Friedel–Crafts alkylation of indoles in deep eutectic solvent†
Atul Kumar*ab,
Ratnakar Dutt Shuklaa,
Dhiraj Yadavc and
Lalit Prakash Guptaa
aMedicinal and Process Chemistry Division, CSIR-Central Drug Research Institute (CDRI), Lucknow, 226031, India. E-mail: dratulsax@gmail.com; atul_kumar@cdri.res.in; Fax: +91-522-26-234051; Tel: +91-522-26-12411
bAcademy of Scientific & Innovative Research (AcSIR), New Delhi, India
cNational Institute of Pharmaceutical Education and Research, ITI Campus, Raebareli, U.P., India
First published on 28th May 2015
Abstract
The application of Deep Eutectic Solvent (DES) as a solvent and a working catalyst for Friedel–Crafts alkylation of indoles with isatin as well as homocyclic carbonyl compounds has been developed. The salient features of the present protocol are practical simplicity, excellent yields, product selectivity and reusability of reaction media.
The Friedel–Crafts reactions are common and powerful methods for C–C bond formation and remain the subject of continued improvement. In the present synthesis scenario, the development of green methodologies has emerged as a distinctive field of synthetic chemistry aimed at redesigning chemical processes in an environmentally benevolent way. Medium engineering has become one of the most important ways to tackle this issue in organic reactions by using water,1 ionic liquids2 and DES.3 In this context, several ionic liquids have been exploited to make the Friedel–Crafts reactions greener, but each ionic liquid contains Lewis acids (such as ZnCl2,4 AlCl3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and InCl3
5 and InCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6). The use of Lewis acid (metal halides) causes problems associated with the strong complex formed between the ketone product and the metal halide itself which provokes the use of Lewis acid catalyst.7 Therefore, a facile and practical Lewis acid free methodology is desirable for Friedel–Crafts reactions. Taking into account the utility of DES in number of reactions,8 we have exploited Lewis acid free DES in Friedel–Crafts reactions with indole and electron-deficient carbonyl compounds.
6). The use of Lewis acid (metal halides) causes problems associated with the strong complex formed between the ketone product and the metal halide itself which provokes the use of Lewis acid catalyst.7 Therefore, a facile and practical Lewis acid free methodology is desirable for Friedel–Crafts reactions. Taking into account the utility of DES in number of reactions,8 we have exploited Lewis acid free DES in Friedel–Crafts reactions with indole and electron-deficient carbonyl compounds.
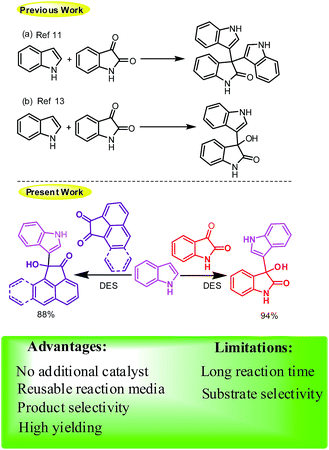
Indole has become a privileged structure in numerous research areas such as: pharmaceuticals, fragrances, agrochemicals, pigments and material science.9,10 Among several reactions of indole, the reactions involving C-3 functionalization of indole with electron-deficient carbonyl compounds especially isatins has attracted and continues to attract interest from synthetic community. However, efforts have taken in this context resulted in the formation of symmetrical 3,3-di(indolyl)indolin-2-one which have limited applications.11 Synthesis of selective 3-substituted-3-hydroxyoxindoles is highly desirable because they are privileged scaffold in various natural products and pharmaceutical lead compound (Fig. 1).12
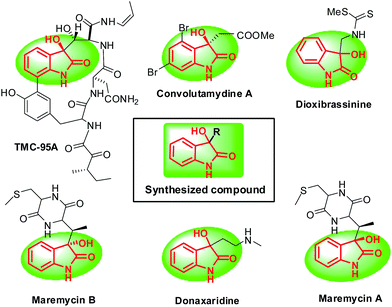 | ||
| Fig. 1 Selected naturally occurring and pharmaceutical molecules with 3-substituted 3-hydroxy-oxindoles. | ||
A protocol has been reported for selective synthesis of 3-substituted-3-hydroyoxindoles using stoichiometric amounts of β-cyclodextrin13 but the search for more economical and environmentally benign catalytic medium is yet desirable. In continuation to our research endeavors in development of greener protocols,14 here we wish to report application of DES in field of Friedel–Crafts reaction of indoles with isatin as well as aceanthrylene-1,2-dione/acenaphthylene-1,2-dione.
