Arylsulfonylmethyl isocyanides: a novel paradigm in organic synthesis
Tanpreet Kaur
,
Preeti Wadhwa
and
Anuj Sharma
*
Department of Chemistry, Indian Institute of Technology Roorkee, Roorkee-247667, India. E-mail: anujs77@gmail.com; Tel: +91-1332-284751
First published on 1st June 2015
Abstract
p-Tosylmethyl isocyanide (TosMIC), an α-acidic isocyanide, has emerged as a privileged reagent to access biologically relevant scaffolds. The present review highlights the significant advancements of TosMIC in the construction of fused heterocycles viz. pyrroles, benzimidazoles, imidazopyridines, quinolones, quinolines and some natural products such as (−)-ushikulide A, variolin B, porphobilinogen and mansouramycin B. The review article encompasses literature from the period starting from 2010 onwards and covers novel synthetic methodologies involving TosMIC. A wide range of reaction strategies have been reported involving TosMIC during this period such as Michael additions, cycloadditions and many cascade/tandem/multicomponent reactions.
1. Introduction
In recent time when premium is put on speed, diversity and efficiency in the drug discovery process, multicomponent reactions (MCRs) represent one of the most powerful tools owing to easy accessibility of molecules in terms of both skeletal and decorative diversity.1 Amongst various MCRs, isocyanide-based multicomponent reactions (IMCRs) are quite prolific due to isocyanide's peculiar reactivity and its tendency to act both as nucleophile and electrophile, which has given it a prominent role in various chemical transformations in the past.2 The last couple of decades have witnessed a renaissance in the chemistry of IMCRs via several twists and turns in the classical age old Passerini (P-3CC) and Ugi (U-4CC) IMCRs, thereby insinuating several new methodologies.2 IMCR chemistry is a significant contributor to the contemporary organic synthetic tools such as diversity-oriented synthesis (DOS), high throughput screening and combinatorial chemistry.3 Due to a tremendous growth in this field, IMCRs have been the subject of different reviews.2–4 Of late, the incorporation of additional functionalities into the isocyanide moiety has gained significant development that would naturally increase the versatility of these synthons, and indeed many new functionalized isocyanides have also been successfully synthesized.5 A number of isocyanides bearing additional functionalities are known such as sulfonylmethyl isocyanides, vinyl isocyanides, isocyanoacetates, and isocyanophosphonoacetates.5,6 Among them, α-acidic isocyanides, in particular, have gained considerable attention due to their 1,3-dipolar character, thus interacting with a wide range of dipolarophiles in cycloaddition reactions.7 Typically, these reaction sequences utilize nucleophilic addition of the α-carbanion, rather than the divalent carbon of the isocyanide as in the P-3CR and U-4CR, onto the respective electrophiles.5 The names of Schöllkopf and Van Leusen stand for many pioneering developments in this field of α-acidic isocyanides. From all that pertinent chemistry, three primary α-acidic isocyanide templates have emerged to the mainstream of organic synthesis: (1) arylsulfonylmethyl isocyanides (I), (2) α-isocyano esters (II) and (3) α-isocyanamides (III) (Fig. 1).Amongst these three types, TosMIC reagents (template I), originally introduced by Van Leusen, are most versatile and valuable synthons in organic chemistry.7,8 TosMIC reagents are densely functionalized moieties with three groups that can engage in a multitude of reactions: the isocyano functionality can undergo α-addition reactions whereas the acidic α-carbon atom and the sulfonyl group in the α-position can act as a leaving group thereby further enhancing the acidity of α-carbon.7 TosMIC, due to its remarkable properties in synthesis, such as ease of preparation, tolerance to various functionalities and effortless removal of the tosyl group, has facilitated a wide range of chemical transformations, both in the chemical and biological sphere through synthesis of molecules such as oxazolidinones, oxazoles, thiazoles, imidazoles, indoles, triazoles, pyrroles, benzofurans, quinoxalines, and pyrrolopyrimidines.6–8 Moreover, they also have been implied in the synthesis of natural products such as variolin B, porphobilinogen and mansouramycin B.9–11 In view of its widespread role in synthesis, TosMIC has been documented in the form of elegant reviews to date, which have largely accounted for the reactions of TosMIC reagents till the year 2010.6,12 Therefore, the present review article summarizes progress in the chemistry of TosMIC reagents reported after 2010 till 2015. Moreover, the emphasis in writing this manuscript has been on the method development from a pure synthesis perspective, and hence the use of TosMIC in the preparation of biologically/medicinally active compounds appearing in the medicinal chemistry and other application based journals was not considered.
The pioneering and revolutionary work on the synthesis of TosMIC reagents was carried out by Van Leusen either by irradiating the solution of corresponding p-tosyl diazomethane in liquid hydrogen cyanide13 or by treating p-tosylfluoride with isocyanomethyl lithium.14 These methods have several disadvantages, for instance, use of poisonous HCN gas, fluorides, harsh reaction conditions and lesser yields. Later on, these shortcomings were overcome by a more facile two-step three component approach involving sulfinates, aldehydes and formamides.15 This modified protocol was started with Mannich condensation to synthesize N-(p-tosylmethyl)formamide 2, followed by its dehydration with phosphoryl chloride to furnish TosMIC 1a (Scheme 1). This method was further modified by Sisko to improve the scope of aldehyde input in the same approach.16
Owing to TosMIC's reactivity, its various synthetic analogues were prepared. One such protocol to access the functional mono-substituted TosMICs was exploited by Van Leusen; it utilized deprotonation and alkylation of α-acidic isocyanides using phase transfer catalyst (PTC) conditions (Scheme 2).17
TosMIC reagents have profound applications towards the construction of N-heterocycles (Fig. 2). Therefore, based upon the types of heterocycles that they synthesized, this review has been subdivided into (i) synthesis of five membered heterocycles, (ii) synthesis of six membered heterocycles and (iii) miscellaneous.
2. Synthesis of five membered heterocycles
2.1 Pyrroles
Pyrroles are privileged scaffold exhibiting an array of pharmacophoric activities viz. antitumor, antibacterial, antifungal, and anti-inflammatory.18 Moreover, they are useful building blocks not only in the field of natural product synthesis but also in the area of heterocyclic chemistry.19 To access this bio-dynamic core, several syntheses, for instance, Knorr, Paul–Knorr, Hantzsch and Van Leusen have been reported.20,21 The latter approach involves base mediated 1,3-dipolar cycloaddition of TosMIC with various Michael acceptors e.g. electron deficient alkenes, alkynes, ketene and dithioacetals.22–24 However, the need for improving Van Leusen's pyrrole synthesis is evident. Subsequently, Adib and coworkers described an efficient and novel synthesis of dialkyl 2-[(4-methylphenyl)sulfonyl]-1H-pyrrole-3,4-dicarboxylates 4 by the reaction of dialkylacetylene dicarboxylates 3 with TosMIC 1a using 1-methylimidazole as a catalyst.23 Interestingly, the cycloaddition reaction was performed at room temperature under mild conditions and in excellent yields (Scheme 3).A detailed mechanistic rationalization for the reaction is provided in Scheme 4. First, the nucleophilic addition of 1-methylimidazole on the acetylenic ester generated the zwitterion A, which deprotonated the TosMIC to form intermediate B. This species B underwent 1,3-cycloaddition followed by the removal of the catalyst and subsequent aromatization to yield the desired product 4.
Similarly, Yu and co-workers also reported the synthesis of multi-functionalized pyrroles 6 from TosMIC 1a and vinyl azides 5.22 The synthesis protocol proceeded via in situ generation of vinyl azides from the respective aldehydes and alkyl azides under mild basic conditions and its subsequent 1,3-dipolar cycloaddition on TosMIC to afford the desired product 6 in 36–94% yield (Scheme 5).
