upgrading of heavy oils, the development of new heavy oil processing technologies has been attracting wide attention. Using supercritical fluids (SCFs) as the reaction medium has great advantages for upgrading heavy oils. The upgrading processes which employ supercritical water (SCW) without catalysts and the effects of different operating conditions are summarized in this work. The temperature and the density of SCW are of great significance to upgrading processes. The upgrading process through partial oxidation and the water gas shift reaction is further discussed. The processes employing other SCFs with different kinds of catalysts are also summarised. Additionally, the role of the SCFs during the upgrading is discussed. According to the development of the upgrading of heavy oils with SCFs, some strategies are proposed to improve the quality of the products and to reduce the coke yield.
Introduction
Recently, with the reduction of petroleum reservoirs and deeper extraction, crude oil is becoming heavier all around the world. The heavy and extra-heavy oils comprise almost two thirds of the crude oil around the world.1 Furthermore, the price of crude oil of high quality, has been kept at a high level for a long period of time. Moreover, refineries are facing new challenges due to crude oil with an increasing sulfur content. In order to meet the increasing demands for excellent quality motor fuels and petrochemical materials, it is essential to make better use of crude oils, especially the heavy oils (bitumen, asphaltene, etc.) and residues (coal tar, residual oil, etc.), which have a low H/C atomic ratio, high viscosity and a high content of heteroatoms (sulfur, nitrogen, nickel, etc.).The conventional processing technologies for upgrading heavy oils can be categorized as carbon rejection processes and hydrogen addition processes, as shown in Table 1.2 The former includes thermal cracking, delayed coking, residue fluid catalytic cracking (RFCC), etc. The latter includes hydrocracking, hydrotreating and hydrorefining. The thermal cracking process is carried out under high temperatures of over 1273 K and is always accompanied with severe condensation. Moreover, the yield of coke in the total product can reach up to 80%, which causes a huge waste of resources.3,4 Compared with thermal cracking, catalytic cracking and hydrogen addition processes require lower reaction temperatures and have a higher selectivity to produce light products. However, these processes are subject to the critical problem of catalyst deactivation caused by coking and the deposition of heavy metals.5 Besides, catalytic cracking processes have high requirements for feedstock. Meanwhile, a large amount of hydrogen is required for hydrocracking processes. It is well known that hydrogen production is one of the most expensive operations of refineries. Additionally, the present upgrading processes in industry mainly aim at the cracking of a certain range of boiling points of heavy oils and the processes have to be carried out in a set of complex and expensive multi-stage reactors.6–8 Therefore, several new technologies should be developed to convert the heavy and extra-heavy oils into light products efficiently and environmentally.
| Temperature (K) | Strengths | Weaknesses | ||
|---|---|---|---|---|
| Carbon rejection process | Gasification | >1273 | Complete cracking of residue | Uncontrolled selectivity for coal conversion |
| Flexicoking | 1103–1273 | Short residence time | Low quality of products | |
| High yield of liquids | ||||
| Less coke | ||||
| RFCC | 933–973 | High selectivity towards high gasoline production | High quality of feedstock needed to avoid coke deposition and high catalyst consumption | |
| Low gas yield | ||||
| Hydrogen addition process | Fixed bed residue catalytic processes | 623–703 | High selectivity of liquid yield (85%) | Poisoning of catalysts |
| Moving/ebullated bed residue catalytic process | 650–730 | High adaptability for feedstock | High hydrogen consumption | |
| High quality of products | ||||
Fluids whose temperature and pressure are above its critical points are called supercritical fluids (SCFs). They have special physical and chemical properties and are suitable for using as a reaction medium.
(1) The viscosity and diffusivity of SCFs are similar to those of gas, in which mass transfer is faster than conventional solvents.
(2) The solubility is analogous with that of liquids, in which a heterogeneous reaction can proceed in a homogeneous phase at high speed.
(3) The density of a SCF is sensitive to changes in pressure and temperature. As a result, the distribution of the reaction products can be controlled by manipulating the temperature and pressure.8
(4) The simple and fast downstream separation and the efficient solvent recycle operations of SCFs are conductive to their application on a practical scale.9
The comparison of gas, SCF and liquid properties is shown in Table 2.
During the past decade, more and more attention has been focused on upgrading the full-range of heavy oils and heavy fractions like asphaltene and bitumen with SCFs as the reaction medium in a one-stage reactor.10–13 From tar, naphthalene and phenol were easily extracted by supercritical CO2 at a temperature slightly higher than room temperature.14 Svetlana Rudyk et al. studied the upgrading and extraction of bitumen from Nigerian tar sand with supercritical CO2.15 Ding et al. investigated the extraction of high temperature coal tar with supercritical n-pentane. The extraction rate reached 78.36%, and the asphalt yield in raffinate was 21.46%, which is significantly lower than that prepared by conventional distillation.16
Methanol is another kind of SCF applied in the upgrading of heavy oils. The critical points of methanol are 513 K and 8.9 MPa. According to the literature, supercritical methanol can perform acid and alkali catalysis.17 Besides, the hydroxyl group of an alcohol under supercritical conditions can cut off an ether bridge bond.18 Supercritical methanol has been widely used in the degradation of polymer compounds and in biodiesel recycling.19–25 Mandal et al. used supercritical methanol to reduce the total acid number of naphthenic acid present in crude oil.26 The experimental results showed that the removal of the total acid number was 96.87% at 623 K, 10 MPa and with a residence time of 30 min. When the reaction was extended to 60 min, the removal increased to 99.77%. The extraction of coal tar with supercritical methanol has also been investigated. The ratio of light oil increased from 65.10 wt% to 78.19 wt% and the H/C ratio of the light oil products increased by 109.62% compared with that of the raw coal tar.25
This review summarizes recent studies on upgrading heavy oils with supercritical fluids and discusses the effects of the operating conditions and additives on this technology. In addition, the mechanism of the involved reactions and the role of water are elaborated. This review intends to clarify both the improvements and the difficulties in this research area. Moreover, some feasible suggestions are proposed for future efforts to reduce the coke yield and improve the quality of the products.