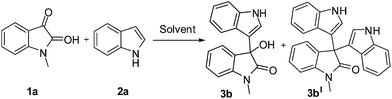
To look into the prospect of new C–C bond formation, a model reaction using easily available and reasonably priced 1-methylisatin (1a) and indole (2a) was investigated in detail by varying the common solvents as well as DESs in order to develop appropriate reaction conditions (Table 1).
| Entry | Solvent | Temp (°C) | Time (h) | Yield (%) | |
|---|---|---|---|---|---|
| 3b | 3bI | ||||
| a Reaction conditions: a mixture of 1a (1.0 mmol), 2a (1.0 mmol) and solvent (5 ml) was stirred. ChCl-choline chloride, CA–citric acid, DMU-1,3-dimethyl urea.b Reaction conditions: a mixture of 1a (1.0 mmol), 2a (2.0 mmol) and solvent was stirred at 80 °C. | |||||
| 1 | H2O | Reflux | 4 | — | — |
| 2 | EtOH | Reflux | 4 | — | — |
| 3 | CH3CN | Reflux | 4 | — | — |
| 4 | CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ChCl ChCl |
70 | 7 | 42 | 24 |
| 5 | Imidazole![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ChCl ChCl |
70 | 8 | 64 | 18 |
| 6 | Urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) ChCl ChCl |
r.t. | 8 | 94 | – |
| 7 | Urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ChCl ChCl |
80 | 5 | — | 42 |
| 8 | Urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ChCl ChCl |
80 | 5 | — | 92b |
| 9 | DMU![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ChCl ChCl |
75 | 5 | 68 | 12 |
When reaction was carried out in water, ethanol and acetonitrile without using catalyst at refluxed condition, desired product was not obtained indicating the necessity of catalyst. Furthermore, screening was continued by using different types of DESs, for example ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (1
CA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), ChCl
1), ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) imidazole (3
imidazole (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7), ChCl
7), ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Urea (1
Urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and ChCl
2) and ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMU (1
DMU (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to determine their catalytic efficacies. Out of all of the trials, an exceedingly high product yield (94%) was obtained in ChCl
2) to determine their catalytic efficacies. Out of all of the trials, an exceedingly high product yield (94%) was obtained in ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Urea (1
Urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at room temperature (Table 1, entry 6), whereas ChCl
2) at room temperature (Table 1, entry 6), whereas ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (1
CA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), ChCl
1), ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) imidazole (3
imidazole (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) and ChCl
7) and ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMU (1
DMU (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) gave just to satisfactory yields.
2) gave just to satisfactory yields.
These results reveal that DES is not only solvent but also a catalyst of the reaction. Besides this, we have also studied the effect of temperature for model reaction with optimized condition. Next, to examine the versatility of the present standardized protocol, we explored the reactions of substituted isatins (benzene ring substituted isatins as well N-substituted isatins) with substituted indoles. To our delight, substituted isatins and indoles participated well in the reaction, diverse range of selective 3-hydroxy-3-indolylindolin-2-ones were obtained (Scheme 1) (Fig. 2).
