Pathways involved in acetaminophen hepatotoxicity with specific targets for inhibition/downregulation
Govindarajan Karthivashana,
Palanisamy Arulselvana and
Sharida Fakurazi*ab
aLaboratory of Vaccines and Immunotherapeutics, Institute of Bioscience, Universiti Putra Malaysia, Serdang, Selangor 43400, Malaysia
bDepartment of Human Anatomy, Faculty of Medicine and Health Sciences, Laboratory of Vaccines and Immunotherapeutics, Institute of Bioscience, Universiti Putra Malaysia, Serdang, Selangor 43400, Malaysia. E-mail: sharida@upm.edu.my; Fax: +60 3 8942 2341; Tel: +60 3 8947 2117
First published on 23rd June 2015
Abstract
The liver, a vital organ in our body, is highly prone to numerous diseases/disorders. The most prolific among the extensive array of liver disorders and challenging to the clinical/pharmaceutical industries is drug-induced hepatotoxicity. Acetaminophen (APAP), recommended as a first-line analgesic therapy and prescribed as an over-the-counter (OTC) medication, possesses an excellent safety profile when administered in therapeutic dosages, but hepatotoxicity can occur when overdosed during misuse/combinational therapy, that can be disastrous. It is well recognized that the pathogenesis of APAP induced hepatotoxicity involves the contribution of the parent drug/its metabolites [NAPQI (N-acetyl-p-benzoquinone imine)] that directly and/or indirectly modulate the bioenergetics of cells through various metabolic disturbances and immune reactions. In spite of the many recent scientific reports which have established various key signal transduction pathways, the streaming events involved in this mechanism are not easily monitored, which have the profound impact of challenging the clinical/pharmaceutical research. In compiling recent research perspectives, in this review, a flow of the chronological events involved in APAP hepatotoxicity, its molecular pathways and triggered immunological responses have been elucidated. This scientific information provides strong hypothetical rationale to create a vivid picture regarding the underlying pathogenesis of this disease and thereby establish new prophylactic/therapeutic interventions by targeting a few specific molecular targets, which have also been established from the signalling pathways. In brief, this review constructs/establishes the underlying mechanism of APAP induced hepatotoxicity and its molecular/immunological aspects based on the recent advancements in this research area.
Introduction
The liver, being the largest internal organ in the body, characteristically performs various pivotal roles. Its multiple tasks include: processing and storing body nutrients, secretion of bile for fat digestion, synthesizing various important proteins, including blood clotting factors and transporter proteins such as lipoproteins and albumin, and converts potentially toxic substances into innocuous forms through the (xenobiotic) process of excretion.1 The xenobiotic metabolism of the liver is interrupted when toxins (i.e., therapeutic drugs/hepatotoxic chemicals) begin to enter the bloodstream at a rate faster than the liver’s ability to metabolize them.2 Drug induced hepatotoxicity (DIH) is a frequent cause of liver impairment. It is responsible for more than one and a half percent of the worldwide acute liver failure cases and causes all sorts of acute and chronic hepatic complications.3 The most commonly quoted cause for drug-induced hepatotoxicity is acetaminophen [APAP].4 It is available under different brand names as an over-the-counter (OTC) or prescribed medication. At normal therapeutic doses, acetaminophen is a good analgesic, though it produces toxic by-products, the liver usually detoxifies them and flushes them out in the bile. However, at a very high dosage or in combination with alcohol/combinational therapy, acetaminophen ingestion turns out to be an impending cause for life-threatening acute liver damage.5 According to the WHO Collaborating Centre for International Drug Monitoring, APAP is the ultimate amongst the top 10 drugs associated with fatal liver injury.6 In the United States, amidst the reported APAP induced hepatotoxicity cases, few are intentional (suicide attempts) whereas around 50% of deaths and emergency room visits are due to unintentional overdoses of acetaminophen.7 Acetaminophen has also been reported as the most common drug overdosed either accidentally or intentionally in the United Kingdom (UK), with an estimated 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cases annually; Canada had a yearly occurrence of 46 per 100