In a similar way, 1,3′-bipyrrole motifs, a basic constituent of a number of polypyrrole pigments, have relevance in medicinal chemistry and various marine natural products e.g., antibacterial bipyrrole marinopyrroles A and B.25 Owing to its importance in medicinal chemistry, Wu and coworkers attempted its synthesis by treating multisubstituted olefins with TosMIC 1a via classical Van Leusen's methodology.26 The reaction mixture was stirred at room temperature under basic conditions to afford the 1,3′-bipyrroles 8 in 43–78% yield (Scheme 6).
A plausible reaction mechanism of the reaction has been described in Scheme 7. Possibly, the reaction proceeded through the iterative process of cascade 1,3-dipolar cycloaddition through the intermediates (A–D) as discussed in Scheme 4.
On a similar line, Wang and coworkers also reported the methodology for the synthesis of 3,3′-bipyrroles 10 using dienones 9 and TosMIC 1a.27 This reaction involved C–C bond cleavage promoted by water. Moreover, the reaction tolerated a broad range of functional groups and conformationally restricted dienones (Scheme 8).
A plausible mechanism for the reaction has been depicted in Scheme 9. The first step involved the Michael addition of 1a to dienone 9 in the presence of KOH furnished enolate intermediate A, which subsequently underwent intramolecular cyclization to yield a spirocyclic intermediate B. Furthermore, the same iterative step occurred again and generated the bis-spirocyclic intermediate C. The intermediate C underwent cascade reaction involving ring-opening, decarboxylation and protonation in the presence of aqueous KOH to furnish the desired product 10.
Afterwards, Padmaja et al. utilized E-1-(arylsulfonylethylsulfonyl)-2-arylethene 11 as Michael acceptor to synthesize pyrroles 12 and pyrazoles 13 via 1,3-dipolar cycloaddition of TosMIC 1a and diazomethane, respectively (Scheme 10).28
Similarly, Padmavathi and co-workers also synthesized sulfone-linked bis-heterocycles (16–19) containing either similar or different pendant heterocyclic rings such as bis-pyrroles 16, bis-pyrrolyl pyrazoles (17–18), or bis-pyrrolyl isoxazole 19. The reaction involved 1,3-dipolar cycloaddition of dipolarphile bis(styryl)sulfone 14 with TosMIC 1a, diazomethane, nitrile imines and nitrile oxides.29 The reaction is highly regiospecific yielding the desired products in 65–82% yield (Scheme 11).
Moreover, the same group further exploited the reaction of 1-aroyl-2-styrylsulfonylethenes 20 with TosMIC 1a and synthesized the sulfone-linked bis-heterocycles 21–22. The reaction proceeded via the common intermediate A, which was readily accessed by the reaction of 1-aroyl-2-styryl sulfonylethenes 20 and hydrazine hydrate (Scheme 12).30
Ketene dithioacetals are important intermediates in organic synthesis.24 Liu and coworkers expanded the synthesis of polysubstituted fused bicyclic pyrrole systems 25 via the domino reaction of 1,5-dielectrophilic substrates such as α-alkenoyl ketene dithioacetals 23 with α-acidic isocyanides 1a using mild conditions in 18–90% yield (Scheme 13). It was observed that isocyanoacetate in the presence of NaOH and TosMIC under DBU conditions gave excellent yields.31
The reaction mechanism involved [5 + 1] annulation of TosMIC (or ethyl isocyanoacetate) with 1,5-dielectrophile under basic conditions, which initially provided anion intermediate A. The intermediate A underwent intramolecular cyclization followed by further protonation, isomerization and elimination (in case of TosMIC) to furnish the desired product 25 (Scheme 14).
Similarly, Pan et al. also employed α-formyl ketene dithioacetals 26 as a common precursor for the synthesis of oxazoles 27, 5-alkylthio-pyrroles 28–29, 4-alkylthiocarbonyl-pyrroles 30 and 2-alkylthio-4-tosyl-furans 31 through their regiodivergent cyclization with α-acidic isocyanides by changing catalysts and promoters under mild reaction conditions and in good to excellent yields (Scheme 15).32
Based on the above mentioned results, a probable mechanism has been depicted in Scheme 16. Initially, compound 32 was formed presumably via DBU mediated 1,3-cycloaddition. Next, in the presence of Lewis acid and water, 32 underwent hydrolysis to afford intermediate A, which served as a common intermediate for the formation of various derivatives 27–31. Under the presence of Zn(II)/H2O, sequential hydrolysis followed by cyclization and elimination afforded 30. On the other hand, Cu(I) facilitated the formation of complex B, thereby affording derivative 28, which was further hydrolyzed to pyrrole derivative 29 in water. Moreover, in the case of TosMIC, the hydrolysis of the carbonyl group occurred first to afford C, which subsequently underwent intramolecular cyclization to produce intermediate D. Finally, 1,2-sulfonyl migration and subsequent elimination afforded furan derivative 31.
In continuation, Ila and co-workers disclosed a domino process for the diversity oriented synthesis of annulated pyrroles involving a base-induced reaction of cyclic α-oxoketene dithioacetals with activated methylene isocyanides.33 The generality and scope of this method is described in Fig. 3. This methodology facilitated the synthesis of biologically relevant and structurally diverse pyrroles, for instance, pyrrolonapthoquinones, pyrroloquinolones, tetracyclic fused indoles, pyrrole annulated dibenzooxocinones and dibenzothiocinones.
Mechanistically, it was suggested that initial Michael addition of α-acidic isocyanide on substrate 33 was facilitated by intramolecular cyclization to yield spiro-aza-allyl intermediate A. Further nucleophilic attack on the carbonyl group resulted in the strained tricyclic alkoxide intermediate B. Next, ring expansion followed by elimination and aromatization afforded the desired product 34 (Scheme 17).
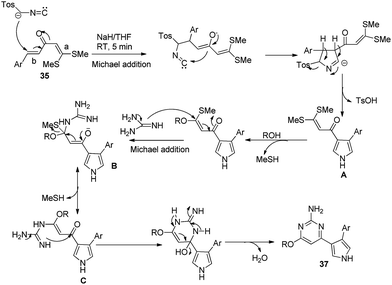
In a recent example, Shanmugam and co-workers reported a multicomponent route towards construction of 6-pyrrolyl pyrimidin-2-amine analogues 37 in good to excellent yields.34 The reaction proceeded via the cycloaddition reaction of α-aroylidine ketene dithioacetals 35, guanidine nitrate 36, alcohols and TosMIC 1a in the presence of NaH/THF in a highly chemo- and regioselective manner (Scheme 18).
A plausible mechanistic pathway is depicted in Scheme 19. The reaction proceeded via the 1,3-dipolar cycloaddition of TosMIC selectively on the α-aroylidine ketene dithioacetals 35. It is postulated that attack of the nucleophile is favored at side b of the double bond rather than side a, because the latter double bond is more electron rich due to the presence of two electron donating methyl sulfonyl groups. Subsequently, tosylic acid was eliminated to yield intermediate A, which followed the nucleophilic substitution of alcohol and iterative Michael addition and intramolecular cyclization to afford the desired product 37.
Similarly, Liu and co-workers described a tandem Michael addition/isocyanide insertion protocol on acyl C–C bond allowing an access to polyfunctionalized 2-acylpyrroles and seven-/eight-membered ring fused pyrroles from the reaction of α-acidic isocyanides with enones.35 As depicted in Scheme 20, the reaction of acyclic enones with isocyanide furnished two products 38 and 39. The formation of product 39 was favored in the presence of NaOH/DMF, while product 38 was formed exclusively under the presence of CuCl and DBU/ACN conditions. On the other hand, cyclic enedione exclusively afforded the desired product 40 in good yields.
