DOI:
10.1039/C5RA08129G
(Paper)
RSC Adv., 2015,
5, 57082-57089
Polyethylenimine/grapefruit peel hybrid biosorbent for the removal of toxic CdTe quantum dots from water†
Received
3rd May 2015
, Accepted 16th June 2015
First published on 16th June 2015
Abstract
The use of engineered nanoparticles would increase their release into wastewater treatment plants, leading to the risk of contaminating water and ultimately causing environmental and health concerns. The removal of such nanoparticles from wastewater is highly needed. In this text, the polyethyleneimine (PEI)-decorated grapefruit peel (GP) composite adsorbent was facilely prepared for this purpose, which was based on PEI grafting onto the GP through a simple one-step reaction. The as-prepared PEI–GP adsorbent was characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR) and X-ray photoelectron spectrometry (XPS), showing that PEI was successfully grafted onto GP. The as-prepared PEI–GP adsorbents were used for the removal of CdTe quantum dots (QDs). Batch adsorption experiments were performed to investigate the influences of pH, contact time, initial CdTe QDs concentration, ionic strength and humic acid concentration. The results show that the adsorption kinetics could be fitted well to the pseudo-second-order model and the adsorption process could be described well by the Freundlich adsorption isotherm. The ionic strength caused a significant effect on the adsorption of CdTe QDs onto the PEI–GP adsorbents due to aggregation of the CdTe QDs in the presence of salt. The presence of humic acid had a minor effect on the sequestration of CdTe QDs by the PEI–GP biosorbents. The results indicate that the PEI–GP composite can be used as a promising adsorbent for the removal of CdTe QDs from aqueous media.
1. Introduction
Quantum dots (QDs) are particularly interesting nanoparticles (NPs) owing to their tunable optical properties.1,2 They have a size of about 2–10 nm in diameter. In particular, cadmium-telluride (CdTe) QDs as semiconductor nanocrystals are gradually showing unimaginable commercial potential; being used in sensor development,3–5 therapy of cancer,6 food analysis2,7 and biochemical analysis.8–10 However, due to the toxicity of CdTe QDs,11,12 usage of such tiny NPs would increase the release of them into wastewater treatment plants, leading to the risk of contaminating water and ultimately causing environmental and health concerns.13 For instance, previous studies have demonstrated that CdTe QDs could easily enter the living body through skin contact or the respiratory system, causing oxidative stress, DNA damage, cell inflammation or damage and even death.14,15 Thus, the removal of such NPs from wastewater is urgently needed, and it is important to design functional materials capable of removing these NPs from the environment. However, approaches to the latter problem have not been well developed until now.
On the other hand, coagulation has been considered as a promising technology for the removal of various pollutants from contaminated waters due to their low cost, the ease with which they can be chemically modified for increased efficiency, and their environmentally friendly characteristics.16–21 This provides a potential way to remove engineered NPs. However, only a limited effort has been devoted to investigate the removal of engineered NPs by coagulation. Up to now, rare bioadsorbents have been evaluated for this purpose. For example, Khan et al. illustrated that the resistant bacterial species Aeromonas punctata can be used for the effective removal of silver NPs.22 Valiyaveettil’s group reported that chitosan coated cellulose nanofibers (isolated from raw sugarcane bagasse by treatment with bleach and alkali solution) were highly efficient in extracting Au and Ag NPs.23 These studies demonstrated that the adsorption of engineered NPs onto bioadsorbents is an economical and efficient method for their removal. However, the related studies received little attention to date. To our knowledge, there have been no reports thus far regarding the utilization of bioadsorbents for the removal of toxic CdTe QDs from contaminated water.
Here, we report results of a polyethyleneimine (PEI)-decorated grapefruit peel (GP) composite adsorbent for extracting CdTe QDs from the aqueous solution. The composite adsorbent was facilely prepared by grafting PEI onto the GP through a simple one-step reaction. Grapefruit peel was employed as the host bioadsorbent, mainly because it was a readily available agricultural waste product, a sustainable material containing several water soluble and insoluble monomers and polymers,24 and rich in carboxyl and hydroxyl functional groups (alcohols, phenols, and carboxylic acids) as in pectin, cellulose and lignin.24 Branched polyethyleneimine (PEI) promises good metal chelation characteristics due to the presence of electron-rich amine groups in the molecule.25 Thus, it was selected as the functionalized reagent and can be grafted on GP via an amidation reaction to obtain a PEI–GP composite adsorbent for the immobilization of CdTe QDs. The functionalization process of GP with PEI is simple and easy, and does not require a toxic linking agent such as glutaraldehyde.
2. Experimental materials and methods
2.1. Chemicals and reagents
Sodium tetraborate (NaB4O7), citric acid (C6H8O7·H2O) and CdCl2·2.5H2O were purchased from Chongqing Taixin Chemical Reagents Company (Chongqing, China). Sodium tellurite (Na2TeO3) and mercaptosuccinic acid (MSA, C4H6O4S) were purchased from Aladdin (Beijing, China). Branched polyethyleneimine (PEI, with molecular weight of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was purchased from Sigma-Aldrich (Shanghai, China). The pH of the solution was adjusted by using 0.1 M NaOH or 0.1 M HCl. All the chemicals were of analytical grade and all the solutions were prepared with ultra pure water.
000) was purchased from Sigma-Aldrich (Shanghai, China). The pH of the solution was adjusted by using 0.1 M NaOH or 0.1 M HCl. All the chemicals were of analytical grade and all the solutions were prepared with ultra pure water.
2.2. Synthesis of PEI grafted GP (PEI–GP)
Grapefruits were purchased from a local market. After thorough washing with pure water, the grapefruit peel was cut into small pieces by scissors. After the GP was dried at 50 °C overnight, it was ground to a powder. For the preparation of PEI–GP, 1.0 g of GP was immersed in 150 mL of methanol solution with different concentration of PEI for 4 h under shaking at 60 °C. Then, the mixture was stirred overnight at room temperature. Finally, the as-prepared products were isolated and washed with hot water several times and dried at 50 °C for 12 h in a vacuum oven. In this work, different levels of PEI (0.2, 0.4, 0.6, 0.8, 1.0 g L−1) were selected to vary the PEI content. The obtained products of PEI–GP were marked as PEI–GP-0.2, PEI–GP-0.4, PEI–GP-0.6, PEI–GP-0.8 and PEI–GP-1.0, respectively.