The upgrading process of heavy oils with SCW
At present, supercritical water (SCW, Tc = 647 K and Pc = 22.1 MPa) is the SCF that has been studied the most as a solvent for upgrading heavy oils, due to its special physical and chemical properties. First of all, water is a relatively cheap solvent which can act as a hydrogen donor. Different from ordinary water, the dielectric coefficient of SCW is much lower, about 10, which means that SCW has good solubility in organics and gas. Meanwhile, SCW has a certain level of acid and alkali catalytic activity, which is conducive to remove heteroatoms.10,17,27 However, serious fundamental questions regarding the chemical mechanism, the catalysis of SCW and the role of SCW are still unclear, and are attracting more and more researchers to this area.Current progress of the research on upgrading with SCW
According to the experimental results, using SCW as the medium of heavy oil pyrolysis can improve the light product yield, suppress coke formation and enhance the removal of heteroatoms without an external hydrogen supply or catalyst, simultaneously.10–15 The upgrading process with SCW has no special requirements for the feedstock, and is suitable for a variety of kinds of heavy oil. Ding et al. studied the upgrading of vacuum residue with a batch autoclave and found that the feedstock showed a high mass transfer rate and good solubility in SCW.16 The viscosity of the residue decreased to 4 mm2 s−1. The content of sulfur and nickel decreased by 32 wt% and 83 wt%, respectively. A similar desulfurization rate of vacuum residue, 40 wt%, was obtained by Yuan.28 Patwardhan et al. investigated the desulfurization of sulfur compounds with different molecular structures. They found that the decomposition rate of aliphatic sulfides reached 90 wt%, whereas that of aromatic sulfides was less than 3 wt%. The delocalization of a lone pair of electrons on the sulfur atom into the aromatic π-electron system resulted in the poor activity of thiophenes.29 Sasaki et al. studied the liquefaction of tar in SCW and sub-critical water. The tar was constituted by the residue of atmospheric and vacuum distillation. The main products after liquefaction were phenol (3.44 wt%), biphenyl (2.23 wt%), diphenylether (13.70 wt%) and diphenylmethane (1.30 wt%).30Kishita et al. carried out experiments concerning the visbreaking of bitumen in SCW. The products were mainly alkanes and aromatics. No sulfur compounds such as benzothiophene and dibenzothiophene were detected in the products, which means that light and desulfurized products were prepared.31 Watanabe et al. investigated the upgrading process of Canada oil sand bitumen in a batch reactor. The conversion of asphaltene in SCW was higher than that of neat pyrolysis, and the coke yield was lower than that of neat pyrolysis.13
Ma et al. studied the conversion of coal tar with SCW. They found that SCW promoted the formation of light oils, leading to an increase in the light oil yield, to about 51.55 wt%, and suppressed the formation of coke, simultaneously.17 The yield of light oil from the cracking of high temperature coal tar and low temperature coal tar were both improved.6 They found that hydrocarbons with weak bonds, such as tert-butyl benzene, heptyl-benzene and heptyl-naphthalene can be cracked in SCW easily. Additionally, C–O, C–S and C–N bonds can be easily cleaved to generate small molecule compounds. Whereas, the C–C bond energy is too high to break: biphenyl methyl naphthalene was reacted at 733 K for 1 h without any obvious chemical change. Furthermore, the presence of maltene could suppress the coke formation, and the coke formed has the same effect on the formation of maltene.27 The Institute of Coal Chemistry (Chinese Academy of Science) has done much work in this area. Han et al. employed high temperature coal tar and heavy fractions of coal tar (pitch and asphaltene) for the refining process in SCW and in N2. The asphaltene conversion, the maltene yield and the H/C atom ratio of the products were all higher in SCW than in N2.11,32,33
I. V. Kozhevnikov et al. studied the conversion of petroleum asphaltene in SCW at 653 K, 22.8 MPa and with a water density of 0.33 g cm−3. The hexane soluble fraction of the products was similar to mixtures of diesel fractions and vacuum gas oil.10
The co-pyrolysis of heavy oils and polyolefins was investigated. Through the H-abstraction of low density polyethylene, the radicals prepared by heavy oil pyrolysis can be quickly saturated, because the activation energy of reaction is less than 40 kJ mol−1. The saturation of the radicals prevented the light oil fractions from transforming into coke precursors and promoted the decomposition of the heavy oils. As a result, the further condensation of coke precursors (resins, asphaltenes) was significantly suppressed.34,35
The effects of the operating parameters and properties of the SCF
SCW has a good solubility, which is controlled by temperature and pressure, for heavy oils. As a result, the product distribution can be controlled by manipulating the temperature and pressure.17 The yield of light products and valuable chemicals are affected by operating parameters, such as temperature, pressure and residence time, etc. In order to obtain the desired upgraded product distribution, researchers have studied the effects of different operating parameters.The experimental results indicated that the effect of the temperature on the upgrading of coal tar in SCW is more significant than that of pressure and residence time. According to previous studies, temperature has the most significant influence on the upgrading process, which is dominated by a free radical mechanism.11,33 With the temperature rising, the selectivity of asphaltene to convert into maltene increases. But if the temperature reaches too high, the selectivity towards forming coke increases over that of converting into maltene, and maltene can also transform into coke. A similar phenomenon showed that light oil yield increases gradually with increasing temperature in supercritical gasoline until the temperature is increased up to 653 K. Then the light oil yield starts to decrease.6
The density of SCW is another significant parameter that can control the upgrading result. The solubility of heavy oils increases with the density of SCW. The density is affected by the synergy of temperature and pressure.36
The mixing state of heavy oils and SCFs has an important influence on the cracking of heavy oils. Morimoto et al. conducted an extraction to optimize the miscibility of SCW with asphaltene at 673–723 K and 20–35 MPa. The extraction was thought to be controlled mainly by the dielectric constant (DC) and the Hansen solubility parameters (HSPs) of water.37 According to the results, when DC = 2.2, δp (dispersive force) = 6.4, and δh (polar interactions) = 9.7, then water had the highest solubility for asphaltene, and the state of the solution was close to the homogenous phase.38 A similar result of DC = 2.2, δp = 6.3, and δh = 9.9 was obtained by Cheng et al.39
Chang et al. studied the lumping macro-kinetics of high temperature coal tar hydrocracking in supercritical xylene, demonstrating the relationship between the variation of light oil yield with several parameters, such as the reaction time, temperature, hydrogen pressure, and the ratio of solvent to tar. The work provided basic data for the further development of high temperature coal tar hydrocracking in supercritical xylene.40
Intensification of upgrading by additives
To increase the quality of the upgraded products, several kinds of additives have been added into the reaction system. The additives and their effects are shown in Table 3. NaOH was added into SCW to increase the solubility of asphaltene.41 The synergistic effects of H2 and CO2 have also been studied.42 The hydrogenation and desulfurization of heavy oils were studied by introducing catalysts.12,43| Additives | Solvents | Effects | Reference |
