The first dye-sensitized solar cell with p-type LaOCuS nanoparticles as a photocathode†
Abstract
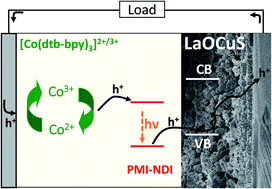
Layered LaOCuS oxysulfide is a well-known wide band gap p-type semiconductor that has attracted strong interests for transparent electronics. We report here that nanoparticles of this material can also be used as a substitute for the widely used NiO compound to fabricate photocathodes for p-type dye sensitized solar cells.

 Please wait while we load your content...
Please wait while we load your content...