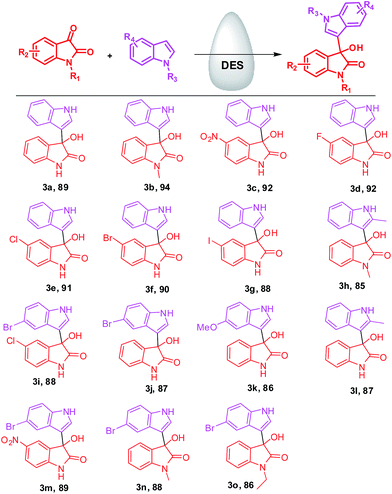 | ||
Scheme 1 Synthesis of 3-hydroxy-3-indolylindolin-2-ones. Reaction conditions: reactions were performed with isatin (1.0 mmol), indole (1.0 mmol) and DES (urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ChCl-1 ChCl-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at room temperature. 2) at room temperature. | ||
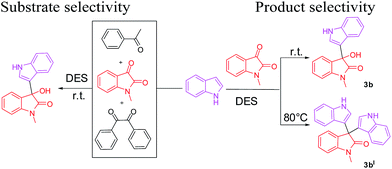
Moreover, with standardized conditions, substrate selectivity was studied for present protocol (Fig. 3). We performed a competitive reaction of indole (1.0 mmol) with the mixture of a number of carbonyl compounds such as acetophenone, 1-methyl isatin and benzil (1.0 mmol each).
Interestingly, only the desired product 3-hydroxy-1-methyl-3-(1H-indol-3-yl)indolin-2-one (3b) was obtained as a sole product and the other starting carbonyl compounds were recovered intact. Hence, the present protocol seems to have a preferential substrate-selective property.
Additionally, we have also studied the effect of temperature on product selectivity (Fig. 3). In this regard, when reaction (1-methyl isatin and indole, 1 mmol each) was carried out at room temperature, the desired product 3-hydroxy-1-methyl-3-(1H-indol-3-yl)indolin-2-one (3b, 94%) was obtained, whereas the same reaction was explored at 80 °C, the selectivity was destroyed and resulted in simple ABB type pseudo multicomponent reaction i.e. the formation of 3,3-di(1H-indol-3-yl)-1-methylindolin-2-one (3bI, 42%). When we performed the reaction of 1-methyl isatin and indole in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric ratios at 80 °C, the yield of product 3bI was increased to 92%.
2 stoichiometric ratios at 80 °C, the yield of product 3bI was increased to 92%.
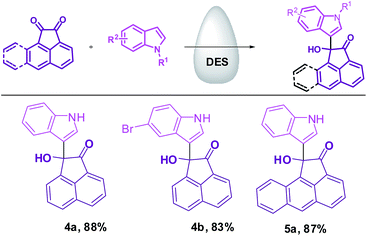
To examine the efficacy and scope of the present protocol for Friedel–Crafts reaction, next, we explored the reaction of indole with homocyclic electron-deficient carbonyl compounds such as acenaphthylene-1,2-dione and aceanthrylene-1,2-dione. To our delight, the expected products were obtained in good yields (Scheme 2).
For model reaction, a complete study was done to assess the reusability of DES (Urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ChCl). After completion of reaction, water was added to the reaction mixture. The DES being soluble in water comes in the water layer and product was precipitated. The solid product was separated by filtration. The DES was recovered from the filtrate by evaporating the water phase at 80 °C under vacuum. For compound 3b an alternative way was tried for recovery of DES using of 2-methyl THF instead of water. After completion of reaction 2-methyl THF was added to reaction mixture, product went to organic phase and DES settled down. Organic phase was separated and product was obtained by evaporating organic phase under vacuum and settled DES was used for next batch syntheses.
ChCl). After completion of reaction, water was added to the reaction mixture. The DES being soluble in water comes in the water layer and product was precipitated. The solid product was separated by filtration. The DES was recovered from the filtrate by evaporating the water phase at 80 °C under vacuum. For compound 3b an alternative way was tried for recovery of DES using of 2-methyl THF instead of water. After completion of reaction 2-methyl THF was added to reaction mixture, product went to organic phase and DES settled down. Organic phase was separated and product was obtained by evaporating organic phase under vacuum and settled DES was used for next batch syntheses.
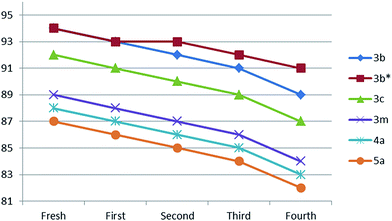
The recovered DES was then successfully used for the next 5 batches. Furthermore, the reusability of DES was confirmed for the syntheses of compounds 3c, 3m, 4b, and 5a with five cycles and small losses in catalytic activity were observed (Fig. 4).