|---|---|---|---|
| NaOH | SCW | Increases the solubility | 41 |
| CO | SCW | WGSR | 44–48 |
| CO2–H2 | Improves the solubility | ||
| HCOOH | |||
| NaY zeolite | SC-gasoline | Hydrogenation | 6 |
| SC-xylene | |||
| ZnO | SCW | Desulfurization | 43 |
| MoO3 | |||
| MoS | |||
| Activated carbon | SC-n-hexane | Hydrogenation | 12, 72 and 75 |
| SC-n-dodecane | H transfer | ||
| Ni/Mo/Al2O3 | SCW | Desulfurization | 44 and 71 |
Upgrading through the water gas shift reaction (WGSR) and partial oxidation
SCW is a potentially inexpensive source of hydrogen. But according to previous studies, SCW is dominated by solvent and dispersion effects during the upgrading process rather than the supply hydrogen. The water gas shift reaction (WGSR: CO + H2O → CO2 + H2) is an efficient method to extract hydrogen atoms from water for the hydrogenation of heavy oils. Adschiri et al. studied the catalytic desulfurization of dibenzothiophene using Ni/Mo/Al2O3 as catalysts in H2–SCW, CO–SCW, CO2–H2–SCW and HCOOH–SCW. The conversion of dibenzothiophene in CO–SCW, CO2–H2–SCW and HCOOH–SCW is higher than that in H2–SCW.44 The H/C atomic ratio was in the order of SCW–H2–CO > SCW > raw asphaltene. The number of naphthene rings of asphaltene extracted in SCW–H2–CO2 was the most.45 The hydrogenation of dibenzothiophene, carbazole and naphthalene was studied at 673 K in the presence of SCW–CO, SCW–H2 and SCW–H2–CO2.46 The rate of hydrogenation was in the order of SCW–CO > SCW–H2–CO2 > SCW–H2. The above indicates that the activity of the hydrogen obtained through WGSR was stronger than that of molecular hydrogen (H2). The reason is that the in situ-H prepared through WGSR can react with hydrocarbons directly. On the contrary, H2 needs energy to break the H–H bond before saturating the intermediates formed by the pyrolysis of the hydrocarbons. The conversion of Gudao residual oil through WGSR was investigated and the increased H/C ratio showed that hydrogen was incorporated into the products.47SCW oxidation has been used in industrial wastewater treatment. The organics are converted into CO2 and H2O. The partial oxidation of heavy oils and hydrocarbons in SCW is an important supply of CO for hydrogenation through the WGSR in order to avoid releasing the poisonous gas, CO.48–51 The partial oxidation of n-hexadecane and hexylbenzene was studied in SCW.52 The selectivity for CO and H2 increased with the water density. The yield of the compounds containing oxygen atoms (aldehyde and ketone) prepared by the partial oxidation process increased and the yield of 1-alkene/n-alkane decreased with increasing water density simultaneously.44,46
The reaction pathway of CO formation proposed by Sato is shown as follows:
First, the hydrocarbons and heavy oils are partially oxidized to form aldehydes and ketones.
Secondly, the aldehydes and ketones decompose to form CO.53
The conversion of asphalt with SCW at 613–673 K and water density of 0–0.5 g cm−3 was studied. The gaseous products of the partial oxidation were CO2, H2S, CO, CH4, C2H6 and C3H8 (no H2 was detected). It has been observed that the CO2 yield and the conversion rate of asphaltene increases with the water density in previous studies. In the high density region of water, a change in the fugacity of the radical or the ionic reactions might promote the partial oxidation of the hydrocarbons in SCW.54
The effects of temperature and air pressure have also been examined.48,49 Higher CO/(CO + CO2) ratios and less coke were obtained at lower temperatures. The high water/oil ratio improved the selectivity towards partial oxidation to produce CO. The increased air pressure promoted gas formation, especially CO2 and CO, but caused a decrease in the ratio of CO/(CO + CO2). A large amount of oxygen tended to promote the total oxidation route. Low air pressure was advantageous for asphaltene modification in the view of CO selectivity.
Sato et al. proposed a feasible route towards upgrading heavy oils through partial oxidation and the WGSR in SCW based on the previous studies as shown in Fig. 1.53 First, the partial oxidation of the heavy oil should be operated at below about 673 K with the appropriate amount of oxygen. High density water should be utilized for the high selectivity towards CO. The oxygen is completely consumed to form CO in order to suppress the CO2 and coke formation. Secondly, the hydrogenation of the residual oil through the WGSR should be operated under a higher temperature than partial oxidation, which is above 673 K. In some special cases, additional CO is introduced into the system to compensate for the shortage of CO derived from the partial oxidation of heavy oils.
 | ||
| Fig. 1 A possible process for upgrading heavy oils with partial oxidation and hydrogenation through the WGSR in SCW. | ||
During the partial oxidation process, hydrogen sources such as CO–H2O, H2–CO2 and HCOOH are injected into the oil rich phase directly. This operation inhibits the coke formation significantly because the amount of hydrogen introduced into the oil rich phase promotes the formation of lighter oils so that more asphaltene core is dispersed in it. Besides, the coexisting gases such as CO, CO2 and H2 with SCW promote the extraction of coke precursors from the oil rich phase to the water rich phase, which can also suppress the coke formation. From the experimental results, the direct injection of a hydrogen source (active hydrogen through the WGSR) into the oil rich phase was an effective method for inhibiting coke formation and increasing the production of light oils.
Current progress of the research on upgrading using catalysts
Although SCW can promote light oil formation and restrain the coke formation during the upgrading process of heavy oils, the high critical point of water requires a large amount of energy to carry out the process. Besides, water can cause the rapid corrosion of the apparatus when it is under the subcritical or supercritical state.33 Therefore, it is reasonable to apply other solvents with lower critical points and no corrosion concerns to refine heavy oils. However, the poorer activity of such solvents compared with SCW means that introducing catalysts into the reaction system is needed.There are additional requirements for catalysts that are introduced into the SCF environment. Some kinds of SCFs can deactivate many catalysts due to their aggressive chemical activity. The heteroatoms in heavy oils, especially sulfur have a poisoning effect on metal catalysts.28,44,55–60 Besides, conventional catalyst support materials, such as Si and Al, can be degraded in a SCF reaction environment.61 According to previous research, three types of heterogeneous catalysts, including activated carbon, and transition metals and their oxides, have been used in the severe SCW environment.57,62 In detail, the oxides of Ce, Co, Fe, Mo, and Zn catalysts have been commonly used in SCW.6,63 In the catalytic cracking process, Ni–Mo loaded on a Y zeolite support is effective for the reaction, and the noble metals Pt and Pd have a high activity for the hydrogenation of aromatics.64–70
Gu et al. selected xylene and gasoline as the solvents to modify high and low temperature coal tar using NaY zeolite (Na56 [(AlO2)56(SiO2)136]·264H2O) as the catalyst.6 The results showed that gasoline was a more appropriate solvent in comparison with xylene. The upgrading process of the high temperature coal tar needed more catalyst to supply sufficient active sites for a high yield of light oil. However, an excessive catalyst amount accelerated the rate of light oil cracking over that of light oil formation and caused excessive modification, as a result the light oil yield decreased.