2.3. Preparation of MSA-capped CdTe QDs
CdTe QDs were synthesized according to the method reported by Ying et al.26 with a modification. Briefly, 200 mL of 15 mM Na2B4O7–citrate acid buffer solution (pH 7.0) was transferred into a suitable three-neck flask, then 0.0457 g CdCl2·2.5H2O, 0.011 g Na2TeO3 and 0.090 g MSA were added and mixed well. After vigorously stirring for 5 min, 230 mg of solid potassium borohydride was added quickly to the above mixture. The resulting solution was stirred for another 5 min at room temperature, then heated to 100 °C and refluxed for 1.5 h under open-air conditions. After cooling to room temperature naturally, the as-prepared CdTe QDs were stored in a refrigerator. The concentration of the as-prepared CdTe QDs was 500 mg L−1.26
2.4. Instrumentation
UV-visible (UV-vis) measurements were taken on a UV-2450 Shimadzu spectrophotometer (Suzhou, China). Scanning electron microscopy (SEM) images were taken on a Hitachi model S4800 field emission scanning electron microscope (Hitachi, Tokyo, Japan). The solution pH was detected by a PHS-3D pH meter (Shanghai Precision Scientific Instruments Co., Ltd., China). Fourier transform infrared (FT-IR) spectra were recorded on a Nicolet 170SX instrument (Madison, WI) in the transmission mode using KBr pellets of the sample. An XSAM800 X-ray photoelectron spectrometer (Kratos, Manchester, Britain) was used to carry out the surface analysis of the as-prepared PEI–GP adsorbent. Dynamic light scattering (DLS) determination was carried out on a Zetasizer Nano-ZS90 (Malvern, UK). A XS-105 Mettler Toledo analytical balance (Mettler-Toledo, Switzerland) was used to accurately weigh the amount of the PEI–GP biosorbents.
2.5. Batch experiments
The adsorption experiments were carried out in a series of 100 mL stoppered conical flasks, each of which contained 25 mL of CdTe QDs solution. 5 mg of the PEI–GP biosorbent was added to each flask and shaken at 180 rpm in a thermostatic shaker. To examine the influence of pH on the CdTe QDs adsorption, the solution pH was adjusted in the range of 6.0–11.0 by 0.1 M NaOH or 0.1 M HCl. After adsorption, the solid phase was filtered using a 0.22 μm membrane, then the concentration of the resulting CdTe QDs was determined using a UV-vis spectrophotometer. The amount of CdTe QDs adsorbed per unit mass of the adsorbent was calculated from the following equation:
where qt (mg g−1) is the amount of CdTe QDs adsorbed per gram of adsorbent at time t (h), C0 and Ct are the initial CdTe QDs concentration and the CdTe QDs concentration at time t in the solution (mg L−1), m is the mass of the as-prepared PEI–GP adsorbents used in the experiments (g) and V is the volume of CdTe QDs solution (L). Note that all experiments were performed in triplicate and the data were recorded as a mean.
2.6. Adsorption kinetics and isotherms
Adsorption kinetics experiments were carried out by adding 5 mg of the PEI–GP adsorbent into a series of 100 mL stoppered conical flasks containing 25 mL of CdTe QDs solution (pH = 8.4) with a contact time ranging from 0 to 24 h. The flasks were shaken at 180 rpm and 25 °C. At specific time intervals, samples were taken for measuring the concentration of CdTe QDs. The adsorption isotherm experiments were performed by adding 5 mg of the PEI–GP adsorbent into a series of 100 mL stoppered conical flasks containing 25 mL of different concentrations of CdTe QDs solution (pH = 8.4). After the flasks were shaken at 180 rpm in a thermostatic shaker at 25 °C for 12 h, the samples were taken and the concentration of CdTe QDs was measured.
2.7. Breakthrough experiments
Different water samples were used to investigate the adsorption ability of PEI–GP through a continuous column breakthrough experiment for practical application. A tap water sample was collected from our laboratory after the tap had been run for 10 min. Wastewater was from the effluent of the sewage treatment plant in Beibei (Beibei, Chongqing). The tested water samples were prepared by adding CdTe QDs solution into the applied water samples to reach a final CdTe QDs concentration of 25 mg L−1. The breakthrough experiments were carried out on a mini plastic column (0.9 cm in diameter and 6.0 cm in height), which was filled with 100 mg of the PEI–GP-0.8 biosorbent. The breakthrough experiments were realized by passing the various water samples containing CdTe QDs through the mini-column at a flow rate of 3.0 mL min−1, and the effluent samples were manually collected and used for determining the concentration of CdTe QDs at a different bed volume (BV). The BV represents the volume of a water sample solution equivalent to the adsorbent volume in the mini-column. The breakthrough curves were then obtained by plotting the ratio of the effluent CdTe QDs concentration (C) to the initial CdTe QDs concentration (C0) against the BV.