The mechanistic pathway for the formation of acetyl pyrroles A and deacetyl pyrroles B is depicted in Scheme 21. First, TosMIC was co-ordinated with CuCl under the basic conditions, which generated carbanion by Michael addition. Afterwards, it followed two different pathways to form either 38 or 39. The formation of product 38 (path A) involved intramolecular α,α-addition through the cyclopropane intermediate A. Its subsequent ring opening followed by elimination furnished the desired product 38. However, the formation of 39 was rendered by the intramolecular cyclization of the intermediate B, elimination of the tosylic acid and hydrolysis of the acetyl group (path B) (Scheme 21).
In order to diversify annulated pyrrole scaffolds, Kelly and Leeper synthesized cycloalkano[c]-pyrroles 40–42 using 1,3-dipolar cycloaddition of Michael acceptor cycloalkenones and α-acidic isocyanides.10 This method is highly compatible to a variety of Michael acceptors as shown in Scheme 22. Moreover, this method has been utilized for the synthesis of conformationally constrained analogues of porphobilinogen, which is an important precursor of natural tetrapyrroles.
In continuation, Costi et al. utilized quinolinones 43 for the synthesis of annulated pyrroles. However, it involved subsequent steps of protection and deprotection. In order to further improve the yield and selectivity, a variety of protecting groups such as acetyl, mesyl, and Boc were used.36 Amongst them, Boc gave the best results under microwave irradiation and furnished the desired 2H-pyrrolo[3,4-b]quinolin-9(4H)-ones 44 with a yield of 55% (Scheme 23).
Initially, authors were attempting to synthesize 2H-pyrrolo[3,4-b]quinolin-9(4H)-one 44 in one step by the reaction of N-alkyl derivative A and TosMIC 1a. Unfortunately, the reaction yielded multiple products 43 (1.5%), B (30%) and 44 (2%). Out of these, the formation of product B could be rationalized on the basis of cleavage of the Boc group from the starting material and sequentially shifting it to the pyrrole ring (Scheme 24).
Moreover, Wang and co-workers utilized a C–C bond cleavage strategy for the construction of 2H-pyrrolo[3,4-c]quinoline derivatives 45 in 19–83% yields.37 The present protocol demonstrated a non-classical Van Leusen's pyrrole synthesis where C–C bond cleavage occurred during the final aromatization step. Initially, 1,3-dipolar cycloaddition of 3-phenacylideneoxindoles 45 with TosMIC 1a furnished highly strained spiro intermediate A, which subsequently underwent C–C bond cleavage, followed by aromatization and its reaction with MeOH, to afford the desired product 36. Here, t-BuOK functioned both as base and additive. The methodology was tolerant to a variety of functional groups present on the phenacylideneoxindole moiety. However, substituted TosMIC derivatives failed to give the desired product probably due to steric hindrance (Scheme 25).
Next, Zhao et al. utilized allenoates to access 3H and 1H pyrroles scaffolds, catalyzed by phosphine conditions.38 It is an attractive method due to its significant advantages such as highly efficient, use of cheap PPh3, easy to handle, and reactions occur under air. Different substituents on allenoates 47, isocyanoacetates as well as TosMIC are well tolerated and product 48 was formed in highly chemo- and regio-selective fashion with comparable yields (Scheme 26).
Mechanistically, intermediate ylide A was initially formed by the addition of PPh3 to allenoate, which consequently deprotonated and generated the ylide A followed by intramolecular cyclization, protonation and elimination furnished intermediate F, which eventually transformed to the final product 48 (Scheme 27).
Furthermore, Nair and co-workers studied a range of effective bases in Van Leusen's pyrrole synthesis and found that LiOH·H2O/EtOH can act as an effective base and also with cinnamaldehyde and thiophene-2-carboxaldehyde derivatives. Under optimized conditions, enolizable ketone and aromatic aldehydes in situ generated chalcones 48, which subsequently underwent 1,3-dipolar cycloaddition with TosMIC to afford the pyrrole derivatives 49 in 75–88% yield (Scheme 28).39
A plausible mechanism is depicted in Scheme 29. The first step involved the abstraction of a proton, followed by its stabilization by LiOH·H2O, which underwent [3 + 2] cycloaddition and afforded cycloadduct B. It was observed that polar solvents accelerated the equilibrium step by stabilizing the ionized species and facilitated the reaction rate (Scheme 29).
Similarly, Bi et al. also reported silver-catalyzed cyclization reactions between tertiary or secondary 2-pyridyl alkynyl carbinols and isocyanides, which divergently afforded 2,4-disubstituted pyrroles 50 and indolizines 51, respectively, in good to excellent yields.40 Herein, the reaction involved regioselective [3 + 2] cycloaddition of terminal alkynes with isocyanides to afford the 2,4-disubstituted pyrroles (Scheme 30). The present method showed wide substrate tolerance both on isocyanides and terminal alkynes.
The authors hypothesized that the coordination effect of pyridyl and alkynyl units facilitated the formation of complex A, which afforded complex B through further coordination with isocyanide (Scheme 31). It was further followed by the [3 + 2] cycloaddition of the alkynyl unit with isocyanide, to afford 2,4-disubstituted pyrroles C regioselectively, which oxidizes to furnish the desired product 50. On the other hand, desired indolizines 51 were generated from tertiary 2-pyridyl alkynyl carbinols via their silver acetylide intermediate. Next, 1,1′-insertion of the isocyanide into the Ag–C bond catalyzed to afford the acetylenic imido complex E, which subsequently underwent intramolecular rearrangement to produce 2,3-allenamides F followed by intramolecular cycloisomerization to afford the indolizines 51. The different reactivities of tertiary and secondary alkynyl carbinols can be attributed to the steric hindrance and easy cleavage of the hydroxyl group in case of tertiary alkynyl carbinols.
2.2 Oxazoles
Oxazoles have gained attraction due to their presence in various biologically active natural products and their utility as valuable precursors in many useful synthetic transformations.41 Basically, this core can be synthesized from Hantzsch reaction,42 Schmidt rearrangements,43 intramolecular alkyne additions,44 and the use of isocyanides as toluenesulfonylmethyl isocyanide (TosMIC).45 Due to their potential in diverse areas, still there is need for more diversity oriented synthesis for these heterocycles.Moreover, Ila and co-workers in 2013 demonstrated Cu(I)-catalyzed domino process for the diversity-oriented synthesis of 2,5,4′-trisubstituted-4,5′-bisoxazoles 44.46 The reaction displayed broad substrate scope and excellent functional group compatibility by employing a variety of heteroaryl and aryl-substituted oxazolones and activated methylene isocyanides (Scheme 32).
On the basis of preliminary results, the authors postulated a plausible mechanism for the above mentioned reaction, depicted in Scheme 33. Initially, α-cuprioisocyanide species A or its tautomer was generated in situ by the reaction of isocyanide with Cu catalyst. Next, intermediate A catalyzed the nucleophilic ring opening of oxazolone and in turn generated acyclic α-acylisonitrile intermediate B, which exists in equilibrium with its enolate counter C. Subsequently, intramolecular cyclization of intermediate C followed by protonation furnished oxazole. After the generation of oxazole, the same iterative process was repeated to give bis-oxazoles 53.