000 cases annually; Canada had a yearly occurrence of 46 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 population between 1997–2002 and a low profile of around 2% among the European countries.8,9 In the general population, a substantial percentage of patients consume an excessive amount of acetaminophen as they misread the dosing instructions or are unaware of the presence of acetaminophen as a combinational therapy with other drugs, and is more common among illiterates or heavy acetaminophen consumers.10 The incidence of APAP overdose in the population with other medical complications, such as metabolic syndrome including hypertension, dyslipidemia, obesity, diabetes mellitus and insulin resistance, furthermore exacerbate the primary medical complication. Recent studies reported the protective role of APAP at therapeutic doses against metabolic disorders.11,12 However, the incidence of an APAP overdose caused elevated oxidative stress and inflammation, thereby inducing more severe liver injury in obese, type 2 diabetic and NAFLD induced animals.13–15 This indicates the impact of an APAP overdose in both general and vulnerable populations with other medical complications and hence drives the FDA to propose ways to limit the hepatotoxicity of acetaminophen via reducing its therapeutic index/minimizing combinational therapy.16
000 population between 1997–2002 and a low profile of around 2% among the European countries.8,9 In the general population, a substantial percentage of patients consume an excessive amount of acetaminophen as they misread the dosing instructions or are unaware of the presence of acetaminophen as a combinational therapy with other drugs, and is more common among illiterates or heavy acetaminophen consumers.10 The incidence of APAP overdose in the population with other medical complications, such as metabolic syndrome including hypertension, dyslipidemia, obesity, diabetes mellitus and insulin resistance, furthermore exacerbate the primary medical complication. Recent studies reported the protective role of APAP at therapeutic doses against metabolic disorders.11,12 However, the incidence of an APAP overdose caused elevated oxidative stress and inflammation, thereby inducing more severe liver injury in obese, type 2 diabetic and NAFLD induced animals.13–15 This indicates the impact of an APAP overdose in both general and vulnerable populations with other medical complications and hence drives the FDA to propose ways to limit the hepatotoxicity of acetaminophen via reducing its therapeutic index/minimizing combinational therapy.16
Acetaminophen hepatotoxicity passes the preclinical/clinical trial investigations due to genetic variations among individuals and its infrequent occurrence among the tested study candidates. Though, the therapeutic window of acetaminophen has been narrowed down by the FDA, the occurrence rate of toxicity still remains the same.17
Presently, only a few blood-based tests on total bilirubin content and alkaline phosphatase (ALP), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) enzyme activity are available as biomarkers to assess acetaminophen overdose, though they are not precise for APAP toxicity and can take place due to other medical complications, such as hepatitis, steatosis and liver cancer, still they are utilised for preliminary analysis of the extent of liver damage.18 In terms of treatment, currently the most acceptable and effective treatment for acetaminophen hepatotoxicity is supplement therapy using the clinically accepted antidote, N-acetyl-cysteine (NAC).18 However, NAC therapy involves a dose-dependent prothrombin time prolongation and is effectual in restoring depleted glutathione levels only when administered within a stipulated period of the APAP ingestion.19,20
The inaccessibility of an effective treatment against acetaminophen hepatotoxicity is confined due to the obscure mode of action of acetaminophen hepatotoxicity. These observations clearly suggest that further insight into the immunological and molecular mechanisms of APAP hepatotoxicity would help researchers/clinicians/pharmaceutical industries to identify novel biomarkers, effective bioactive candidates and fruitful therapies against acetaminophen hepatotoxicity.
Though there are a few excellent reviews on acetaminophen induced hepatotoxicity, in this review we mainly discuss the existing pharmaceutical strategies with emphasis on the immunological mechanism and molecular targets involved in acetaminophen induced hepatotoxicity and also propose the pathway, with recent updates on its biomarkers.
