Design, synthesis and antifungal activity of carboxylic acid amide fungicides: part 2: substituted 1-phenyl-2-phenoxyethylamino valinamide carbamates†‡
Abstract
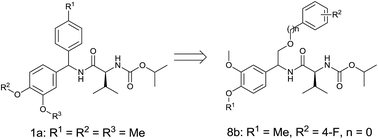
Valinamide carbamates are important agricultural fungicides with anti-oomycete activity and low toxicity toward mammalian cells. To find valinamide carbamates with high activity against resistant pathogens, a series of substituted 1-phenyl-2-phenoxyethylamino derivatives were designed and synthesized by introducing substituted phenyloxy or benzyloxy into valinamide carbamate leads. Bioassays showed that many title compounds exhibited good anti-oomycete activity. Compound 8b had much higher in vitro fungicidal activity against Phytophthora capsici than the control compound iprovalicarb and had a similar potency to the control mandipropamid. At 6.25 μg mL−1 the fungicidal activities of many compounds against in vivo Pseudoperonospora cubensis were greater than 30%, while the control dimethomorph gave only 27% activity at this concentration. The effect of 8b against Pseudoperonospora cubensis in the field indicates promise for a new candidate for controlling cucumber downy mildew.

 Please wait while we load your content...
Please wait while we load your content...