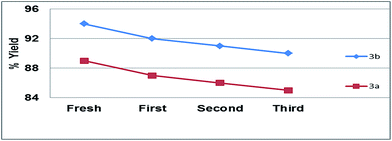
Beside reusability test, cross reusability test was also performed by taking fresh DES for the synthesis of 3a, after completion of reaction DES was recovered then it was used for the first batch synthesis of 3b and vice versa. The cross reusability was carried out for 3 cycles with minute loss of catalytic activity of DES (Fig. 5). Furthermore, no substrate product contamination was observed during cross reusability experiment.
In conclusion, we have developed Lewis acid free Friedel–Crafts reaction using low-priced, non-toxic, easily available DES as a solvent and as a catalyst. The selective syntheses of 3-hydroxy-3-indolylindolin-2-ones/2-hydroxy-2-(1H-indol-3-yl)acenaphthylen-1-(2H)-ones/1-hydroxy-1-(1H-indol-3-yl)aceanthrylen-2(1H)-ones and reusability/cross reusability of the reaction media are added advantages to this protocol, thus provides a better and practical alternative to existing methods. This research confers a step towards development of more active greener catalytic media.
Acknowledgements
Authors RDS, DY and LPG are thankful to CSIR, New Delhi, for financial assistance as SRF and CSIR-Network project BSC 0104. Furthermore, authors are grateful to the SAIF-CSIR-CDRI for providing the spectroscopic data and analytical facilities. CDRI communication no. 9003.Notes and references
- (a) C.-J. Li, Acc. Chem. Res., 2010, 43, 581–590 CrossRef CAS PubMed; (b) C. I. Herrerías, X. Yao, Z. Li and C.-J. Li, Chem. Rev., 2007, 107, 2546–2562 CrossRef PubMed; (c) C.-J. Li and L. Chen, Chem. Soc. Rev., 2006, 35, 68–82 RSC; (d) C. J. Li, Chem. Rev., 2005, 105, 3095–3166 CrossRef CAS PubMed; (e) A. K. Nezhad and F. Panahi, Green Chem., 2011, 13, 2408–2415 RSC.
- (a) P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772–3789 CrossRef CAS; (b) M. Petkovic, K. R. Seddon, L. P. N. Rebelo and C. S. Pereira, Chem. Soc. Rev., 2011, 40, 1383–1403 RSC; (c) J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS PubMed; (d) T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef CAS PubMed.
- (a) Q. Zhang, K. D. O. Vigier, S. Royer and F. Jerome, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC; (b) E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed; (c) A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2014, 2, 1063–1071 CrossRef CAS; (d) C. Ruß and B. König, Green Chem., 2012, 14, 2969–2982 RSC.
- Z. Duan, Y. Gu and Y. Deng, Catal. Commun., 2006, 7, 651–656 CrossRef CAS.
- C. O. Kangani and B. W. Day, Org. Lett., 2008, 10, 2645–2648 CrossRef CAS PubMed.
- M. J. Earle, U. Hakala, C. Hardacre, J. Karkkainen, B. J. McAuley, D. W. Rooney, K. R. Seddon, J. M. Thompson and K. Wahala, Chem. Commun., 2005, 903–905 RSC.
- G. Sartori and R. Maggi, Chem. Rev., 2006, 106, 1077–1104 CrossRef CAS PubMed.
- (a) P. M. Pawar, K. J. Jarag and G. S. Shankarling, Green Chem., 2011, 13, 2130–2134 RSC; (b) F. Jérôme, M. Ferreira, H. Bricout, S. Menuel, E. Monflier and S. Tilloy, Green Chem., 2014, 16, 3876–3880 RSC; (c) S. Handy and N. M. Westbrook, Tetrahedron Lett., 2014, 55, 4969–4971 CrossRef CAS; (d) N. Azizi and Z. Manocheri, Res. Chem. Intermed., 2012, 38, 1495–1500 CrossRef CAS.