2.3 Naphthoxazoles
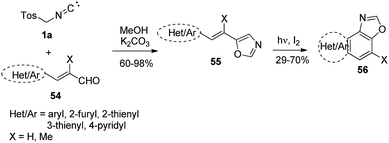
Naphthoxazole derivatives occupy a large domain of biologically significant activities exhibiting anti-trypanosidal, bacteriostatic, and cysteine protease inhibitor.47Kulyk and co-workers explored the synthesis of 5-(aryl/furyl/thienyl/pyridylethenyl)oxazoles, 55 via the reaction of α,β-unsaturated aldehydes 54 with TosMIC 1a (Scheme 34) in refluxing under basic conditions. Furthermore, the synthesized oxazoles 55 underwent photo-irradiation in the presence of iodine to afford fused heterobenzoxazoles 56. The present method not only provided access to naphthoxazoles but also to furyl, thienyl, and pyridyl substituted oxazoles in good yields.48
A plausible mechanism for the synthesis of naphthoxazoles is depicted in Scheme 35. Initially, the Ar/Het-ethenyloxazole derivatives 55 in trans-conformation was generated by the reaction of α,β-unsaturated aldehydes 54 with TosMIC 1a, which upon photochemical irradiation in a Rayonet reactor having 300 nm lamp afforded the cis-55 derivative. The generated dihydro-intermediate aromatizes in the presence of iodine to afford the title 5-(aryl/furyl/thienyl/pyridylethenyl)oxazoles 56.
2.4 Imidazoles
Imidazoles and their derivatives play an important role in the synthesis of natural products and exhibit biologically significant activities, for instance, anti-inflammatory, antiallergic, analgesic, antitumor agents and glucagon receptor antagonists.49 This class of compounds has wide application in biology, material science, and catalysis. This core is presumably present in a number of enzymes and metallo-enzymes in the biological systems and is a valuable drug candidate.50Furthermore, Cai et al. described an efficient en route towards the construction of indolyl imidazole derivatives 58 via base catalyzed reaction of N-[2-(1-alkynyl)phenyl]carbodiimides 57 with isocyanides. This reaction involved [3 + 2] cycloaddition of isocyanide to carbodiimide and its intramolecular cyclization to afford the desired products in good yields (Scheme 36).51
A possible pathway for the above mentioned transformation is depicted in Scheme 37. Initially, the reaction involved a base catalyzed reaction of isocyanides with the carbodiimide 57, which first generated the anion A, followed by its proton abstraction to form B and subsequently isomerized to 2-amino substituted imidazoyl derivative C, followed by its intramolecular cyclization to afford the indolyl imidazole derivative 58.
Bunev and co-workers also exploited the Van Leusen reaction for the construction of multisubstituted imidazoles 60. The reaction involves condensation reaction between substituted trifluoroacetimidoyl chlorides 59 and TosMIC 1a. This protocol provided a quick access to trifluoromethyl substituted 1,4,5-trisubstituted imidazoles 60 in good yields (Scheme 38) and tolerant to both electron-donating as well as electron-withdrawing groups.52
A possible mechanistic route is displayed in Scheme 39. It is presumed that the TosMIC molecule generated stable carbanion under basic conditions, which attacked on the substituted trifluoroacetimidoyl chlorides 59, resulted in the iminium intermediate B and its subsequent cyclization afforded the imidazoles 60.
Very recently, Zhu and co-workers attempted an efficient CuI catalyzed synthesis of unique heterocyclic core of 5-acetamidoimidazoles 63 by reacting isocyanides 1a, carbodiimide 61 and acyl chlorides 62 (Scheme 40) in 39–82% yield.53
The mechanism towards construction of the 5-substituted imidazoles is depicted in Scheme 41. Initial mixing of carbodiimide 61 and acyl chloride 62 generated N-acyl chloroformamidine intermediate A, which upon addition of copper iodide formed the hypothetical N-acyliminium intermediate B. On the other hand, copper(I)-coordinated isocyanide yielded the enolate anion. The later derivative attacked on the intermediate B and initiated a cascade of reactions, for instance, proton abstraction led by the intramolecular cyclization to furnish the desired derivative 63.
2.5 Benzofurans
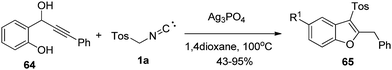
Benzofurans are known as a potent biodynamic class of molecules, which not only exhibits antiparasitic, antibiotic, antitumor, fluorescent molecules, antihyperglycemic, analgesic activities but also gained importance as privileged synthons in organic synthesis.54Bi and co-workers again attempted [3 + 2] cycloaddition reaction of α-acidic isocyanides and propargyl alcohols. They observed the dual role of TosMIC 1a both as a sulfonyl source and as a ligand in heteroaromatization of propargylic alcohols 64. The reaction went through the remarkable deoxysulfonylation/hydration/condensation cascade pathway for the formation of the sulfonyl benzofurans 65 (Scheme 42).55
A probable mechanistic pathway for the synthesis of benzofurans is outlined in Scheme 43. The first step involved the nucleophilic replacement of hydroxyl compound 64 by TosMIC 1a to generate intermediate A, which further reacted with water to form derivative B. It was found that conversion of 64 to 65 needed both TosMIC as well as a water molecule, where TosMIC acts as a ligand in silver catalyzed reaction. The derivative C followed keto-enol tautomerisation to generate intermediate D, which again went through a sequential addition/elimination cascade to afford the desired product 65.
2.6 Imidazo[1,2-a]pyridines
Imidazo[1,2-a]azines are drug prejudice scaffolds, which demonstrate a wide spectrum of biological activities such as antifungal, antiinflammatory, antitumor, analgesic, antibacterial, antiviral, hypnoselective, and antipyretic.56 There are many drugs such as zolpidem for insomnia, alpidem, necopidem and saripidem as anxiolytic agents, and minodronic acid to control osteoporosis in the market, which contain the imidazo[1,2-a]pyridine moiety. Due to the pharmaceutical importance of imidazo[1,2-a]azines, there is continuous effort to develop new methods for their synthesis.In continuation, Rahmati and co-workers developed a novel and efficient one-pot, four-component synthetic route of N-arylidene-2-aryl-imidazo[1,2-a]pyridin-3-amines/N-arylidene-2-arylimidazo[1,2-a]pyrazin-3-amines 68 from readily and cheaply available 2-aminopyridine 66, aldehydes 67, and TosMIC 1a (Scheme 44).57
3. Synthesis of six membered heterocycles
3.1 Pyrrolo[1,2-c]pyrimidine
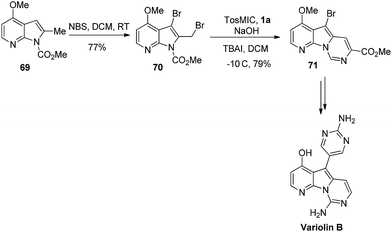
Similar to other nitrogenated heterocycles, the pyrrolo [2,3-d] pyrimidine or 7-deazapurine is an important scaffold, which exists in a vast number of biologically active natural compounds and synthetic drugs.58 In comparison to purine alkaloids, only rare reports have been published for their synthesis.To fill this gap, Vaquero and co-workers reported a formal synthesis of anti-proliferative natural alkaloid variolin B. The synthetic approach was started by treating analogue 69 with N-bromo succinimide (NBS) to form derivative 3-bromo-2-(bromomethyl)-4-methoxypyrrolo[2,3-b]pyridine 70. The latter derivative 70 was reacted with TosMIC 1a under basic conditions which resulted in the synthesis of 7-carboxylated pyrido[3′,2′,4,5]pyrrolo[1,2-c]pyrimidine derivative 71 (Scheme 45).9
The mechanistic pathway for the synthesis of derivative 71 is demonstrated in Scheme 46. Initially, TosMIC was allowed to react under basic and phase-transfer conditions to form the N-tosylmethyl dichloroformimide. The formation of 71 was started with the treatment of bromomethyl pyrrole 70 on the N-tosylmethyl dichloroformimide, followed by intramolecular transfer of methoxycarbonyl protecting group to form B. Furthermore, the removal of the chloride from the intermediate B and 1,2-elimination of p-tosylic acid led the cyclized product 71.