Ates et al. studied the desulfurization of Arabian heavy whole crude oil and two model compounds (hexyl sulfide in hexadecane and dibenzothiophene (DBT) in hexadecane). The experiments were performed in the absence and presence of ZnO, MoO3 and MoS2. They found that 6–7 wt% of the sulfur in crude oil was removed by SCW alone. When MoS2 was added into the system, the sulfur removal increased to 12%. SCW can convert aliphatic sulphide compounds effectively. 25% DBT decomposition was observed when introducing the ZnO catalyst.43 Fedyaeva et al. studied the conversion of sulfur-rich asphaltite with Zn and Al. When Al was employed, the yield of volatile and liquid products increased from 56.3 to 98.3 wt% and the desulfurization rate increased from 20.3 to 72.3 wt%. Zn decreased the formation of H2S, converting S to ZnS.71
In order to solve the problem of metal catalyst deactivation by carbon deposition, activated carbon has attracted certain attention as a catalyst used in heavy oil upgrading.12 It was reported that the hydrocarbon–carbon system could supply hydrogen more efficiently than tetralin (conventional hydrogen source).72 One definition of the concept of “hydrogen shuttling” is given as “the promoted transfer of hydrogen between the gaseous phase and the catalyst surface through intermediate hydrogen rich solvents”.12 As a result, an alkane-rich hydrocarbon–activated carbon system could supply hydrogen for the hydrocracking of heavy oils efficiently. According to the literature, the activated carbon high surface area, variable pore structure, and surface functional groups are advantageous to prepare high quality products under mild conditions.12,73,74 The upgrading of bitumens using activated carbon catalysts was investigated by Scott in hydrogen-rich solvents under supercritical states.12 The solvents used are shown in Table 4. The operating conditions for promising results decreased to 7 MPa and 673–723 K. The yield of distillable liquids achieved was 82–88 wt%, with only a 6–8 wt% coke yield. The rates of demetallization and desulfurization were almost 100% and 80%, respectively. Tran Tan Viet et al. conducted the hydrocracking of vacuum residue in supercritical aromatic hydrocarbons (m-xylene and toluene) and normal alkane hydrocarbons (n-hexane and n-dodecane) by applying acid-treated activated carbon as a catalyst.72 The conversion of vacuum residue was in the order of n-dodecane > m-xylene > toluene > n-hexane. The reactions proceeded at 673 K and 6.89 MPa, and the partial pressure of H2 was 3.45 MPa. High residue conversion (69.2 wt%), low coke formation (13.5 wt%), and high quality light oils (13.0 wt% of naphtha, 34.9 wt% of middle distillate, 27.1 wt% of vacuum gas oil and 11.2 wt% of residue) were obtained in supercritical m-xylene with the modified activated carbon catalyst. The experiments revealed that meso-pores, macro-pores and the surface functional groups all have a significant effect on the hydrocracking of vacuum residue. Compared with the upgrading process using conventional Co/Mo catalysts, a lower hydrogen consumption and a higher pitch conversion were obtained in the process using the activated carbon catalysts. Tran et al. studied the hydrocracking of real vacuum residue from distilled vacuum units using four kinds of activated carbon catalyst in supercritical m-xylene.75 Catalyst A was activated carbon based on coal tar pitch and catalyst B was A treated with sulfuric acid. Catalyst C was activated carbon based on petroleum pitch and catalyst D was B treated with sulfuric acid. The conversion of the vacuum residue was in the order of catalyst D > catalyst B > catalyst C > catalyst A. Fe2O3, NiSO4, and LiC2H3O2 were added to modify the activated carbon catalysts. Catalyst D impregnated with 10 wt% Fe had the best catalytic effect: the conversion and the light product (naphtha and middle distillate) yield were the highest. The steric hindrance in the mesopores of the activated carbon based on petroleum pitch had a positive effect on the conversion and coke suppression in the hydrocracking of vacuum residue.
The influence of the operating conditions was studied simultaneously. The effect of temperature is the most important for the process proceeding in SCW. Hydrogen pressure has limited effect on hydrocracking and hydrogenation. Excess hydrogen cannot promote these reactions. It was confirmed that both of the activated carbons with and without modification showed excellent catalysis. The mesopore and macropore structures are effective to restrain the coke formation. Activated metal loaded carbon catalysts can enhance desulphurization and denitrification.12,72
Summarized from the previous studies, the necessary rules for obtaining high-quality products are:
(1) An activated carbon catalyst must be introduced.
(2) The solvent must be highly saturated paraffinic or naphthenic hydrocarbons.
(3) Hydrogen is necessary.
The cracking mechanism of activated carbon is significantly different from that of metallic catalysts. Cracking using activated carbon has a high selectivity to break the alkylene bridge between aromatic moieties. The bond cleavage can take place at a lower temperature than the corresponding reaction with metallic catalysts.76 Besides, the free radicals initially generated from asphaltene pyrolysis can diffuse in the mesopores and can be adsorbed on the active sites to prevent condensation.73
Reaction mechanism of the upgrading of heavy oils
Extensive amounts of literature have confirmed that asphaltene pyrolysis is the main reaction in the upgrading process of heavy oils. The process is dominated by a free radical mechanism in subcritical water and SCW. Besides, only resins undergo hydrolysis in high density SCW. However, that has limited influence on the upgrading performance.36 There are two reaction routes of asphaltene thermal cracking as shown in Fig. 2: asphaltene is decomposed to form light fractions as light oils and aggregated to form coke and char, simultaneously.When asphaltenes thermal crack in a SCF, at the beginning, they are dispersed in the solvent. And then, hydrocarbon radicals are generated by the cleavage of the aliphatic side chains and the weak bonds (C–O, C–S, C–N) connecting aromatic groups. The thermal cracking of hydrocarbons consists mainly of elementary reactions as follows: C–C cleavage, β-scission, isomerization, H-abstraction and addition to olefins, as shown in Fig. 3.77
 | ||
| Fig. 3 Key reactions involved in the upgrading of residual oil.36 | ||
Comprehensively, in mechanism 1, the aromatic radicals abstract hydrogen from hydrogen donors to become saturated. Subsequently, aromatics and saturates are generated through the random C–C bond cleavage of aliphatic substituents. In mechanism 2, the aliphatic substituents of the aromatic radicals are shortened further. The methylated aromatics and short olefins are formed through mono-molecular β-scission. The specifics depend on not only the intrinsic kinetics of the involved reactions, but also on their mass transfer environment. The mass transfer environment has the most significant influence on which dealkylation reaction aromatic radicals form.34
With the progression of the upgrading process, the radicals are inclined to recombine together to form coke preferentially. The coke formed by the aggregation and polymerization decreases the conversion of the feedstocks and the quality of the products, and limits the operability and efficiency of the upgrading process. Towfighi et al. concluded that coke was formed through radical propagation reactions on the active sites of the catalyst, asphaltene recombination occurs intermolecularly, and that activated asphaltene cores react with small molecular radicals.78 According to theoretical calculations, methylated aromatics and short olefins have a high activity for coke production.77–83 Rahmani et al. found that the yield of coke was strongly influenced by the hydrogen offering ability of the solvent and the hydrogen capturing ability of the asphaltenes.84
In order to clarify the mechanism of coke formation in SCFs, Watanabe et al. observed the coke formed during asphaltene pyrolysis in the absence and presence of SCW through scanning electron microscopy (SEM).13 The major part of the coke formed in the absence of SCW had a coalescent structure that was made of small coke particles, while that formed in the presence of SCW had a porous structure, as shown in Fig. 4.