3. Results and discussion
3.1. Characterizations of PEI–GP and the key role of PEI in the removal of CdTe QDs
PEI can be adsorbed or covalently linked to the GP surfaces because it contains a large number of amine groups.25 In the present study, the PEI–GP composites were prepared with different PEI levels (see Experimental section). As shown in Scheme 1, the GP was first dispersed in methanol solution with desired amounts of PEI. After stirring at 60 °C for 4 h, the color of the suspension changes from light yellow to deep yellow, suggesting that the PEI penetrated into the GP. This is because, due to the presence of carboxyl and hydroxyl functional groups,24,27 the negatively charged GP can bind with positively charged PEI molecules by electrostatic interactions, leading to the formation of amide bonds.25,28 On the basis of the XPS analysis (Table S1 ESI†), the nitrogen content was increased from 4.3 at% in GP to 7.1 at% at the PEI–GP interface, demonstrating the presence of PEI at the surface of the GP. Fig. 1A shows the FT-IR spectra of GP and PEI–GP-0.8. For the GP, the broad peak located at 3410 cm−1 can be assigned to the O–H stretching band, suggesting the presence of –OH groups (alcohols, phenols, and carboxylic acids) as in pectin, cellulose and lignin on the GP surface.24,27 The peaks at 1748 and 1630 cm−1 correspond to the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band and the C–O band,27 respectively. The peak at 1437 cm−1 is attributed to the N–H stretching.27 After modification with PEI, the characteristic band of the GP at 1748 cm−1 nearly disappears, while a new peak at 1410 cm−1 appears, corresponding to the C–N stretching vibration.29 These observations confirm that PEI was successfully grafted onto the GP. The XRD pattern of GP and PEI–GP displays two peaks at 2θ of about 22° and 18° (Fig. 1B), respectively. The peaks at 22° and 18° correspond to the presence of crystalline cellulose and a polysaccharide structure,27 respectively. The observation suggests that PEI functionalization has almost no effect on the structure of GP. The SEM images reveal that the original structure of GP was retained after PEI functionalization (Fig. 2A and B and S1 ESI†). However, as compared to the GP, there exists more irregular and porous structures on the surface of the PEI functionalized GP. Clearly, more cavities appear in its structure after PEI treatment (Fig. 2B and S1 ESI†). Interestingly, after the adsorption of the CdTe QDs, the PEI–GP biosorbents turn out to be orange-red (Scheme 1). Furthermore, the surface of PEI–GP, after adsorption of the CdTe QDs, appears to be relatively flat, as compared to PEI–GP before adsorption (Fig. 2C). This indicates that CdTe QDs are indeed adsorbed on the PEI–GP adsorbent.
O band and the C–O band,27 respectively. The peak at 1437 cm−1 is attributed to the N–H stretching.27 After modification with PEI, the characteristic band of the GP at 1748 cm−1 nearly disappears, while a new peak at 1410 cm−1 appears, corresponding to the C–N stretching vibration.29 These observations confirm that PEI was successfully grafted onto the GP. The XRD pattern of GP and PEI–GP displays two peaks at 2θ of about 22° and 18° (Fig. 1B), respectively. The peaks at 22° and 18° correspond to the presence of crystalline cellulose and a polysaccharide structure,27 respectively. The observation suggests that PEI functionalization has almost no effect on the structure of GP. The SEM images reveal that the original structure of GP was retained after PEI functionalization (Fig. 2A and B and S1 ESI†). However, as compared to the GP, there exists more irregular and porous structures on the surface of the PEI functionalized GP. Clearly, more cavities appear in its structure after PEI treatment (Fig. 2B and S1 ESI†). Interestingly, after the adsorption of the CdTe QDs, the PEI–GP biosorbents turn out to be orange-red (Scheme 1). Furthermore, the surface of PEI–GP, after adsorption of the CdTe QDs, appears to be relatively flat, as compared to PEI–GP before adsorption (Fig. 2C). This indicates that CdTe QDs are indeed adsorbed on the PEI–GP adsorbent.
 |
| | Scheme 1 Schematic diagram of the preparation process of the PEI–GP biosorbents. | |
 |
| | Fig. 1 (A) FT-IR spectra of GP and PEI–GP-0.8. (B) XRD patterns of GP and PEI–GP-0.8. | |
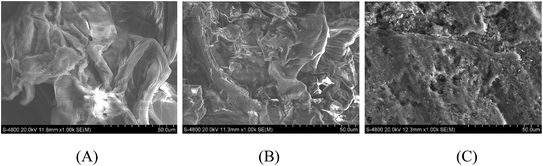 |
| | Fig. 2 SEM images of GP (A), PEI–GP-0.8 before adsorption of CdTe QDs (B) and PEI–GP-0.8 after adsorption of CdTe QDs (C). | |
Fig. 3A shows the effect of the PEI content on the adsorption capacity of the as-prepared PEI–GP for CdTe QDs. The PEI–GP composites with different PEI contents were prepared by simply varying the amount of PEI (0–1.0 g L−1) while keeping the amount of the GP unchanged (1.0 g in this work). As can be seen, no removal of CdTe QDs was found with the GP alone (Fig. 3A), indicating that the direct adsorption of CdTe QDs by the GP was negligible. In contrast, adsorption of the CdTe QDs increases as the PEI content increases from 0.2 to 0.8 g L−1, above which it decreases. The higher amount (above 0.8 g L−1) of PEI does not improve the sequestration of CdTe QDs. This might be attributed to the fact that the amount of PEI on the surface of the GP becomes saturated. These results indicated that PEI plays an important role in the adsorption process and the amount of PEI anchored on the surface of the GP is a key factor in determining the adsorption capacity of CdTe QDs by the PEI–GP.