3.2 Quinolinones
Many alkaloids exhibit potent biological activities invariably due to the presence of quinolinone moiety. These analogues possess antioxidant, anti-inflammatory, and enzyme–inhibitor activities.59Captivated by the promising activities of this biologically elegant core, Cai and co-workers attempted its synthesis. The synthesis of functionalized quinolinones 73 was carried out by reacting N-(2-haloaryl)propiolamide 72 with isocyanides efficiently through copper-catalyzed tandem cycloaddition reaction (Scheme 47).60
Mechanistically, initially Cu–isocyanide was generated by the reaction of isocyanide with copper iodide, which attacked on N-(2-haloaryl)propiolamide 72 to form cyclic organocopper intermediates A–B through the formal [3 + 2] cycloaddition and generated C–C bond. Finally, tautomerism of the intermediate afforded the desired product 73 (Scheme 48).
3.3 Pyrrolo[2,3-c]quinoline
Pyrrolo[2,3-c]quinoline derivatives are another interesting class of biologically active heterocyclic natural products comprising antibacterial and antimalarial activities.61To attempt the first concise total synthesis of pyrroloquinoline natural product marinoquinoline 76, Yao and co-workers employed the key reaction between TosMIC 1a and α,β-unsaturated ester 74 under basic conditions. Initially, the reaction resulted in the formation of intermediate 75 and its subsequent cyclization to furnish the natural product marinoquinoline 76 by Morgen–Walls reaction (Scheme 49).62
Ji and co-workers developed an efficient and regioselective approach arising from 2-aminoarylacrylates/2-aminochalcones 77 and TosMIC 1a via Van Leusen reaction under basic conditions. In this regioselective transformation, 2-aminoarylacrylates and 2-aminochalcones resulted in the generation of 2H-pyrrolo[3,4-c]quinolines 78 and 2H-pyrrolo[3,4-c]quinolone 79 derivatives, respectively (Scheme 50).63
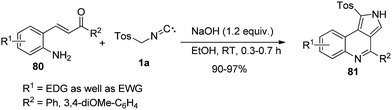
Moreover, Xu and co-workers developed tandem [3 + 2] cycloaddition followed by cyclization reaction of aminochalcones 80 with TosMIC 1a derivatives to furnish the diverse tricyclic pyrrolo[3,4-c]quinolones 81 (Scheme 51) in 90–97% yield under basic conditions.64
Mechanistically, the pathway involved Michael addition of TosMIC to aminochalcones 80 under the basic conditions providing carbanion intermediate A, followed by its intramolecular cyclization to generate the imidazoyl anion intermediate B; further elimination of tosylic acid provided the intermediate C; afterwards, intramolecular condensation of ketone with amine furnished the desired product 81 (Scheme 52).
 | ||
| Scheme 52 Mechanistic route towards the synthesis of tricyclic-pyrrolo-A-[3,4-c]quinolone derivatives. | ||
Another method to synthesize fused pyrrolo[2,3-c]quinolinones 83 was described by Wang and co-workers wherein (Z)-3-(2-oxo-2-ethylidene)indolin-2-one derivatives 82 were allowed to react with functionalized TosMIC under basic conditions in excellent yield (82–94%) (Scheme 53).65
The mechanism for the synthesis of 3H-pyrrolo[3,2-c]quinolinones 84 involved generation of an anion from 1-((cyclohexylidene(isocyano)methyl)sulfonyl)-4-methylbenzene, which attacked on the indoline derivative A, followed by the elimination of tosylic acid, which resulted in the generation of spiro derivative C. The latter derivative C induced base mediated ring opening and its subsequent cyclization to yield the target compound 84 (Scheme 54).
Liu and co-workers successfully implemented the tandem [3 + 2] cycloaddition/intramolecular imidoyl anion trapping strategy for the synthesis of 6,7-dihydro-1H-indol-4(5H)-ones 87 from alkenoyl-bis-(ketene dithioacetals) 86 and TosMIC 1a. These alkenoyl-bis-(ketene dithioacetals) 86 were isolated from the corresponding ketones 85 via the basic Knoevenagel condensation (Scheme 55).66
Mechanistically, first alkenoyl-bis-(ketene dithioacetals) 86 were allowed to react with TosMIC 1a under the basic conditions, which underwent [3 + 2] cycloaddition to generate imidoyl anion A. This was further trapped by intramolecularly tethered terminal carbonyl group, eliminated tosylic acid to yield B and spontaneous 1,5-H shift led to indol-4(5H)-ones 87 (Scheme 56).
3.4 Imidazo[1,5-a]quinoxalines
Imidazo[1,5-a]quinoxalines derivatives constitute a biologically important class and exhibit a wide variety of biological activities viz. antibacterial, antianxiolytic, and anticancer.67Hulme and coworkers adopted a modified Van Leusen protocol by employing arylglyoxaldehydes 88, masked amino nucleophile 89, and TosMIC 1a using deprotection-cyclisation strategy. The method provided a diversity oriented synthesis of biologically appealing imidazo[1,5-a]quinoxalines 90 (Scheme 57).68
3.5 Pyrazinones
Pyrazinones are important scaffolds from the therapeutic point of view and have been reported for applications in the areas of organic and medicinal chemistry.69 Similarly, mesoionic compounds have always fascinated chemists due to their masked 1,3-dipolar character.70In order to synthesize 2-(1H)-pyrazinones 92, Kawase and coworkers transformed mesoionic 1,3-oxazolium-5-olates 91 or münchnones with TosMIC 1a under oxygen atmosphere (Scheme 58).71
The mechanistic pathway is postulated in Scheme 59. Initially, TosMIC anion attacked on the C-2 position of the oxazole ring 91, followed by decarboxylation, which is due to the electron withdrawing effect of trifluoro group, furthermore addition of oxygen generated superoxide anion, followed by hydroperoxide anion G and finally extrusion of trifluoroacetate anion H to afford 2-(1H)-pyrazinones 92.
3.6 Benzoimidazothiazines
Benzoimidazothiazines core of many bioactive molecules have potential as valuable synthetic intermediates in organic chemistry.72 Cai and co-workers explored an efficient route for the synthesis of 5H-benzo[d]imidazo[5,1-b][1,3]thiazines 94 using the copper(I)-catalyzed tandem reaction of o-alkynylphenyl isothiocyanates 93 with isocyanides 1a with Cs2CO3 as a base. The reaction is based on [3 + 2] cycloaddition of the acidic isocyano group with the isothiocyanate and followed by the ring formation with the intervention of copper (Scheme 60).73Herein, we have discussed several examples of [3 + 2] cycloaddition of isocyanides, but the first example of [3 + 3] cycloaddition reactions of isocyanide was attempted by Liu and co-workers. In this reaction, α-metalated isocyanides74 were allowed to react with azomethine imine 95 and 1,3-dipoles 96, which in turn provided regio- and diastereoselectivity to a variety of 1,2,4-triazine 97 and 98 derivatives, respectively, in good yields (Scheme 61).75
Mechanistic details derived from experimental results are depicted in Scheme 62. The reaction was started with the initial formation of α-cuprioisocyanide, and it tautomerized to A, which underwent [3 + 3] cycloaddition on the imine and generated intermediate B. This intermediate B showed N-isocyanide insertion and formed imidoyl–copper complex C, which protonated to yield the 1,2,4-triazines, 97.