Acetaminophen-induced hepatoxicity background
Acetaminophen is an effective analgesic at prescribed therapeutic doses (Fig. 1a), almost 95% of the acetaminophen dose is predominantly metabolized by glucuronic acid/sulfotransferases to form glucuronate and sulfate conjugates and are excreted through urine. Approximately 5 to 10% of the acetaminophen dose is oxidized by cytochrome P450 2E1 (CYP2E1) to generate a highly reactive, toxic, short-lived metabolite, N-acetyl-p-benzoquinone imine (NAPQI). This NAPQI in turn conjugates with glutathione (GSH) and is detoxified by the formation of 3-glutathionyl acetaminophen (non-toxic conjugate) and is eliminated through urine.21In the case of an acetaminophen overdose (Fig. 1b), the acetaminophen fraction which is usually metabolized by the glucuronidation and sulfation process, becomes saturated and thereby, oxidation mediated by cytochrome P450 2E1 is increased. These unbalanced metabolization events result in elevated levels and accumulation of NAPQI (a toxic metabolite). The rate of formation of NAPQI overwhelms hepatic GSH, resulting in hepatic GSH depletion in the cytosol and mitochondria. Moreover, the excessive amount of NAPQI covalently binds with the available cellular protein thiols as well as non-protein thiols and causes protein dysfunction and cell death, and thus results in triggering the hepatic immune system.22
Liver immune system
The human liver is a site of intense immunological activity and involves up to 1010 inhabitant lymphocytes that includes B lymphocytes, T lymphocytes, natural killer cells and natural killer T cells.23 Inflammation causes the activation of lymphocytes and their deployment at the intra-hepatic site, thereby regulating the disease progression. APAP-induced hepatotoxicity is habitually linked with the lymphocytic infiltration mediated inflammation,24 which determines the progression/severity of the hepatic injury. Hepatic cellular dysfunction and/or death induced by APAP, initiates sequestered immune reactions, comprising both innate and adaptive immune cascades.APAP-induced innate immune response
APAP overdose-induced tension i.e., NAPQI-induced hepatic damage, leads to the innate immune cell activation and subsequently stimulates hepatic inflammatory infiltration. Activated innate immune cells develop an array of pro-inflammatory mediators such as cytokines, chemokines, and oxidative stress markers that are involved in the further exacerbation of the hepatic injury. Among them, IFN-γ/Fas/Fas ligands have been reported for their direct involvement in the induction of hepatic damage; Fas receptor knockout mice exhibited a protective effect against a 300 mg kg−1 APAP overdose but failed at higher doses. However in this study the very possible markers involved in the APAP overdose mechanism such as the GSH levels were not evaluated.25 In another work, mutant mice lacking Fas factors were reported to partially reduce the hepatic injury resulting from APAP induced hepatotoxicity.26 Nevertheless the results of these studies were not promising as these investigations were carried out using DMSO, that might have falsified the results. But if Fas/its ligand is directly involved in APAP-induced cell death, this would be through apoptosis, which remains controversial for it to occur in acetaminophen-induced hepatotoxic models. On the other hand, recent research articles have reported that apoptosis plays a vital role in initiating the events that lead to a parallel time-dependent hepatic necrosis caused by APAP in vivo (Fig. 5), which indicatively represent the co-existence of apoptotic and necrotic cell death in APAP hepatotoxicity.27–29Besides, the innate immune cells also produce hepatoprotective factors such as COX-2, IL-6 and IL-10 to retain the homeostasis, which was proven by a study where the transgenic mice deficient of these factors were more susceptible to APAP-induced liver damage (Fig. 2).30 The frequently reported innate immune cells in APAP hepatotoxicity