 |
| | Fig. 3 (A) Effect of PEI content; (B) effect of solution pH. | |
3.2. Effect of pH
A previous study indicated that a lower pH value caused the increased aggregation of QDs.30 In order to eliminate the interference of possible aggregation of CdTe QDs derived from pH variance, selecting a suitable pH range is important to study the effect of pH. In a preliminary study, the effect of solution pH on the zeta potential and hydrodynamic diameter of CdTe QDs was investigated. As shown in Fig. S2,† the zeta potentials of the CdTe QDs were lower than −24 mV at pH ≥ 6. It was observed that yellow-green CdTe QD suspensions were clear at pH ≥ 6, and that no QD aggregation was observed (Fig. S3 ESI†). However, at pH ≤ 5, the zeta potentials were less negative than −20 mV (Fig. S2 ESI†), and brown CdTe QD aggregates formed (Fig. S3 ESI†) due to the protonated capping ligands on the CdTe QD surface.30 In particular, black QD aggregates formed at a pH of 2 (Fig. S3 ESI†). The aggregation of CdTe QDs was confirmed by the results of DLS measurements (Fig. S4 ESI†). As shown, solution pH values ranging from 6 to 11 did not change the hydrodynamic diameter of the CdTe QDs significantly (Fig. S4 ESI†). No aggregation was observed for these samples (Fig. S3 ESI†). However, the hydrodynamic diameter of the CdTe QDs increased significantly when the solution pH ranged from 5.0 to 2.0 (Fig. S4 ESI†), suggesting that CdTe QD aggregates were formed due to the protonated capping ligands on the CdTe QD surface.30 Hence, a pH of <6 was not considered to prevent the CdTe QDs from aggregating. The effect of the solution pH on the adsorption of CdTe QDs was studied by varying the solution pH from 6.0 to 11.0. As shown in Fig. 3B, the adsorption capacity is greatly enhanced with an increase in the amount of PEI up to 0.8 g L−1. For the PEI–GP-0.2 and PEI–GP-0.4 adsorbents, the adsorption of CdTe QDs increased with increasing pH from 6.0 to 8.4, however it decreases with the increase of solution pH above 8.4. For other adsorbents, the adsorption of CdTe QDs increased with increasing pH from 6.0 to 8.0, and then remained almost unchanged with increasing pH from 8.0 to 8.4. However, it decreases with the increase of solution pH above 8.4. Such pH dependent sequestration of CdTe QDs is likely to be related to the structure of the PEI–GP adsorbents and CdTe QDs. As indicated previously, the GP alone did not adsorb the CdTe QDs. Hence, the amino groups of PEI in PEI–GP are responsible for the retention of the CdTe QDs, probably through a nonspecific electrostatic interaction or a specific metal coordination.31,32 The amino groups on the PEI–GP adsorbent would be protonated at low pH values.33 Taking PEI–GP-0.8 as an example, at pH 8.4 and lower, the PEI–GP was positively charged (Fig. S2 ESI†). In contrast, the MSA capped CdTe QDs exhibit a negative surface at pH values ranging from 6.0 to 11.0 (Fig. S2 ESI†). Hence, the electrostatic repulsion between the PEI–GP adsorbents and CdTe QDs increased as the pH increases from 6.0 to 8.4. However, the retention of the CdTe QDs onto the PEI–GP adsorbents was enhanced in the pH range from 6.0 to 8.4, indicating that a specific metal coordination plays a major role in the sequestration of CdTe QDs. Considering that the optimum pH for CdTe QDs coagulation by PEI–GP was found to be about 8.4, this pH value was used for further studies.
3.3. Adsorption kinetics and isotherms
To understand the adsorptive performance, the time-dependent adsorption capacity was obtained to study the kinetics for the adsorption of CdTe QDs on the PEI–GP adsorbents. Fig. 4A shows the adsorption capacity of CdTe QDs over the PEI–GP adsorbents as a function of contact time. The results suggest a high rate of adsorption during the first hour, during which the adsorption capacity increased rapidly. Then the adsorption capacity increased gradually with contact time up to 4 to 12 h, depending on the PEI content. Based on the adsorption kinetics data in Fig. 4A, it is concluded that the adsorption equilibrium was finally reached at 12 h for the tested PEI–GP adsorbents. Two kinetic models, namely pseudo-first-order34 (eqn (1)) and pseudo-second-order35 (eqn (2)), were used to fit the adsorption kinetics data.| |
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − kt qe − kt
| (1) |
where qe (mg g−1) and qt (mg g−1) represent the adsorption capacity at equilibrium and at time t, k is the pseudo-first-order adsorption rate constant, and v0 is the initial adsorption rate (mg g−1 h−1). As shown in Table S2,† the correlation coefficient for the pseudo-second-order kinetics model is quite high (>0.99) (Table S2 ESI†). This indicates a better fit of the pseudo-second-order model with the experimental data, as compared to the pseudo-first-order model. Therefore, the adsorption of CdTe QDs over PEI–GP adsorbents can be fitted more favorably by the pseudo-second-order model.
 |
| | Fig. 4 (A) Adsorption kinetics of CdTe QDs onto PEI–GP; (B) adsorption isotherm for adsorption of CdTe QDs onto PEI–GP. | |
Also, the adsorption isotherm was obtained after adsorption for 12 h, which was considered sufficient time to reach adsorption equilibrium (Fig. 4A). The absorption isotherms of CdTe QDs on the as-prepared adsorbents are shown in Fig. 4B. The Langmuir and Freundlich models were used to fit the data (see ESI†). As can be seen, the qm values for PEI–GP in Langmuir model are all negative, which is not in accordance with the actual values (Table S3 ESI†). However the Freundlich isotherm fits well to the experimental data with R2 > 0.9 (Table S3 ESI†). This suggests that the adsorption of CdTe QDs onto the PEI–GP adsorbents can be considered as a multilayer adsorption process. Interestingly, if the two-stage adsorption progress is considered, each stage can be fitted well by the Freundlich model, with correlation coefficients of above 0.99 for all of the tested adsorbents (Table 1 and Fig. S5 ESI†). A similar result was also reported by Liu et al. who illustrated that Cu2+ adsorption by TEA-GO (triethanolamine modified graphene oxide) exhibited the characteristics of a two-stage multilayer adsorption.36 For each stage, Cu2+ adsorption onto TEA-GO can be well described by the Freundlich model.36 As for the n calculated from the two stages, the latter one is higher than the former one for all tested adsorbents (Table 1), showing a higher affinity of PEI–GP to the CdTe QDs in the latter stage than in the former one. This is in agreement with the consensus that if the values of n are in the range of 1–10, the adsorption is favorable.37
Table 1 The two-stage parameters of the Freundlich adsorption isotherm
| Adsorbents |
Stage 1 |
Stage 2 |
| n |
k |
R2 |
n |
k |
R2 |
| PEI–GP-0.2 |
0.42 |
0.009 |
0.9997 |
1.62 |
2.538 |
0.9984 |
| PEI–GP-0.4 |
0.54 |
0.016 |
0.9988 |
1.94 |
17.027 |
0.9941 |
| PEI–GP-0.6 |
0.65 |
0.103 |
0.9957 |
3.74 |
91.829 |
0.9907 |
| PEI–GP-0.8 |
0.67 |
0.151 |
0.9911 |
3.16 |
82.779 |
0.9973 |
| PEI–GP-1.0 |
0.65 |
0.145 |
0.9994 |
1.51 |
11.655 |
0.9902 |
3.4. Effect of ionic strength
Fig. S6† shows the effect of NaCl on the adsorption of CdTe QDs onto PEI–GP. The adsorption of CdTe QDs onto PEI–GP was increased with the addition of NaCl in the range of 1 to 100 mM for the tested PEI–GP adsorbents. It can be concluded that the adsorption of CdTe QDs onto PEI–GP is strongly dependent on the ionic strength. The observed trend is probably attributed to the aggregation of the CdTe QDs in the presence of salt. As indicated in previous studies, an increase in the electrolyte concentration led to an increase in size of the QDs,30,38,39 thus finally inducing aggregation of the QDs. In order to confirm this, the resonance light scattering (RLS) technique was used to monitor the interaction between the CdTe QDs and NaCl. The RLS signals continue to increase as the concentration of NaCl increases (Fig. S7 ESI†). This phenomenon indicates that the CdTe QDs aggregate in solution with the addition of salt, resulting in the enhanced light scattering signal. The aggregation of the CdTe QDs was also confirmed by the result of the DLS measurement. It is obvious that the hydrodynamic diameter of the CdTe QDs increased significantly in the presence of 100 mM NaCl (Fig. S8 ESI†).