4. Miscellaneous reactions
To check the reactivity of TosMIC on Baylis–Hillman acetates, Yadav and co-workers reported an efficient synthesis of E-trisubstituted olefins 100 by allylic nucleophilic substitution of Baylis–Hillman acetates 99 with TosMIC 1a in the presence of BF3·OEt2 in good yields (Scheme 63). The present protocol worked well with both electron donating as well as electron withdrawing groups.76Moreover, Garima and co-workers observed an unexpected reaction of Baylis–Hillman alcohols 101 and TosMIC 1a catalyzed by Brønsted acidic ionic liquid-[Hmim]HSO4 resulting in the formation of corresponding sulfone derivatives 102 through nucleophilic attack of p-toluene-sulfinate anion (Scheme 64).77
To study the reaction of TosMIC towards Passerini reaction, Ramazani and co-workers synthesized highly substituted 2,2-disubstituted indane-1,3-dione derivatives 105. The reaction followed Passerini reaction of indane-1,2,3-trione 103, TosMIC 1a and substituted benzoic acids 104 at room temperature and in quantitative yields (Scheme 65).78
Furthermore, Ganem and co-workers reported an efficient method for the synthesis of alkoxymethylamides. The synthetic oxidation protocol started from TosMIC 1a, which generated tosylmethylisocyanate 106. The latter derivative 106 involved in nucleophilic addition reactions with alcohols, amines, and thiols, as well as with benzoic acid 107 to form N-tosylmethylbenzamides 108. The generated N-tosylmethylbenzamides 108 underwent an unusual substitution reaction in the presence of organocopper and organo-magnesium reagents to furnish N-(alkoxymethyl)benzamides 109 (Scheme 66).79
Mechanistically, N-tosylmethylbenzamides 109 generated anion A in the presence of an organometallic reagent and eliminated tosylsulfinate anion B, which attacked the imine and cyclized to generate acyloxaziridine intermediate D. Furthermore, the attack of the organometallic reagent at the oxaziridine oxygen furnished N-(alkoxymethyl)benzamides 110 (Scheme 67).
Vaquero and co-workers reacted 2-bromo-benzylbromide 111 with alkylated TosMIC derivatives 1i in the presence of organolithium and observed unexpected cascade processes for the synthesis of aryl-(or heteroaryl)cyano derivatives 112 (Scheme 68) in good yields.80
The reaction involved isocyanide-cyanide rearrangement. It initiated with the treatment of isocyanides with t-BuLi yielded 2,3-dihydro-1H-indenimines D, followed by further treatment with t-BuOK in t-BuOH to afford the E-vinylnitriles 112 (Scheme 69).
Williams and co-workers studied the reactivity of acyl anion equivalents (umpolung) en route towards the 1,3-hydroxy keto compounds. In short, they treated TosMIC 1a with butyl lithium to generate the imidazolyl anion 113, which further attacked on the epoxide ring to generate the enols 114. On the other hand, in the presence of BF3·Et2O, monoalkylated TosMIC derivative 115 was formed, which was subsequently dialkylated 117 under basic conditions (Scheme 70). Therefore, their investigation evaluated computationally and experimentally the reactivity of acyl anion equivalents in the epoxide ring opening.81
Yadav and co-workers reported the stereoselective synthesis of the segment C38–C54 of a marine metabolite halichondrin B. The key feature of the synthesis involved reaction of iodo derivative 118 with mono-alkylated TosMIC 1j, which resulted in the formation of double alkylated derivative 119. The latter derivative 119 was treated with boron tribromide to remove the acidic protecting groups and its subsequent cyclization to yield the product 120, which upon further functional group transformations yielded the halichondrin B (Scheme 71).82
The same group undertook and developed a highly stereoselective total synthesis of attenols A and B. The key feature of the synthesis involved double alkylation of TosMIC derivative 1k to construct the spiroacetal segment 122. This spiroacetalization strategy is one-pot, simple and efficient in contrast to dithiane mediated spiroacetalization protocol. The generated spiroacetal 188 after functional group transformations gave attenol A, 123 (Scheme 72).83
Yadav and co-workers discussed the stereoselective synthesis of the spiroketal fragment of the immune suppressant (−)-ushikulide A, 127. The key feature of this synthesis involved the construction of the spiroketal moiety, which was formed by the subsequent hydrolysis of a dialkylated TosMIC 126 derivative that is generated from monoalkylated TosMIC 125 (alkylation of TosMIC 1a) with suitably substituted iodohydrin derivatives 124 (Scheme 73).84
5. Conclusions
The present survey has clearly reflected that activated isocyanides have become part and parcel in the synthesis of diversified heterocyclic systems. Although tremendous advances have been achieved in this field, it is firmly believed that the applications of the TosMIC and its analogues to deliver novel scaffolds will continue to grow. These synthetically viable reagents possess high synthetic utility in modern organic, combinatorial and medicinal chemistry. There are many opportunities to tame the potential of this synthetically viable reagent. Among other isocyanides, these acidic isocyanides occupy an important place due to their exciting chemistry and for opening new avenues for other classes of molecules. They do help in advancing new cascade reactions/domino reactions in order to synthesize complex acyclic and cyclic systems viz. peptides, peptide molecules, and nitrogen heterocycles. These complexes not only have useful potential in synthetic organic chemistry, but also in the areas of inorganic, coordination and polymer chemistry. In a nut shell, they are perspective monomers with diverse functional groups and have unique qualities of isocyanide and carboxylic groups. Therefore, the exploration of α-acidic isocyanides will open new paradigms of synthetically challenging molecules.Acknowledgements
This work was financially supported by Department of Science and Technology (DST), Govt. of India (Grant no. SR/FT/CS-55/2011). The authors T.K. and P.W. thank MHRD for the award of postdoctoral and JRF fellowship, respectively.Notes and references
- (a) I. Ugi, A. Dömling and W. Hoerl, Endeavour, 1994, 18, 115–122 CrossRef CAS; (b) R. W. Armstrong, A. P. Combs, P. A. Tempest, S. D. Brown and T. A. Keating, Acc. Chem. Res., 1996, 29, 123–131 CrossRef CAS.
- I. Ugi and A. Dömling, Angew. Chem., Int. Ed., 2000, 39, 3168–3210 CrossRef.
- A. Dömling, Comb. Chem. High Throughput Screening, 1998, 1, 1–22 Search PubMed.
- (a) I. Ugi, Angew. Chem., Int. Ed., 1982, 94, 826–835 CrossRef CAS PubMed; (b) I. Ugi, A. Dömling, B. Gruber and M. Almstetter, Croat. Chem. Acta, 1997, 70, 631–647 CAS.
- U. Schöllkopf, Angew. Chem., Int. Ed., 1977, 89, 351–360 Search PubMed.
- V. K. Tandon and S. Rai, Sulfur Rep., 2003, 24, 307–385 CAS.
- Encyclopedia for Organic Synthesis, ed. L. A. Paquette, Wiley, New York, 1995, vol. 7, pp. 4973–4979 Search PubMed.
- A. M. Van Leusen and J. Strating, Q. Rep. Sulfur Chem., 1970, 5, 67–78 CAS.
- A. Baeza, J. Mendiola, C. Burgos, J. A. Builla and J. J. Vaquero, Eur. J. Org. Chem., 2010, 5607–5618 CrossRef CAS PubMed.
- J. M. Kelly and F. J. Leeper, Tetrahedron Lett., 2012, 53, 819–821 CrossRef CAS PubMed.
- A. Coppola, D. Sucunza, C. Burgos and J. J. Vaquero, Org. Lett., 2015, 17, 78–81 CrossRef CAS PubMed.
- D. Van Leusen and A. M. Van Leusen, Org. React., 2001, 57, 417–679 CAS.
- A. M. Van Leusen, G. J. M. Boerma, R. B. Helmholdt, H. Siderius and J. Strating, Tetrahedron Lett., 1972, 13, 2367–2368 CrossRef.
- U. Schöllkopf, R. Schröder and E. Bhime, Justus Liebigs Ann. Chem., 1972, 766, 130–141 CrossRef PubMed.
- B. E. Hoogenboom, O. H. Oldenziel and A. M. Van Leusen, Org. Synth., 1977, 57, 102–106 CrossRef CAS.