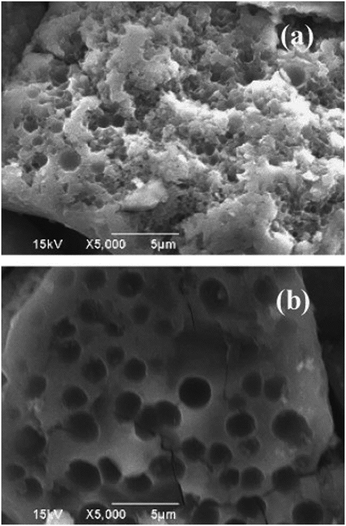 | ||
| Fig. 4 SEM images of coke obtained after 15 min of reaction ((a): pyrolysis without water and (b): pyrolysis in the presence of SCW at 200 kg m−3 water density).13 | ||
The pores were formed by the light fractions dissolving into the asphaltene cores. According to a previous study, Sato et al. assumed that there existed a water rich phase and an oil rich phase in the reaction system.85 The mechanism of coke formation during the upgrading of heavy oils in SCW can be elucidated as follows: when the reaction is proceeding, the lighter fractions are extracted into the water rich phase and their concentration decreases in the oil rich phase, while the concentration of heavier fractions in the oil phase increases. In the water rich phase, the lighter fractions can be cracked further to then react with small radicals to form light products rather than aggregate. When heavy fractions in the oil rich phase exceed the solubility, the heavy fractions are inclined to aggregate together to form coke because of their higher concentration and lower amount of small radicals.
The chemical effect of SCW on the heavy oil upgrading process
Although SCW is the most common solvent studied as the heavy oil upgrading medium, the role of SCW is still not clear. It is confirmed that SCW has a high solubility for heavy oils in order to form an approximately homogeneous phase for the proceeding reaction. But there still exists controversy as to whether the SCW participates in the reaction as a hydrogen donor.To understand the role of SCW, many researchers have studied several model compound reactions with SCW. Benzyl phenyl ether (BPE), quinoline and dibenzylsulphide (DBS) were chosen as model compounds to define the chemical effects of SCW on the pyrolysis of heavy oils.33 The main products obtained from the BPE, quinoline and DBS pyrolysis in SCW are shown in Table 5. The products containing O atoms show the portion of free radicals generated when the model compounds capture H˙ or OH˙ from water and transform into light aromatic hydrocarbons such as phenol and aromatic aldehydes. In other words, SCW acts as a reactant to supply hydrogen and oxygen atoms for the pyrolysis of model compounds.
| BPE | Quinoline | DBS | |||
|---|---|---|---|---|---|
| Compound | Formula | Compound | Formula | Compound | Formula |
| Toluene | C7H8 | Toluene | C7H8 | Toluene | C7H8 |
| Ethylbenzene | C8H10 | Ethylbenzene | C8H10 | Ethylbenzene | C8H10 |
| Xylene | C8H10 | Xylene | C8H10 | Xylene | C8H10 |
| Benzaldehyde | C7H6O | Phenol | C6H6O | Benzaldehyde | C7H6O |
| Phenol | C6H6O | Dimethylbenzenamine | C8H11N | Diphenyl | C12H10 |
| Diphenylmethane | C13H12 | Tetrahydro-quinoline | C9H11N | Diphenylmethane | C13H12 |
Kida et al. studied the decomposition of hexyl sulphide with and without SCW. The products were dramatically different. Hexane, isomers of hexene and hexanethiol were detected in both systems, meaning that free radical reactions were dominating. However, pentane and CO + CO2 were only detected in the products of the SCW system. Similar experimental results were obtained from using other linear di-n-alkyl sulphides. The experimental results and quantum calculations all showed that Cm thioaldehydes were hydrolysed to form geminal mercaptoalcohols. Then, the mercaptoalcohols were catalysed by water to form aldehydes and H2S. The H atom of H2S was supplied by H2O. It can be concluded that water behaves as a reactant in the hydrolysis of thioaldehydes and as a H donor for H2S formation.86
However, an opposite conclusion was obtained by Xu.87 He selected p-benzoquinone, naphthalene and azobenzene as probes to verify the hydrogen donation mechanism of SCW. The experimental results showed that the products of p-benzoquinone and naphthalene hydrogenation were not detected. Besides, the radical initiator azobenzene cannot abstract H˙ or HO˙ from SCW. D2O and H218O were used as tracers to clarify the role of SCW during the cracking of polyethylene. The results showed that SCW is inclined to be an inert reaction medium for the free radical mechanism depending on pyrolysis.88,89 Calculations based on density functional theory showed, from the viewpoint of thermodynamics, that the H-abstraction between hydrocarbon radicals and SCW is impossible.90
Morimoto et al. analysed the properties and composition of the upgraded products of oil sand bitumen, comprehensively.38 The products were prepared in SCW, supercritical toluene (a radical capping medium) and N2 (an inert medium). The products included gas, middle distillate, distillation residue and coke. The composition and properties of middle distillate were almost the same in the SCW and N2 systems, but different from that prepared in supercritical toluene. The high solubility of SCW for the heavier fractions caused a difference in the product yield between the middle distillate formed in SCW and in N2. Moreover, the distillation residue formed in SCW had a lower molecular weight distribution, a smaller number of units, a larger number of aromatic rings and shorter side chains, compared with the distillation residue formed in N2.
These results suggest that the hydrocarbons were chiefly dehydrogenated intramolecularly in SCW. The coke formed in SCW had a lower H/C ratio and a higher aromaticity, which indicates that the heavy oils were decomposed in an approximately homogeneous phase. It can be concluded that the high dispersion of heavy fractions in SCW promotes the intramolecular dehydrogenation and suppresses the recombination of heavier components, simultaneously. According to previous research, some conclusions can be summarized as follows.
(1) SCW is not expected to provide H˙ and HO˙ radicals for hydrocarbon hydrogenation.
(2) Some of the reaction behaviour of SCW may be caused by residual O2 in the system. The partial oxidation of hydrocarbons or heavy oils and the water–gas shift reaction (WGSR) produce the hydrogen for hydrogenation.
(3) Free radicals generated from the pyrolysis of hydrocarbons are not expected to capture H˙ and couple with HO˙ from the SCW.
(4) In SCW, some detected oxygen-containing compounds can be attributed to the ionic effect in the reaction.
The diversity in the transportation properties between an inert atmosphere and SCW results in the different reaction behaviours of the carbonaceous materials.