3.5. Effect of humic acid
Previous works have demonstrated that, as one of the ubiquitous dissolved organic matters, humic acid retards the aggregation of engineered nanoparticle suspensions.40–42 In our case, the result of the effect of humic acid on the adsorption of CdTe QDs onto PEI–GP suggested that the adsorption capacities of the as-prepared PEI–GP biosorbents for CdTe QDs were almost independent on the concentration of humic acid (Fig. S9 ESI†). The observation suggests that presence of humic acid has a minor effect on the sequestration of CdTe QDs by the PEI–GP biosorbents. This is likely attributed to the strong affinity of the PEI–GP biosorbents to the CdTe QDs, confirming our speculation that a specific metal coordination plays a major role in the sequestration of CdTe QDs. Another possible explanation for the observed trend is due to the weak electrostatic attraction of the negatively charged humic acid with the neutral surface of the PEI–GP biosorbents (Fig. S2 ESI†) under the used condition (pH 8.4).
3.6. Breakthrough curves of adsorption of CdTe QDs onto a PEI–GP biosorbent packed column
Fig. S10† presents the breakthrough curves for different water samples containing CdTe QDs (Fig. S10 ESI†). The curves show that the mini-column packed with the PEI–GP biosorbent had the capability to deal with 890 BV, 1210 BV and 1320 BV of CdTe QDs solution in ultra pure water, tap water and wastewater samples, respectively. In the present work, the breakthrough point of the PEI–GP packed mini-column was set as the point at which the concentration of CdTe QDs in the effluent of the PEI–GP packed mini-column was above zero. The breakthrough volume of the PEI–GP column for CdTe QDs in the tested water samples follows the order of wastewater > tap water > pure water. The result is probably attributed to the difference in the ion strength in these water samples because the concentration levels of Ca2+, Mg2+, Na+ and K+ in the tested water samples also follow the order of wastewater > tap water > pure water (Table S4 ESI†). Also, the high concentration of divalent cations (Ca2+ and Mg2+) in wastewater and tap water is responsible for the observed result because they can cause aggregation of CdTe QDs even at low concentration levels,30 which was confirmed by the DLS result (Fig. S8 ESI†). The above result clearly demonstrated that PEI–GP could be used as a low-cost and effective biosorbent for the removal of CdTe QDs from water and wastewater.
3.7. Possible mechanism of CdTe QDs adsorption onto PEI–GP
XPS survey spectra indicated that Cd and Te are present in the PEI–GP biosorbent after adsorption of CdTe QDs (Fig. 5A). The high resolution XPS spectrum of the Cd 3d and Te 3d region (Fig. 5B and C) suggested that the Cd 3d3/2 and Cd 3d5/2 line peaks are located at 411.8 and 405.0 eV, and the Te 3d3/2 and Te 3d5/2 peaks are located at 572.3 and 582.7 eV respectively.43 For PEI–GP, the broad peak of O 1s can be fitted by three peaks at binding energies of 531.3, 532.6 and 533.3 eV (Fig. 5D). The peak at 532.6 eV is characteristic of the O atom in the C–O group of the GP, and the peak at around 531.3 eV suggested the presence of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group.44 After CdTes adsorption, a new peak appeared at about 530.5 eV for the O 1s level of PEI–GP. This may be attributed to the structural oxygen in the metal oxides45 of Cd–O and Te–O. In addition, the peak located at 163.5 eV in the S 2p region (Fig. 5E) was attributed to the SH groups in the MSA ligands attached to the surface Cd sites. The binding energy at 162.0 eV in the S 2p region (Fig. 5E) demonstrates the existence of chemical bonds (Cd–SR) between thiols and cadmium ions on the surface of the CdTe QDs.46 After adsorption of the CdTe QDs, the peak intensity at a binding energy of 163.5 eV was reduced significantly. At the same time, the peak intensity at a binding energy of 162.0 eV was enhanced greatly. This was probably due to the enrichment of CdTe QDs on the surface of the PEI–GP biosorbent (Fig. 2C). The N 1s spectrum of PEI–GP can be divided into three peaks (Fig. 5F). The peak situated at 399.4 eV can be assigned to –NH– or –NHCO groups.44 The other peak at 400.2 eV is attributed to a C–N group.44 The peak at 401.7 eV is attributed to the charged-N sites.47–49 After the adsorption of the CdTe QDs, the peak of the charged-N site disappeared, indicating that the reaction involved electrostatic interaction. Furthermore, the intensities of the peaks at 399.4 eV and 400.2 eV were reduced, suggesting that these nitrogen containing groups were involved in the adsorption of CdTe QDs.
