Relating elasticity and graphene folding conformation
Abstract
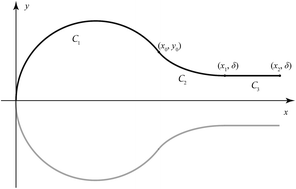
Variational calculus is employed to determine the folding behaviour of a single graphene sheet. Both the elastic and van der Waals energies are taken into account, and from these considerations the shape of the curve is determined. By prescribing that the separation distance between the folded graphene in the parallel region is 3.32 Å, an arbitrary constant arising by integrating the Euler–Lagrange equation is determined, and the full parametric representations for the folding conformation are derived. Using typical values of the bending rigidity in the range of 0.800–1.60 eV, the shortest stable folded graphene sheets are required to be at least 6.5–10 nm in length.

 Please wait while we load your content...
Please wait while we load your content...