The physical effect of SCW during the heavy oil upgrading process
According to former research, it has been suggested that the enhanced degree of heavy oil upgrading in SCW is attributable to the physical properties of SCW: the solvent effects and the dispersion effects.39The conventional pyrolysis of heavy oils is a multiphase reaction. The heat and mass transfer resistance during the reaction would limit the hydrogen transfer from the hydrogen donor to the free radicals, promoting the condensation of the radicals. Compared with the conventional process, the upgrading of heavy oils in SCFs can apparently restrain the coke formation. The solvent effects make the reaction proceed in a homogeneous phase and the dispersion effects promote the mass transfer of radicals in SCFs.37 Furthermore, a phenomenon called “cage effect” is favorable to suppress the coke formation.53 The light fractions and unreacted oil can be mixed with water as a solvent to dilute the coke precursors. The heavy fractions are surrounded by a cage formed of solvent molecules. The heavy fractions are insulated by the cage effect, which only allows small radicals to pass through and limits the heavy fraction reactions with each other to inhibit the coke formation. The cage effect can be enhanced by increasing the water density.
The solubility of heavy oils can be increased by strengthening the mass transfer. Vilcáez et al. tested the continuous hydrothermal extraction of bitumen in subcritical water with a column flow reactor.91 The study was carried out at 573 K, 3–6 MPa, with a water flow of 3–10 g min−1. Fig. 5 reveals that the modification of bitumen and the suppression of coke formation were achieved simultaneously.
 | ||
| Fig. 5 Comparison of the fractions of maltene, asphaltene and coke in: raw bitumen (A), bitumen treated in a visible-type autoclave reactor at 613 K and 9 MPa (B), residues of bitumen treated in the column flow reactor (C1), and bitumen extracted from the column flow reactor at 613 K and 13 MPa (C2).91 | ||
An asphaltene core was formed from unreacted asphaltene and the by-products, which were unreactive and could not be dissolved in heptane.92 Flowing water can enhance the mass transfer of the asphaltene core in both the water and oil phases, which causes more asphaltene core to be extracted from the oil rich phase into the water rich phase. The asphaltene core left in the oil rich phase is insufficient to combine together to form coke, and that extracted into the water rich phase is inclined to capture small radicals, which are rich in the water phase, to form maltene. Meanwhile, the process can proceed under much milder conditions than that proceeding in a batch reactor. Liu et al. found that in a continuous water phase, the upgrading proceeded in a pseudo single-phase so that the coke formation was suppressed effectively.36 A study of bitumen conversion (gross-formula CH1.47N0.01S0.007) with SCW in a vertically aligned tubular reactor was conducted.93 The bitumen and SCW were supplied at the top and bottom of the reactor, respectively, at 673 K, 30 MPa. The low temperature of the top area of the reactor was insufficient for bitumen to form coke. When the bitumen flowed down, part of it was converted into light hydrocarbons, which were removed by a SCW counter-current flow from the reactor. Heavy bitumen components deposited at the bottom of the reactor were converted in SCW into lighter hydrocarbons by increasing the temperature. The yield of liquid products was 73.3 wt%, and among them, 45.4 wt% liquid was formed at 673–773 K. Furthermore, oils were the major product of the liquid. According to former research, the coke can be burnt by introducing an oxidant to the SCW, which occurs rather than the interruption of the conversion of bitumen.94,95
Conclusion and future research topics
Fossil resources, especially petroleum, will continue to play a crucial role in the global energy mix for many years. The use of heavy crude oils and residues causes many refining and environmental problems. It is evident that heavy and extra-heavy oils can be modified in SCFs. The approximately homogeneous reaction environment can reduce the mass and heat transfer resistance. More light fractions and less coke can be obtained than with conventional processes. The viscosity, and the content of heteroatoms, of the light products decrease simultaneously. SCFs can also supply a reaction medium for the hydrogen donor, promoting the production and transfer of hydrogen, reducing hydrogen consumption. The upgraded products of heavy oils can be further modified as feedstock, some of which can even be used as fuel or high quality fuel additives.However, the intrinsic chemical mechanisms are incompletely understood and the question is hard to solve as the relevant physicochemical properties vary from liquid-like to gas-like. Theoretical studies to express the complicated mixed state of SCFs and heavy oils are necessary to understand the reaction mechanism and to predict the optimized operating conditions. The content of heteroatoms in the light products is still too high to be qualified. A high activity and stability, desulfurization and hydrogenation catalyst should be developed. Selecting a new liquid with a hydrogen donor capacity and low critical points, without corrosion, will have a significant effect on the industrial application of the technology. In line with this, a new kind of continuous reactor that can promote mass transfer is the key to improving the quality of the products and application in industry.
Acknowledgements
The authors would like to acknowledge the following financial supports: PetroChina Innovation Foundation (2014D-5006-0401), National Natural Science Foundation of China (No. 21376186), the Ministry of Education (Doctoral Special Research Foundation No. 20110201110032) China, Fundamental Research Funds for the Central Universities (New Teacher Research Support Plan No. 08141002, International Cooperation Project No. 2011jdhz37 and Integrated Cross Project xjj2014136 in Xi’an Jiaotong University), Natural Science Basic Research Plan in Shaanxi Province of China (No. 2012JM2010) and Sci. & Tech. Project for Overseas Scholars (the Ministry of Human Resources and Social Security of China, No. 19900001).References
- H. Alboudwarej, J. Felix, S. Taylor, R. Badry, C. Bremner, B. Brough, C. Skeates, A. Baker, D. Palmer and K. Pattison, Oilfield Rev., 2006, 18, 34–53 Search PubMed.
- M. S. Rana, V. Samano, J. Ancheyta and J. Diaz, Fuel, 2007, 86, 1216–1231 CrossRef CAS.
- C.-Z. Li and P. F. Nelson, Energy Fuels, 1996, 10, 1083–1090 CrossRef CAS.
- D. Velegol, M. Gautam and A. Shamsi, Powder Technol., 1997, 93, 93–100 CrossRef CAS.
- S. Ebrahimi, J. Moghaddas and M. R. Aghjeh, Fuel, 2008, 87, 1623–1627 CrossRef CAS.
- Z. Gu, N. Chang, X. Hou, J. Wang and Z. Liu, Fuel, 2012, 91, 33–39 CrossRef CAS.
- Ö. Gül, L. R. Rudnick and H. H. Schobert, Energy Fuels, 2006, 20, 2478–2485 CrossRef.
- X. Chu, W. Li, B. Li and H. Chen, Fuel, 2008, 87, 211–215 CrossRef CAS.
- H. Machida, M. Takesue and R. L. Smith, J. Supercrit. Fluids, 2011, 60, 2–15 CrossRef CAS.
- I. V. Kozhevnikov, A. L. Nuzhdin and O. N. Martyanov, J. Supercrit. Fluids, 2010, 55, 217–222 CrossRef CAS.
- L. Han, R. Zhang, J. Bi and L. Cheng, J. Anal. Appl. Pyrolysis, 2011, 91, 281–287 CrossRef CAS.
- D. Scott, D. Radlein, J. Piskorz, P. Majerski and T. J. W. deBruijn, Fuel, 2001, 80, 1087–1099 CrossRef CAS.
- M. Watanabe, S.-N. Kato, S. Ishizeki, H. Inomata and R. L. Smith, J. Supercrit. Fluids, 2010, 53, 48–52 CrossRef CAS.
- C. Ximing, Fuel & Chemical Processes, 2004, 4, 024 Search PubMed.