O group.44 After CdTes adsorption, a new peak appeared at about 530.5 eV for the O 1s level of PEI–GP. This may be attributed to the structural oxygen in the metal oxides45 of Cd–O and Te–O. In addition, the peak located at 163.5 eV in the S 2p region (Fig. 5E) was attributed to the SH groups in the MSA ligands attached to the surface Cd sites. The binding energy at 162.0 eV in the S 2p region (Fig. 5E) demonstrates the existence of chemical bonds (Cd–SR) between thiols and cadmium ions on the surface of the CdTe QDs.46 After adsorption of the CdTe QDs, the peak intensity at a binding energy of 163.5 eV was reduced significantly. At the same time, the peak intensity at a binding energy of 162.0 eV was enhanced greatly. This was probably due to the enrichment of CdTe QDs on the surface of the PEI–GP biosorbent (Fig. 2C). The N 1s spectrum of PEI–GP can be divided into three peaks (Fig. 5F). The peak situated at 399.4 eV can be assigned to –NH– or –NHCO groups.44 The other peak at 400.2 eV is attributed to a C–N group.44 The peak at 401.7 eV is attributed to the charged-N sites.47–49 After the adsorption of the CdTe QDs, the peak of the charged-N site disappeared, indicating that the reaction involved electrostatic interaction. Furthermore, the intensities of the peaks at 399.4 eV and 400.2 eV were reduced, suggesting that these nitrogen containing groups were involved in the adsorption of CdTe QDs.
 |
| | Fig. 5 XPS spectra of CdTe QDs, PEI–GP-0.8 biosorbent before and after adsorption of CdTe QDs: (A) survey, (B) Cd 3d, (C) Te 3d, (D) O 1s, (E) S 2p, (F) N 1s. | |
4. Conclusion
In conclusion, a facile and simple route was proposed to prepare a PEI–GP composite biosorbent. Results on the adsorptive removal of CdTe QDs indicated that the introduction of PEI could greatly enhance the adsorption performance of the GP. The adsorption kinetics could be fitted well by the pseudo-second-order model and the adsorption process fitted well with the Freundlich adsorption isotherm. Interestingly, the ionic strength caused a significant effect on the adsorption of CdTe QDs onto the PEI–GP adsorbents, whereas, the humic acid has a minor effect on the sequestration of CdTe QDs by the PEI–GP biosorbents. The good adsorption ability of the PEI–GP composite for CdTe QDs clearly demonstrated that it can be used as a promising adsorbent for the removal of engineered nanoparticles such as CdTe QDs from water.
Acknowledgements
Financial support from the National Natural Science Foundation of China (no. 21277111) is gratefully acknowledged.
References
- A. J. Nozik, M. C. Beard, J. M. Luther, M. Law, R. J. Ellingson and J. C. Johnson, Chem. Rev., 2010, 110, 6873–6890 CrossRef CAS PubMed.
- H. Liu, D. Wu, Y. Liu, H. Zhang, T. Ma, A. Aidaerhan, J. Wang and B. Sun, J. Agric. Food Chem., 2015, 63, 2545–2549 CrossRef CAS PubMed.
- R. Liang, R. Tian, W. Shi, Z. Liu, D. Yan, M. Wei, D. G. Evans and X. Duan, Chem. Commun., 2013, 49, 969–971 RSC.
- M.-R. Chao, Y.-Z. Chang and J.-L. Chen, Biosens. Bioelectron., 2013, 42, 397–402 CrossRef CAS PubMed.
- M. Amelia, C. Lincheneau, S. Silvi and A. Credi, Chem. Soc. Rev., 2012, 41, 5728–5743 RSC.
- J. Ruan, H. Song, Q. Qian, C. Li, K. Wang, C. Bao and D. Cui, Biomaterials, 2012, 33, 7093–7102 CrossRef CAS PubMed.
- M. Zhang, X. Cao, H. Li, F. Guan, J. Guo, F. Shen, Y. Luo, C. Sun and L. Zhang, Food Chem., 2012, 135, 1894–1900 CrossRef CAS PubMed.
- Y. Shan, J.-J. Xu and H.-Y. Chen, Chem. Commun., 2010, 46, 5079–5081 RSC.
- D.-Y. Li, X.-W. He, Y. Chen, W.-Y. Li and Y.-K. Zhang, ACS Appl. Mater. Interfaces, 2013, 5, 12609–12616 CAS.
- M. Xue, X. Wang, H. Wang and B. Tang, Talanta, 2011, 83, 1680–1686 CrossRef CAS PubMed.
- X. Han, L. Lai, F. Tian, F. L. Jiang, Q. Xiao, Y. Li, Q. Yu, D. Li, J. Wang and Q. Zhang, Small, 2012, 8, 2680–2689 CrossRef CAS PubMed.
- A. Ambrosone, L. Mattera, V. Marchesano, A. Quarta, A. S. Susha, A. Tino, A. L. Rogach and C. Tortiglione, Biomaterials, 2012, 33, 1991–2000 CrossRef CAS PubMed.
- W. Zhang, K. Lin, Y. Miao, Q. Dong, C. Huang, H. Wang, M. Guo and X. Cui, J. Hazard. Mater., 2012, 213–214, 413–420 CrossRef CAS PubMed.
- A. M. Derfus, W. C. Chan and S. N. Bhatia, Nano Lett., 2004, 4, 11–18 CrossRef CAS.
- S. J. Cho, D. Maysinger, M. Jain, B. Röder, S. Hackbarth and F. M. Winnik, Langmuir, 2007, 23, 1974–1980 CrossRef CAS PubMed.