were hepatic residing macrophages, KC, NK and NKT cells.31 Other than these hepatic residing innate immune cells, leukocytes such as neutrophils and monocytes will also be recruited at the inflammatory site. Macrophages are a significant cellular factor of the innate immune system. Phagocytising or inducing toxicity on foreign cells, and eradicating them is the major role of macrophages. It was previously identified that two major classes of macrophages exist – classically stimulated macrophages (M1) and alternatively stimulated macrophages (M2). M1 macrophages are involved in the production of proinflammatory cytokines and bacteriocidal intermediaries, however, M2 plays the most significant role of downregulating inflammation, tissue remodelling, riddance of tissue debris and also the induction of angiogenesis.32,33 A study projected dissimilarities among the APAP-induced infiltrating macrophages and resident Kupffer cells (KC).34 In accordance, the non-parenchymatic hepatocytes of APAP administered mice, expressed two sets of macrophages i.e., the resident KC (CD11b low F4/80 high) and infiltrating macrophage population (CD11b high F4/80 low). These infiltrating macrophages expressed a unique set of genes, such as Ym1, a secretory protein which has an affinity towards heparin; Fizz1, a protein endowed with cysteine; arginase1, which competes with NO synthetase and is collectively recognized as the marker of M2 cells. They also revealed matrix metalloproteinase-12 and 9 (MMP-12 & MMP-9), the macrophage C-type lectin precursor for galactose/N-acetylgalactosamine and the mannose receptor which are reported as key markers in the tissue reconstruction mechanism.34
The Kupffer cells are the largest population of resident macrophages in the liver, which act as a major source of inflammatory mediators i.e., cytokines, nitric oxide, superoxide, chemokines, lysosomal and proteolytic enzymes that are involved in increased cytotoxicity and chemotaxis. Studies have reported that KC induce the increased production of the chemokine MCP-1 in APAP mediated liver injury. KC also play a major role in the activation of NK and NKT cells through specific cytokines.35,36 A few studies have revealed that NK and NKT cell depleted mice showed protective effects against APAP intoxicated liver damage thereby eliminating IFN-γ production and decreasing neutrophil accumulation in the liver,37 which was later reported as the effect of DMSO usage during experimentation.38,39 Though there are conflicting results regarding the role of NK and NKT cells, further studies are required to determine their role in APAP hepatotoxicity.
APAP induced liver injury involves the activation of KC (Fig. 2), resulting in inducing TNF-α-mediated tissue damage, MCP-1 pathogenesis and also inducing the production of IL-12 and IL-18, the foremost activators of IFN-γ and Fas.40,41 In contrast, KC also play a protective role by the production of ELR-CXC chemokines such as MIP-2, which induce hepatocyte proliferation40–43 and also KC extend their protective role by inducing IL-10, IL-6 and prostaglandins which are involved in the stimulation of liver regeneration and inhibit the inflammatory cascade.44 As a result of the natural immune response-inflammatory cascade, the adaptive immune system sequentially becomes activated. In that aspect, various proposed mechanisms explained the adaptive immune system mechanism. The most reliable scenarios have been depicted as follows.
Elucidation of KC mediated-adaptive immune cascade
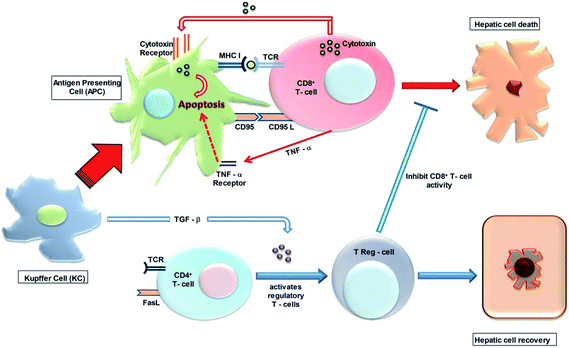