- S. Rudyk and P. Spirov, Appl. Energy, 2014, 113, 1397–1404 CrossRef CAS.
- Y.-H. Ding, C. Hang, D.-F. Wang, J.-F. Wang, D.-P. Xu and Y.-G. Wang, J. Fuel Chem. Technol., 2010, 38, 140–143 CrossRef CAS.
- C.-X. Ma, R. Zhang and J.-C. Bi, J. Fuel Chem. Technol., 2003, 31, 103–110 CAS.
- C. Zhang, H. Zhang, J. Yang and Z. Liu, Polym. Degrad. Stab., 2004, 86, 461–466 CrossRef CAS.
- C. Zhang, L. Xu, H. Zhang, J. Yang, J. Du and Z. Liu, J. Chromatogr. A, 2004, 1055, 115–121 CrossRef CAS PubMed.
- M. Genta, M. Goto and M. Sasaki, J. Supercrit. Fluids, 2010, 52, 266–275 CrossRef CAS.
- L. Chen, Y.-Q. Wu, Y.-H. Ni and Z.-B. Zhu, J. Chem. Eng. Chin. Univ., 2004, 18, 585–589 CAS.
- W. Li, C. Pan, L. Sheng, Z. Liu, P. Chen, H. Lou and X. Zheng, Bioresour. Technol., 2011, 102, 9223–9228 CrossRef CAS PubMed.
- D. Zeng, Y. Li and T. Fang, Adv. Mater. Res., 2012, 560, 555–562 CrossRef.
- T. Fang, Wahyudiono, B. Al-Duri, Y. Shimoyama, Y. Iwai, M. Sasaki and M. Goto, Ind. Eng. Chem. Res., 2007, 46, 5325–5332 CrossRef CAS.
- X. He, T. Li, K. Wang, J. Qin and H. Zhang, Coal Conversion, 2011, 34, 59–63 CAS.
- P. C. Mandal, Wahyudiono, M. Sasaki and M. Goto, Fuel Process. Technol., 2013, 106, 641–644 CrossRef CAS.
- C.-X. Ma, Master, Institute of Coal Chemistry, Chinese Academy of Science, 2002.
- P.-Q. Yuan, Z.-M. Cheng, W.-L. Jiang, R. Zhang and W.-K. Yuan, J. Supercrit. Fluids, 2005, 35, 70–75 CrossRef CAS.
- P. R. Patwardhan, M. T. Timko, C. A. Class, R. E. Bonomi, Y. Kida, H. H. Hernandez, J. W. Tester and W. H. Green, Energy Fuels, 2013, 27, 6108–6117 CrossRef CAS.
- M. Sasaki and M. Goto, Polym. Degrad. Stab., 2008, 93, 1194–1204 CrossRef.
- A. Kishita, S. Takahashi, H. Kamimura, M. Miki, T. Moriya and H. Enomoto, J. Jpn. Pet. Inst., 2003, 46, 215–221 CrossRef CAS.
- L.-N. Han, R. Zhang and J.-C. Bi, J. Fuel Chem. Technol., 2008, 36, 1–5 CrossRef.
- L. Han, R. Zhang and J. Bi, Fuel Process. Technol., 2009, 90, 292–300 CrossRef CAS.
- X.-C. Tan, C.-C. Zhu, Q.-K. Liu, T.-Y. Ma, P.-Q. Yuan, Z.-M. Cheng and W.-K. Yuan, Fuel Process. Technol., 2014, 118, 49–54 CrossRef CAS.
- F. Bai, C.-C. Zhu, Y. Liu, P.-Q. Yuan, Z.-M. Cheng and W.-K. Yuan, Fuel Process. Technol., 2013, 106, 267–274 CrossRef CAS.
- Y. Liu, F. Bai, C.-C. Zhu, P.-Q. Yuan, Z.-M. Cheng and W.-K. Yuan, Fuel Process. Technol., 2013, 106, 281–288 CrossRef CAS.
- R. O. Canıaz and C. Erkey, Chem. Eng. Res. Des., 2014, 92, 1845–1863 CrossRef.
- M. Morimoto, Y. Sugimoto, Y. Saotome, S. Sato and T. Takanohashi, J. Supercrit. Fluids, 2010, 55, 223–231 CrossRef CAS.
- Z.-M. Cheng, Y. Ding, L.-Q. Zhao, P.-Q. Yuan and W.-K. Yuan, Energy Fuels, 2009, 23, 3178–3183 CrossRef CAS.
- C. N. G. Z. H. Xiongpo, L. Zongkuan and W. Zanshe, Coal Conversion, 2010, 2, 012 Search PubMed.
- N. Li, B. Yan, L. Zhang, S.-X. Quan, C. Hu and X.-M. Xiao, J. Supercrit. Fluids, 2015, 97, 116–124 CrossRef CAS.
- T. Sato, S. Kurosawa, R. L. Smith, T. Adschiri and K. Arai, J. Supercrit. Fluids, 2004, 29, 113–119 CrossRef CAS.
- A. Ates, G. Azimi, K.-H. Choi, W. H. Green and M. T. Timko, Appl. Catal., B, 2014, 147, 144–155 CrossRef CAS.
- T. Adschiri, R. Shibata, T. Sato, M. Watanabe and K. Arai, Ind. Eng. Chem. Res., 1998, 37, 2634–2638 CrossRef CAS.
- T. Sato, T. Tomita, P. H. Trung, N. Itoh, S. Sato and T. Takanohashi, Energy Fuels, 2013, 27, 646–653 CrossRef CAS.
- K. Arai, T. Adschiri and M. Watanabe, Ind. Eng. Chem. Res., 2000, 39, 4697–4701 CrossRef CAS.
- J. Cheng, Y.-H. Liu, Y.-H. Luo, G.-X. Liu and G.-H. Que, J. Fuel Chem. Technol., 2003, 31, 574–578 CAS.
- T. Sato, M. Watanabe, R. L. Smith, T. Adschiri and K. Arai, J. Supercrit. Fluids, 2004, 28, 69–77 CrossRef CAS.
- F. Jin, X. Zeng, J. Cao, K. Kawasaki, A. Kishita, K. Tohji and H. Enomoto, Fuel, 2010, 89, 2448–2454 CrossRef CAS.
- U. Armbruster, A. Martin and A. Krepel, J. Supercrit. Fluids, 2001, 21, 233–243 CrossRef CAS.
- J. H. Lee and N. R. Foster, J. Supercrit. Fluids, 1996, 9, 99–105 CrossRef CAS.
- M. Watanabe, M. Mochiduki, S. Sawamoto, T. Adschiri and K. Arai, J. Supercrit. Fluids, 2001, 20, 257–266 CrossRef CAS.
- T. Sato, J. Jpn. Pet. Inst., 2014, 57, 1–10 CrossRef CAS.
- T. Sato, T. Adschiri, K. Arai, G. L. Rempel and F. T. T. Ng, Fuel, 2003, 82, 1231–1239 CrossRef CAS.