- S. Wang, M. Wei and Y. Huang, J. Agric. Food Chem., 2013, 61, 4988–4996 CrossRef CAS PubMed.
- P. Yin, Z. Wang, R. Qu, X. Liu, J. Zhang and Q. Xu, J. Agric. Food Chem., 2012, 60, 11664–11674 CrossRef CAS PubMed.
- M. Panda, A. Bhowal and S. Datta, Environ. Sci. Technol., 2011, 45, 8460–8466 CrossRef CAS PubMed.
- L. X. Zhong, X. W. Peng, D. Yang and R. C. Sun, J. Agric. Food Chem., 2012, 60, 5621–5628 CrossRef CAS PubMed.
- P. Li, Y. J. Su, Y. Wang, B. Liu and L. M. Sun, J. Hazard. Mater., 2010, 179, 43–48 CrossRef CAS PubMed.
- X. Xu, Y. Gao, B. Gao, X. Tan, Y. Q. Zhao, Q. Yue and Y. Wang, J. Hazard. Mater., 2011, 192, 1690–1696 CrossRef CAS PubMed.
- S. S. Khan, A. Mukherjee and N. Chandrasekaran, Colloids Surf., B, 2012, 92, 156–160 CrossRef CAS PubMed.
- N. Mahanta, W. Y. Leong and S. Valiyaveettil, J. Mater. Chem., 2012, 22, 1985–1993 RSC.
- A. Saeed, M. Sharif and M. Iqbal, J. Hazard. Mater., 2010, 179, 564–572 CrossRef CAS PubMed.
- X. Zhou, Z. Chen, D. Yan and H. Lu, J. Mater. Chem., 2012, 22, 13506–13516 RSC.
- E. Ying, D. Li, S. Guo, S. Dong and J. Wang, PLoS One, 2008, 3, e2222 Search PubMed.
- W. Zou, S. Gao, X. Zou and H. Bai, Water Environ. Res., 2013, 85, 466–477 CrossRef CAS.
- J. H. Chen, H. T. Xing, H. X. Guo, W. Weng, S. R. Hu, S. X. Li, Y. H. Huang, X. Sun and Z. B. Su, J. Mater. Chem. A, 2014, 2, 12561–12570 CAS.
- B. Liu and Y. Huang, J. Mater. Chem., 2011, 21, 17413–17418 RSC.
- Y. Zhang, Y. Chen, P. Westerhoff and J. C. Crittenden, Environ. Sci. Technol., 2007, 42, 321–325 CrossRef.
- Y. Chen, B. Pan, S. Zhang, H. Li, L. Lv and W. Zhang, J. Hazard. Mater., 2011, 190, 1037–1044 CrossRef CAS PubMed.
- S. B. Deng and Y. P. Ting, Water Res., 2005, 39, 2167–2177 CrossRef CAS PubMed.
- J. Suh, H. J. Paik and B. K. Hwang, Bioorg. Chem., 1994, 22, 318–327 CrossRef CAS.
- M. Najafi, Y. Yousefi and A. A. Rafati, Sep. Purif. Technol., 2012, 85, 193–205 CrossRef CAS PubMed.
- L. Wang, X. L. Wu, W. H. Xu, X. J. Huang, J. H. Liu and A. W. Xu, ACS Appl. Mater. Interfaces, 2012, 4, 2686–2692 CAS.
- G. Liu, S. Gui, H. Zhou, F. Zeng, Y. Zhou and H. Ye, Dalton Trans., 2014, 43, 6977–6980 RSC.
- A. S. K. Kumar, S. Jiang and W. Tseng, J. Mater. Chem. A, 2015, 3, 7044–7057 Search PubMed.
- I. R. Quevedo and N. Tufenkji, Environ. Sci. Technol., 2009, 43, 3176–3182 CrossRef CAS.
- S. Zhang, Y. Jiang, C.-S. Chen, J. Spurgin, K. A. Schwehr, A. Quigg, W.-C. Chin and P. H. Santschi, Environ. Sci. Technol., 2012, 46, 8764–8772 CrossRef CAS PubMed.
- R. L. Johnson, G. O. B. Johnson, J. T. Nurmi and P. G. Tratnyek, Environ. Sci. Technol., 2009, 43, 5455–5460 CrossRef CAS.
- R. F. Domingos, N. Tufenkji and K. J. Wilkinson, Environ. Sci. Technol., 2009, 43, 1282–1286 CrossRef CAS.
- D. A. Navarro, D. F. Watson, D. S. Aga and S. Banerjee, Environ. Sci. Technol., 2009, 43, 677–682 CrossRef CAS.
- H. Zhang, Z. Zhou, B. Yang and M. Gao, J. Phys. Chem. B, 2003, 107, 8–13 CrossRef CAS.
- S. W. Won, I. S. Kwak and Y.-S. Yun, Bioresour. Technol., 2014, 160, 93–97 CrossRef CAS PubMed.
- Z. Yu, X. Zhang and Y. Huang, Ind. Eng. Chem. Res., 2013, 52, 11956–11966 CrossRef CAS.
- H. Peng, L. Zhang, C. Soeller and J. Travas-Sejdic, J. Lumin., 2007, 127, 721–726 CrossRef CAS PubMed.
- H. S. O. Chan, S. C. Ng, W. S. Sim, K. L. Tan and B. T. G. Tan, Macromolecules, 1992, 25, 6029–6034 CrossRef CAS.
- S. Golczak, A. Kanciurzewska, M. Fahlman, K. Langer and J. J. Langer, Solid State Ionics, 2008, 179, 2234–2239 CrossRef CAS PubMed.
- Y.-Z. Su, W. Dong, J.-H. Zhang, J.-H. Song, Y.-H. Zhang and K.-C. Gong, Polymer, 2007, 48, 165–173 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: ESI tables and figures. See DOI: 10.1039/c5ra08129g |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was purchased from Sigma-Aldrich (Shanghai, China). The pH of the solution was adjusted by using 0.1 M NaOH or 0.1 M HCl. All the chemicals were of analytical grade and all the solutions were prepared with ultra pure water.