Kupffer cells (KC) may cause hepatic impairment by inducing apoptosis through the CD95L-CD95 pathway. KC act as antigen presenting cells (APC) of drug–protein adducts or chemokine secreting injured hepatic cells.45 On activation (Fig. 3), KC induce both cytotoxic T-cells, CD8+ T-cells and CD4+ T-cells, facilitating activation of regulatory T-cells (Treg-cells) via specific chemokines. KC expressed with CD95 and major histocompatibility complex (MHC) class I, act as antigen presenting cells (APC), thereby attracting cytolytic T-cells. CD8+ T-cells possess TLR (Toll like receptor) and CD95L which bind with MHC class I and CD95 of APC respectively. This contact directly activates the CD8+ T-cells to exterminate KC via releasing cytotoxins (e.g. perforin and granzyme)46 or by activating TNF-α-induced apoptosis, which in turn further exacerbate hepatic cell death. Conversely, KC also exude TGF-β (transforming growth factor), which activates T-reg cells, to inhibit CD8+ T-cell activity. In addition, by secreting growth factors and inhibiting pro-inflammatory signals, KC play a hepatoprotective role.47Elucidation of macrophage mediated-adaptive immune cascade
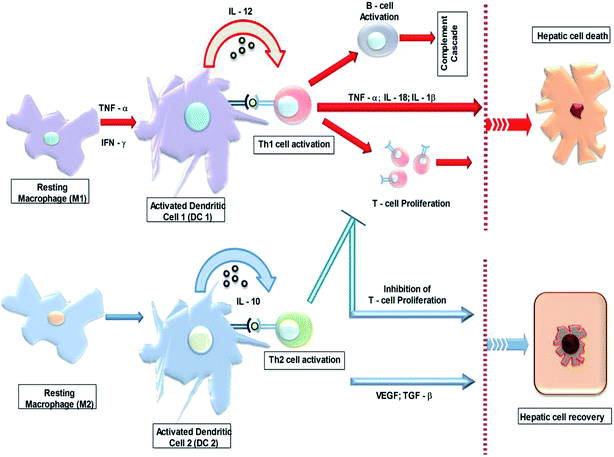
Macrophages can perfectly act as APC/dendritic cells (DCs), by processing the antigen and presenting them via MHC class I and II, thereby triggering the adaptive immune responses.48,49 As previously mentioned there are two classes of macrophages – M1 and M2; here the DCs also have been hypothetically classified as M1 derived DC1 and M2 derived DC 2. Injured hepatic cells secrete cytokines – such as TNF-α to induce DC 1. In Fig. 4, the activated DC 1 releases IL-12, which promotes a sturdy Th1 immune response. Thereby, it may induce B-cell activation and lead to a humoral mediated immune response, through immunoglobulins and activate a complementary cascade. In addition, M1 releases ROS, RNS and pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β).41,50 The synergistic mechanism is directed to the major contribution of the macrophage mediated tissue injury.51,52 On the contrary, the M2 derived DC 2 releases IL-10, which promotes the Th2 immune response which is subsequently followed by an immunosuppressive activity by inhibiting T-cell proliferation. Synergistically, M2 plays a major role in the phagocytising of apoptotic neutrophils and deceasing cell debris, inhibiting pro-inflammatory signals, and increasing the synthesis of mediators (i.e., VEGF and TGF-β) that are important in tissue remodelling, angiogenesis and wound healing.50In brief, hepatic injury is induced by toxic metabolites initiating a sequence of immunological responses such as innate immune cell activation, cytokine and chemokine release and the activation of the adaptive immune response through drug-specific T-cell and B-cell reactions. The macrophages/resident KC play pivotal roles in activating the adaptive immune cells. They are involved both in inducing inflammation via proinflammatory cytokines and also in the hepatoprotective role through tolerogenic factors.
Proposed hepatotoxcity pathway-induced by APAP overdose
APAP-induced hepatotoxicity involves a complex sequestered molecular mechanism which has been clearly elucidated (Fig. 5) as follows.NAPQI mediated GSH depletion and ROS generation
As discussed earlier, APAP overdose leads to the formation and accumulation of the reactive toxic metabolite NAPQI in the hepatocytes. GSH plays the major role in detoxifying H2O2 in hepatocytes, where GSH peroxidase uses the GSH reducing power to reduce H2O2 to H2O. NAPQI detoxification is usually borne out by GSH, at normal doses of APAP. On the contrary, APAP overdose leads to a high level of NAPQI formation. This towering NAPQI production devastates hepatocyte GSH levels, which ultimately results in severe GSH depletion in the cytoplasm and mitochondria.53 These free reactive toxic metabolites (NAPQI) bind covalently with the available cell protein thiols and modify the effector function of the proteins. In particular, NAPQI binds to thiols in the respiratory complex of mitochondria and deplete mitochondrial GSH resulting in increased ROS with severe ATP depletion.45,46 This leads to hepatocyte death (Fig. 5).GSK-3β mediated early phase attack