- J. Sisko, M. Mellinger, P. W. Sheldrake and N. H. Baine, Tetrahedron Lett., 1996, 37, 8113–8116 CrossRef CAS.
- A. M. VanLeusen, R. J. Bouma and O. Possel, Tetrahedron Lett., 1975, 40, 3487–3490 CrossRef.
- (a) W. W. Wilkerson, R. A. Copeland, M. Covington and J. M. Trzaskos, J. Med. Chem., 1995, 38, 3895–3901 CrossRef CAS; (b) H. Lee, J. Lee, S. Lee, Y. Shin, W. Jung, J.-H. Kim, K. Park, K. Kim, H. S. Cho and S. Ro, Bioorg. Med. Chem. Lett., 2001, 11, 3069–3072 CrossRef CAS.
- V. Bhardwaj, D. Gumber, V. Abbot, S. Dhimana and P. Sharma, RSC Adv., 2015, 5, 15233–15266 RSC.
- (a) L. Knorr, Chem. Ber., 1884, 17, 1635–1642 CrossRef PubMed; (b) C. Paal, Chem. Ber., 1885, 18, 367–371 CrossRef PubMed.
- A. Hantzsch, Ber. Dtsch. Chem. Ges., 1890, 23, 1474–1476 CrossRef PubMed.
- W. Chen, J. Shao, Z. Li, M. A. Giulianotti and Y. Yu, Can. J. Chem., 2012, 90, 214–221 CrossRef CAS.
- M. Adib, B. Mohammadi, E. Sheikhi and H. R. Bijanzadeh, Chin. Chem. Lett., 2011, 22, 314–317 CrossRef CAS PubMed.
- 1,1-Bis(organosulfanyl)alk-1-enes (Ketene S,S Acetals), in Science of Synthesis, Compounds with Four and Three Carbon-Heteroatom Bonds, ed. M. B. Nielsen, Georg Thieme, Stuttgart, 2014, p. 245 Search PubMed.
- (a) C. C. Hughes, A. P. Dave, P. R. Jensen and W. Fenical, Org. Lett., 2008, 10, 629–631 CrossRef CAS PubMed; (b) C. C. Hughes, Y. L. Yang, W. T. Liu, P. C. Dorrestein, J. L. La Clair and W. Fenical, J. Am. Chem. Soc., 2009, 131, 12094–12096 CrossRef CAS PubMed; (c) K. C. Nicolaou, N. L. Simmons, J. S. Chen, N. M. Haste and V. Nizet, Tetrahedron Lett., 2011, 52, 2041–2043 CrossRef PubMed.
- F. Qiu, J. Wu, Y. Zhang, M. Hu and Y. Yu, Tetrahedron Lett., 2012, 53, 446–448 CrossRef CAS PubMed.
- R. Wang, S. Y. Wang and S.-J. Ji, Org. Biomol. Chem., 2014, 12, 1735–1740 CAS.
- A. Padmaja, K. Syamaiah, A. Muralikrishna and V. Padmavathi, Indian J. Chem., 2012, 51, 285–290 CrossRef.
- V. Padmavathi, B. J. M. Reddy, K. Mahesh, P. Thriveni and A. E. Padmaja, J. Heterocycl. Chem., 2013, 47, 825–830 CrossRef PubMed.
- V. Padmavathi, N. Revathi, C. Rajasekhar and C. Premakumari, J. Heterocycl. Chem., 2013, 50, 579–584 CrossRef CAS PubMed.
- H. Wang, Y. L. Zhao, C. Q. Ren, A. Diallo and Q. Liu, Chem. Commun., 2011, 47, 12316–12318 RSC.
- T. Wu, L. Pan, X. Xua and Q. Liu, Chem. Commun., 2014, 50, 1797–1800 RSC.
- S. Yugandar, N. C. Misra, G. Parameshwarappa, K. Panda and H. Ila, Org. Lett., 2013, 15, 5250–5253 CrossRef CAS PubMed.
- P. Dhanalakshmi and S. Shanmugam, RSC Adv., 2014, 4, 29493–29501 RSC.
- L. Zhang, X. Xu, Q. R. Shao and L. P. Q. Liu, Org. Biomol. Chem., 2013, 11, 7393–7399 CAS.
- F. Rosi, G. C. Crucitti, A. Iacovo, G. Miele, L. Pescatori, R. D. Santo and R. Costi, Synth. Commun., 2013, 43, 1063–1072 CrossRef CAS PubMed.
- R. Wang, X.-P. Xu, H. Meng, S.-Y. Wang and S. J. Ji, Tetrahedron, 2013, 69, 1761–1766 CrossRef CAS PubMed.
- J.-Y. Liao, P.-L. Shao and Y. Zhao, J. Am. Chem. Soc., 2015, 137, 628–631 CrossRef CAS PubMed.
- R. Sharma, K. Kumar, M. Chouhan, V. Grover and V. A. Nair, RSC Adv., 2013, 3, 14521–14527 RSC.
- X. Meng, P. Liao, J. Liu and X. Bi, Chem. Commun., 2014, 50, 11837–11839 RSC.
- (a) I. J. Turchi and M. J. S. Dewar, Chem. Rev., 1975, 75, 389–437 CrossRef CAS; (b) H. H. Wasserman and R. J. Gambale, J. Am. Chem. Soc., 1985, 107, 1423–1424 CrossRef CAS.
- (a) T. R. Kelly and F. Lang, J. Org. Chem., 1996, 61, 4623–4633 CrossRef CAS PubMed; (b) J. S. Panek and R. T. Beresis, J. Org. Chem., 1996, 61, 6496–6497 CrossRef CAS.
- M. Lautens and A. Roy, Org. Lett., 2000, 2, 555–557 CrossRef CAS PubMed.
- J. Sisko, A. J. Kassick, M. Mellinger, J. J. Filan, A. Allen and M. A. Olsen, J. Org. Chem., 2000, 65, 1516–1524 CrossRef CAS PubMed.
- (a) P. Wipf, L. T. Rahman and S. R. Rector, J. Org. Chem., 1998, 63, 7132–7133 CrossRef CAS; (b) A. Arcadi, S. Cacchi, L. Cascia, G. Fabrizi and F. Marinelli, Org. Lett., 2001, 3, 2501–2504 CrossRef CAS PubMed; (c) P. Wipf, Y. Aoyama and T. E. Benedum, Org. Lett., 2004, 6, 3593–3595 CrossRef CAS PubMed; (d) P. Wipf and T. H. Graham, Org. Biomol. Chem., 2005, 3, 31–35 RSC; (e) M. D. Milton, Y. Inada, Y. Nishibayashi and S. Uemura, Chem. Commun., 2004, 2712–2713 RSC.
- S. Yugandar, A. Acharya and H. Ila, J. Org. Chem., 2013, 78, 3948–3960 CrossRef CAS PubMed.
- (a) F. Haviv, J. D. Ratajczyk, R. W. DeNet, F. A. Kerdesky, R. L. Walters, S. P. Schmidt, J. H. Holms, P. R. Young and G. W. Carter, J. Med. Chem., 1988, 31, 1719–1728 CrossRef CAS; (b) A. M. Osman and I. Bassiouni, J. Org. Chem., 1962, 27, 558–561 CrossRef CAS; (c) M. E. McGrath, P. A. Sprengeler, C. M. Hill, V. Martichonok, H. Cheung, J. R. Somoza, J. T. Palmer and J. W. Janc, Biochemistry, 2003, 42, 15018–15028 CrossRef CAS PubMed.
- I. Sagud, F. Faraguna, Z. Marini and M. S. Kulyk, J. Org. Chem., 2011, 76, 2904–2908 CrossRef CAS PubMed.
- J. G. Lambardino and E. H. Wiseman, J. Med. Chem., 1974, 17, 1182–1188 CrossRef.