000) was purchased from Sigma-Aldrich (Shanghai, China). The pH of the solution was adjusted by using 0.1 M NaOH or 0.1 M HCl. All the chemicals were of analytical grade and all the solutions were prepared with ultra pure water.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band and the C–O band,27 respectively. The peak at 1437 cm−1 is attributed to the N–H stretching.27 After modification with PEI, the characteristic band of the GP at 1748 cm−1 nearly disappears, while a new peak at 1410 cm−1 appears, corresponding to the C–N stretching vibration.29 These observations confirm that PEI was successfully grafted onto the GP. The XRD pattern of GP and PEI–GP displays two peaks at 2θ of about 22° and 18° (Fig. 1B), respectively. The peaks at 22° and 18° correspond to the presence of crystalline cellulose and a polysaccharide structure,27 respectively. The observation suggests that PEI functionalization has almost no effect on the structure of GP. The SEM images reveal that the original structure of GP was retained after PEI functionalization (Fig. 2A and B and S1 ESI†). However, as compared to the GP, there exists more irregular and porous structures on the surface of the PEI functionalized GP. Clearly, more cavities appear in its structure after PEI treatment (Fig. 2B and S1 ESI†). Interestingly, after the adsorption of the CdTe QDs, the PEI–GP biosorbents turn out to be orange-red (Scheme 1). Furthermore, the surface of PEI–GP, after adsorption of the CdTe QDs, appears to be relatively flat, as compared to PEI–GP before adsorption (Fig. 2C). This indicates that CdTe QDs are indeed adsorbed on the PEI–GP adsorbent.
O band and the C–O band,27 respectively. The peak at 1437 cm−1 is attributed to the N–H stretching.27 After modification with PEI, the characteristic band of the GP at 1748 cm−1 nearly disappears, while a new peak at 1410 cm−1 appears, corresponding to the C–N stretching vibration.29 These observations confirm that PEI was successfully grafted onto the GP. The XRD pattern of GP and PEI–GP displays two peaks at 2θ of about 22° and 18° (Fig. 1B), respectively. The peaks at 22° and 18° correspond to the presence of crystalline cellulose and a polysaccharide structure,27 respectively. The observation suggests that PEI functionalization has almost no effect on the structure of GP. The SEM images reveal that the original structure of GP was retained after PEI functionalization (Fig. 2A and B and S1 ESI†). However, as compared to the GP, there exists more irregular and porous structures on the surface of the PEI functionalized GP. Clearly, more cavities appear in its structure after PEI treatment (Fig. 2B and S1 ESI†). Interestingly, after the adsorption of the CdTe QDs, the PEI–GP biosorbents turn out to be orange-red (Scheme 1). Furthermore, the surface of PEI–GP, after adsorption of the CdTe QDs, appears to be relatively flat, as compared to PEI–GP before adsorption (Fig. 2C). This indicates that CdTe QDs are indeed adsorbed on the PEI–GP adsorbent.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − kt
qe − kt

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group.44 After CdTes adsorption, a new peak appeared at about 530.5 eV for the O 1s level of PEI–GP. This may be attributed to the structural oxygen in the metal oxides45 of Cd–O and Te–O. In addition, the peak located at 163.5 eV in the S 2p region (Fig. 5E) was attributed to the SH groups in the MSA ligands attached to the surface Cd sites. The binding energy at 162.0 eV in the S 2p region (Fig. 5E) demonstrates the existence of chemical bonds (Cd–SR) between thiols and cadmium ions on the surface of the CdTe QDs.46 After adsorption of the CdTe QDs, the peak intensity at a binding energy of 163.5 eV was reduced significantly. At the same time, the peak intensity at a binding energy of 162.0 eV was enhanced greatly. This was probably due to the enrichment of CdTe QDs on the surface of the PEI–GP biosorbent (Fig. 2C). The N 1s spectrum of PEI–GP can be divided into three peaks (Fig. 5F). The peak situated at 399.4 eV can be assigned to –NH– or –NHCO groups.44 The other peak at 400.2 eV is attributed to a C–N group.44 The peak at 401.7 eV is attributed to the charged-N sites.47–49 After the adsorption of the CdTe QDs, the peak of the charged-N site disappeared, indicating that the reaction involved electrostatic interaction. Furthermore, the intensities of the peaks at 399.4 eV and 400.2 eV were reduced, suggesting that these nitrogen containing groups were involved in the adsorption of CdTe QDs.
O group.44 After CdTes adsorption, a new peak appeared at about 530.5 eV for the O 1s level of PEI–GP. This may be attributed to the structural oxygen in the metal oxides45 of Cd–O and Te–O. In addition, the peak located at 163.5 eV in the S 2p region (Fig. 5E) was attributed to the SH groups in the MSA ligands attached to the surface Cd sites. The binding energy at 162.0 eV in the S 2p region (Fig. 5E) demonstrates the existence of chemical bonds (Cd–SR) between thiols and cadmium ions on the surface of the CdTe QDs.46 After adsorption of the CdTe QDs, the peak intensity at a binding energy of 163.5 eV was reduced significantly. At the same time, the peak intensity at a binding energy of 162.0 eV was enhanced greatly. This was probably due to the enrichment of CdTe QDs on the surface of the PEI–GP biosorbent (Fig. 2C). The N 1s spectrum of PEI–GP can be divided into three peaks (Fig. 5F). The peak situated at 399.4 eV can be assigned to –NH– or –NHCO groups.44 The other peak at 400.2 eV is attributed to a C–N group.44 The peak at 401.7 eV is attributed to the charged-N sites.47–49 After the adsorption of the CdTe QDs, the peak of the charged-N site disappeared, indicating that the reaction involved electrostatic interaction. Furthermore, the intensities of the peaks at 399.4 eV and 400.2 eV were reduced, suggesting that these nitrogen containing groups were involved in the adsorption of CdTe QDs.