- (a) R. J. Sundberg, The Chemistry of Indoles, Academic Press, New York, 1970 Search PubMed; (b) R. K. Brown, Indoles, ed. W. J. Houlihan, Wiley-Interscience, New York, 1972 Search PubMed; (c) J. T. Li, H. G. Dai and Z. P. Lin, Prog. Chem., 2007, 19, 751 CAS; (d) D. J. Faulkner, Nat. Prod. Rep., 2001, 18, 19 RSC; (e) A. Kleeman, J. Engel, B. Kutscher and D. Peichert, Pharmaceutical Substances, Thieme, New York, 2001 Search PubMed; (f) G. W. Gribble, J. Chem. Soc., Perkin Trans. 1, 2000, 1045 RSC; (g) R. J. Sundberg, Prog. Heterocycl. Chem., 1989, 1, 111 CAS; (h) A. M. Lobo and S. Prabhakar, J. Heterocycl. Chem., 2002, 39, 429 CrossRef CAS; (i) M. Toyota and M. Ihara, Nat. Prod. Rep., 1998, 15, 327 RSC; (j) W. N. Xiong, C. G. Yang and B. Jiang, Bioorg. Med. Chem., 2001, 9, 1773 CrossRef CAS PubMed.
- M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9608–9644 CrossRef CAS PubMed.
- (a) G. Brahmachari and B. Banerjee, ACS Sustainable Chem. Eng., 2014, 2, 2802–2812 CrossRef CAS; (b) S.-Y. Wang and S.-J. Ji, Tetrahedron, 2006, 62, 1527–1535 CrossRef CAS; (c) J. Bergman and N. Eklund, Tetrahedron, 1980, 36, 1445–1450 CrossRef CAS; (d) F. Garrido, J. Ibanez, E. Gonalons and A. Giraldez, Eur. J. Med. Chem., 1975, 10, 143–149 CAS; (e) C. Praveen, Y. W. Sagayaraj and P. T. Perumal, Tetrahedron Lett., 2009, 50, 644–647 CrossRef CAS; (f) K. R. Moghadam, M. S. Kiasaraie and H. T. Amlashi, Tetrahedron, 2010, 66, 2316–2321 CrossRef.
- (a) C. Marti and E. M. Carreira, Eur. J. Org. Chem., 2003, 2209–2219 CrossRef CAS; (b) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748–8765 CrossRef CAS PubMed; (c) H. Lin and S. J. Danishefsky, Angew. Chem., Int. Ed., 2003, 42, 36–51 CrossRef CAS; (d) A. B. Dounay and L. E. Overman, Chem. Rev., 2003, 103, 2945–2963 CrossRef CAS PubMed; (e) S. Peddibhotla, Curr. Bioact. Compd., 2009, 5, 20 CrossRef CAS; (f) F. Zhou, Y. L. Liu and J. Zhou, Adv. Synth. Catal., 2010, 352, 1381–1407 CrossRef CAS.
- V. P. Kumar, V. P. Reddy, R. Sridhar, B. Srinivas, M. Narender and K. R. Rao, J. Org. Chem., 2008, 73, 1646–1648 CrossRef CAS PubMed.
- (a) A. Kumar, V. D. Tripathi and P. Kumar, Green Chem., 2011, 13, 51–54 RSC; (b) A. Kumar, G. Gupta and S. Srivastava, Green Chem., 2011, 13, 2459–2463 RSC; (c) A. Kumar, M. K. Gupta and M. Kumar, Green Chem., 2012, 14, 290–295 RSC; (d) A. Kumar, M. Kumar and M. K. Gupta, Green Chem., 2012, 14, 2677–2681 RSC; (e) A. Kumar, S. Srivastava and G. Gupta, Green Chem., 2012, 14, 3269–3272 RSC; (f) A. Kumar and R. D. Shukla, Green Chem., 2015, 17, 848–851 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra08038j |
| This journal is © The Royal Society of Chemistry 2015 |