- A. P. Philips, H. L. White and S. Rosen, Eur. Pat. Appl., EP 58890, 1982.
- W. Hao, Y. Jiang and M. Cai, J. Org. Chem., 2014, 79, 3634–3640 CrossRef CAS PubMed.
- A. S. Bunev, M. A. Vasiliev, V. E. Statsyuk, G. I. Ostapenko and A. S. Peregudov, J. Fluorine Chem., 2014, 163, 34–37 CrossRef CAS PubMed.
- G. Sapuppo, Q. Wang, D. Swinnen and J. Zhu, Org. Chem. Front., 2014, 1, 240–246 RSC.
- H. Khanam and Shamsuzzaman, Eur. J. Med. Chem., 2015, 97, 483–504 CrossRef PubMed.
- J. Liu, Z. Liu, P. Liao and X. Bi, Org. Lett., 2014, 16, 6204–6207 CrossRef CAS PubMed.
- R. B. Lacerda, C. K. F. de Lima, L. L. da Silva, N. C. Romeiro, A. L. P. Miranda, E. J. Barreiro and C. A. M. Fraga, Bioorg. Med. Chem., 2009, 17, 74–84 CrossRef CAS PubMed.
- A. Rahmati, A. Moazzam and Z. Khalesi, Tetrahedron Lett., 2014, 55, 3840–3843 CrossRef CAS PubMed.
- T. E. Renau, C. Kennedy, R. G. Ptak, J. M. Breitenbach, J. C. Drach and L. B. Townsend, J. Med. Chem., 1996, 39, 3470–3476 CrossRef CAS PubMed.
- (a) S. Angeleska, P. Kefalas and A. Detsi, Tetrahedron Lett., 2013, 54, 2325–2328 CrossRef CAS PubMed; (b) A. Detsi, D. Bouloumbasi, K. C. Prousis, M. Koufaki, G. Athanasellis, G. Melagraki, A. Afantitis, O. Igglessi-Markopoulou, C. Kontogiorgis and D. J. H. -Litina, J. Med. Chem., 2007, 50, 2450–2458 CrossRef CAS PubMed; (c) E. A. Larsson, A. Jansson, F. M. Ng, S. W. Then, R. Panicker, B. Liu, K. Sangthongpitag, V. Pendharkar, S. J. Tai, J. Hill, C. Dan, S. Y. Ho, W. W. Cheong, A. Poulsen, S. Blanchard, G. R. Lin, J. Alam, T. H. Keller and P. Nordlund, J. Med. Chem., 2013, 56, 4497–4508 CrossRef CAS PubMed.
- F. Zhou, J. Liu, K. Ding, J. Liu and Q. Cai, J. Org. Chem., 2011, 76, 5346–5353 CrossRef CAS PubMed.
- P. W. Okanya, K. I. Mohr, K. Gerth, R. Jansen and R. Muller, J. Nat. Prod., 2011, 74, 603–608 CrossRef CAS PubMed.
- L. Ni, Z. Li, F. Wu, J. Xu, X. Wu, L. Kong and H. Yao, Tetrahedron Lett., 2012, 53, 1271–1274 CrossRef CAS PubMed.
- X.-M. Lu, J. Li, Z.-J. Cai, R. Wang, S.-Y. Wang and S.-J. Ji, Org. Biomol. Chem., 2014, 12, 9471–9477 CAS.
- Z. Hu, Y. Li, L. Pan and X. Xu, Adv. Synth. Catal., 2014, 356, 2974–2978 CrossRef CAS PubMed.
- R. Wang, S.-Y. Wang and S.-J. Ji, Tetrahedron, 2013, 69, 10836–10841 CrossRef CAS PubMed.
- Y. Li, X. Xu, H. Shi, L. Pan and Q. Liu, J. Org. Chem., 2014, 79, 5929–5933 CrossRef CAS PubMed.
- (a) P. Sanna, A. Carta, M. Loriga, S. Zanetti and L. Sechi, Il Farmaco, 1998, 53, 455–461 CrossRef CAS; (b) L. E. Seitz, W. J. Suling and R. C. Reynolds, J. Med. Chem., 2002, 45, 5604–5606 CrossRef CAS PubMed.
- F. D. Moliner and C. Hulme, Org. Lett., 2012, 14, 1354–1357 CrossRef PubMed.
- (a) V. P. Mehta, A. K. Sharma, S. G. Modha, S. Sharma, T. Meganathan, V. S. Parmar and E. Van der Eycken, J. Org. Chem., 2011, 76, 2920–2925 CrossRef CAS PubMed; (b) P. Ortqvist, J. Gising, A. E. Ehrenberg, A. Vema, A. Borg, A. Karlen, M. Larhed, U. H. Danielson and A. Sandstrom, Bioorg. Med. Chem., 2010, 18, 6512–6525 CrossRef PubMed.
- Mesoionic Ring Systems, in Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products, ed., A. Padwa and W. H. Pearson, John Wiley & Sons, Hoboken, 2002, vol. 59, p. 681 Search PubMed.
- R. Saijo, K. I. Kurihara, K. Akira, H. Uno and M. Kawase, Tetrahedron Lett., 2013, 54, 4418–4421 CrossRef CAS PubMed.
- (a) T. Besson, G. Guillaumet, C. Lamazzi and C. W. Ress, Synlett, 1997, 704–706 CrossRef CAS PubMed; (b) A. Hari and B. L. Miller, Org. Lett., 2000, 2, 3667–3670 CrossRef CAS PubMed; (c) C. Gimbert and A. Vallribera, Org. Lett., 2009, 11, 269–271 CrossRef CAS PubMed.
- W. Hao, J. Zeng and M. Cai, Chem. Commun., 2014, 50, 11686–11689 RSC.
- (a) A. V. Lygin and A. de Meijere, Angew. Chem., Int. Ed., 2010, 49, 9094–9124 CrossRef CAS PubMed; (b) M. Gao, C. He, H. Chen, R. Bai, B. Cheng and A. Lei, Angew. Chem., Int. Ed., 2013, 52, 6958–6961 CrossRef CAS PubMed.
- J. Du, X. Xu, Y. Li, L. Pan and Q. Liu, Org. Lett., 2014, 16, 4004–4007 CrossRef CAS PubMed.
- J. S. Yadav, B. V. S. Reddy, A. P. Singh and N. Majumder, Tetrahedron Lett., 2010, 51, 2291–2294 CrossRef CAS PubMed.
- Garima, V. P. Srivastava and L. D. S. Yadav, Tetrahedron Lett., 2011, 52, 4622–4626 CrossRef CAS PubMed.
- A. R. Kazemizadeh and A. Ramazani, ARKIVOC, 2008, XV, 159–163 CrossRef.
- H. V. Le and B. Ganem, Tetrahedron Lett., 2014, 55, 2003–2005 CrossRef CAS PubMed.
- A. Coppola, P. S. Alonso, D. Sucunza, C. Burgos, R. Alajaŕın, J. A. Builla, M. E. G. Mosquera and J. J. Vaquero, Org. Lett., 2013, 15, 3388–3391 CrossRef CAS PubMed.
- W. A. Eger, R. L. Grange, H. Schill, R. Goumont, T. Clark and C. M. Williams, Eur. J. Org. Chem., 2011, 2548–2553 CrossRef CAS PubMed.
- J. S. Yadav, C. N. Reddy and G. Sabitha, Tetrahedron Lett., 2012, 53, 2504–2507 CrossRef CAS PubMed.
- J. S. Yadav, P. A. N. Reddy, Y. J. Reddy, S. Meraj and A. R. Prasad, Eur. J. Org. Chem., 2013, 6317–6324 CrossRef CAS PubMed.
- J. S. Yadav, N. M. Reddy and Md. A. Rahman, Eur. J. Org. Chem., 2014, 5574–5581 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |