Friedel–Crafts alkylation of indoles in deep eutectic solvent†
Abstract
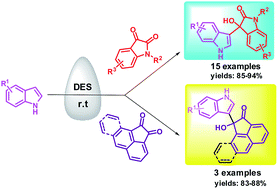
The application of Deep Eutectic Solvent (DES) as a solvent and a working catalyst for Friedel–Crafts alkylation of indoles with isatin as well as homocyclic carbonyl compounds has been developed. The salient features of the present protocol are practical simplicity, excellent yields, product selectivity and reusability of reaction media.

 Please wait while we load your content...
Please wait while we load your content...