- T. Adschiri, K. Kanazawa and K. Arai, J. Am. Ceram. Soc., 1992, 75, 2615–2618 CrossRef CAS.
- T. Adschiri, K. Kanazawa and K. Arai, J. Am. Ceram. Soc., 1992, 75, 1019–1022 CrossRef CAS.
- T. M. Yeh, J. G. Dickinson, A. Franck, S. Linic, L. T. Thompson and P. E. Savage, J. Chem. Technol. Biotechnol., 2013, 88, 13–24 CrossRef CAS.
- P. E. Savage, J. Supercrit. Fluids, 2009, 47, 407–414 CrossRef CAS.
- B. Vogelaar, M. Makkee and J. Moulijn, Fuel Process. Technol., 1999, 61, 265–277 CrossRef CAS.
- T. Adschiri, T. Sato, H. Shibuichi, Z. Fang, S. Okazaki and K. Arai, Fuel, 2000, 79, 243–248 CrossRef CAS.
- D. C. Elliott, M. Phelps, L. J. Sealock Jr and E. G. Baker, Ind. Eng. Chem. Res., 1994, 33, 566–574 CrossRef CAS.
- P. Azadi and R. Farnood, Int. J. Hydrogen Energy, 2011, 36, 9529–9541 CrossRef CAS.
- Z. Y. Ding, M. A. Frisch, L. Li and E. F. Gloyna, Ind. Eng. Chem. Res., 1996, 35, 3257–3279 CrossRef CAS.
- B. Dou, J. Gao, X. Sha and S. W. Baek, Appl. Therm. Eng., 2003, 23, 2229–2239 CrossRef CAS.
- H. Yasuda, T. Sato and Y. Yoshimura, Catal. Today, 1999, 50, 63–71 CrossRef CAS.
- L. McLaughlin, E. Novakova, R. Burch and C. Hardacre, Appl. Catal., A, 2008, 340, 162–168 CrossRef CAS.
- J. Ren, A. Wang, X. Li, Y. Chen, H. Liu and Y. Hu, Appl. Catal., A, 2008, 344, 175–182 CrossRef CAS.
- H. Zhang, X. Meng, Y. Li and Y. Lin, Ind. Eng. Chem. Res., 2007, 46, 4186–4192 CrossRef CAS.
- F. Zhou, X. Li, A. Wang, L. Wang, X. Yang and Y. Hu, Catal. Today, 2010, 150, 218–223 CrossRef CAS.
- J. Zheng, M. Guo and C. Song, Fuel Process. Technol., 2008, 89, 467–474 CrossRef CAS.
- O. N. Fedyaeva, V. R. Antipenko and A. A. Vostrikov, J. Supercrit. Fluids, 2014, 88, 105–116 CrossRef CAS.
- T. T. Viet, J.-H. Lee, J. W. Ryu, I.-S. Ahn and C.-H. Lee, Fuel, 2012, 94, 556–562 CrossRef CAS.
- H. Fukuyama and S. Terai, Pet. Sci. Technol., 2007, 25, 231–240 CrossRef CAS.
- C. Xu, S. Hamilton, A. Mallik and M. Ghosh, Energy Fuels, 2007, 21, 3490–3498 CrossRef CAS.
- T. T. Viet, J. H. Lee, F. Ma, G. R. Kim, I. S. Ahn and C. H. Lee, Fuel, 2013, 103, 553–561 CrossRef CAS.
- M. Farcasiu and C. Smith, Energy Fuels, 1991, 5, 83–87 CrossRef CAS.
- P.-Q. Yuan, C.-C. Zhu, Y. Liu, F. Bai, Z.-M. Cheng and W.-K. Yuan, J. Supercrit. Fluids, 2011, 58, 93–98 CrossRef CAS.
- J. Towfighi, M. Sadrameli and A. Niaei, J. Chem. Eng. Jpn., 2002, 35, 923–937 CrossRef.
- V. van Speybroeck, D. van Neck, M. Waroquier, S. Wauters, M. Saeys and G. Marin, Int. J. Quantum Chem., 2003, 91, 384–388 CrossRef CAS.
- V. van Speybroeck, K. Hemelsoet, M. Waroquier and G. Marin, Int. J. Quantum Chem., 2004, 96, 568–576 CrossRef CAS.
- Y. Xiao, J. Longo, G. Hieshima and R. Hill, Ind. Eng. Chem. Res., 1997, 36, 4033–4040 CrossRef CAS.
- V. van Speybroeck, D. van Neck, M. Waroquier, S. Wauters, M. Saeys and G. Marin, J. Phys. Chem. A, 2000, 104, 10939–10950 CrossRef CAS.
- M. K. Sabbe, A. G. Vandeputte, M.-F. Reyniers, V. Van Speybroeck, M. Waroquier and G. B. Marin, J. Phys. Chem. A, 2007, 111, 8416–8428 CrossRef CAS PubMed.
- S. Rahmani, W. McCaffrey, J. A. Elliott and M. R. Gray, Ind. Eng. Chem. Res., 2003, 42, 4101–4108 CrossRef CAS.
- T. Sato, S. Mori, M. Watanabe, M. Sasaki and N. Itoh, J. Supercrit. Fluids, 2010, 55, 232–240 CrossRef CAS.
- Y. Kida, C. A. Class, A. J. Concepcion, M. T. Timko and W. H. Green, Phys. Chem. Chem. Phys., 2014, 16, 9220–9228 RSC.
- T. Xu, Master, Beijing University of Chemical Technology, 2012.
- T. Moriya and H. Enomoto, Kagaku Kogaku Ronbunshu, 1999, 25, 940–946 CrossRef CAS.
- T. Moriya and H. Enomoto, Jpn. J. Polym. Sci. Technol., 2001, 58, 661–673 CAS.
- C.-C. Zhu, C. Ren, X.-C. Tan, G. Chen, P.-Q. Yuan, Z.-M. Cheng and W.-K. Yuan, Fuel Process. Technol., 2013, 111, 111–117 CrossRef CAS.
- J. Vilcáez, M. Watanabe, N. Watanabe, A. Kishita and T. Adschiri, Fuel, 2012, 102, 379–385 CrossRef.
- I. A. Wiehe, Ind. Eng. Chem. Res., 1993, 32, 2447–2454 CrossRef CAS.
- O. N. Fedyaeva, A. V. Shatrova and A. A. Vostrikov, J. Supercrit. Fluids, 2014, 95, 437–443 CrossRef CAS.
- A. A. Vostrikov, O. N. Fedyaeva, D. Y. Dubov, S. A. Psarov and M. Y. Sokol, Energy, 2011, 36, 1948–1955 CrossRef CAS.
- A. Vostrikov, O. Fedyaeva, A. Shishkin and M. Y. Sokol, Combust., Explos. Shock Waves, 2014, 50, 242–244 CrossRef.
| This journal is © The Royal Society of Chemistry 2015 |