The involvement of glycogen synthase kinase-3beta (GSK-3β) in APAP-induced liver injury is complex as it takes on a pivotal role in JNK activation. In the early phase (<2 h), oxidative stress activated GSK-3β involves JNK activation via mixed-lineage kinase-3 (MLK3).47Previously it was reported that, APAP mediated oxidative stress directly activates JNK. But recent reports on MLK3 knocked out mice revealed that MLK3 is highly involved in H2O2-induced JNK activation in hepatocytes. Surprisingly it was also reported that MLK3-deficient mice inhibited the APAP mediated GSK3β activation however its mechanism remains unclear.48 This portrays that oxidative stress in the early phase activates both GSK-3β and MLK3, whereas MLK3 also plays a role in the activation of GSK-3β. Though the activation mechanism of this loop is unclear, certainly it increases GSK-3β and elevates JNK activation via MAP2K.44 Early phase activated JNK interfere with up-regulation of GSH synthesis by GCL-c (glutamate cysteine ligase catalytic subunit) degradation. In spite of Nrf-2 mediated GCL-c transcription, there is around 40–70% depletion of GCL-c by APAP which is significantly involved in the inhibition and downregulation of GSH synthesis, thereby constraining the recovery of APAP-induced liver injury. Furthermore, JNK translocates to the mitochondria and mediates mitochondrial dysfunction via an increase in mitochondrial permeability.49,54
ASK-1 mediated late phase attack
The upstream events of the early JNK activation mediated mitochondrial insult release a number of inflammatory cytokines, including TNF-α.55 In the liver, TNF-α binds to its receptor protein, tumor necrosis factor receptor-1 (TNF-R1) and activates the cell death pathway. A study showed that mice lacking ASK-1 were more resistant to APAP-induced liver injury.51 Mouse embryonic fibroblast cells from ASK1−/− mice are resistant to oxidant- and TNF-α induced apoptosis and fail to maintain sustained levels of JNK activity upon treatment with H2O2 or TNF-α.52 These lines of evidence suggest that ASK1 is significantly involved in JNK mediated APAP-induced hepatic impairment. ASK1 found in cytosol has been reported in its inactive state with the N-terminal possessing thioredoxin (TRX) and the C-terminal possessing a Ser-967 site to which 14-3-3 (P), a phosphoserine-binding molecule is bonded.56 ROS/H2O2 generated by NAPQI initiatively detach TRX from the ASK1 N-terminal domain by oxidation and form disulfide linkages. This detachment induces a conformational change and exposes Ser-967 to its analogous phosphatase (i.e., Ser-967 phosphatase). This in turn dephosphorylates and dissociates 14-3-3 [P].50 In addition, the immune cell-induced proinflammatory signals (TNF-α) also induce ASK1 activation, by dissociating 14-3-3 [P] from ASK1 through the ASK1-interacting protein (AIP-1). In brief, TNF-α binds to TNF receptor-1 and activates TRAF-2 (TNF receptor-associated factor 2), which in turn causes the binding of the N-terminal C2 domain of AIP-1 with the C-terminus domain of ASK1. This binding leads to the dissociation of 14-3-3 (P) from ASK-1.57 These synergistic/alternate cascades activate ASK-1. Upon activation, ASK-1 undergoes sequential protein phosphorylation and activates MAP2Ks (i.e., MKK4/MKK7) which in turn undergo differential phosphorylation to activate JNK. ASK-1 knockout mice initially exhibited ASK-1 independent JNK activation in the early hours (∼1.5 h) after APAP administration and became gradually involved in ASK-1 dependent JNK activation in the later phase (>2 h).58 In the second phase, activated JNK again translocates to the mitochondria, binds to the mitochondrial proteins (i.e., Sab) and phosphorylates them to induce a mitochondrial permeability transition (MPT).59 MPT results in increased permeability of the mitochondrial inner membrane which leads to mitochondrial swelling and necrotic cell death. JNK also induce ROS generation in the mitochondria to sustain their production level in the cytosol.60 Sustained JNK activation also leads to bid/bax signal mediated mitochondrial outer membrane permeabilization (MOMP) and hepatic apoptosis. But the rate of apoptosis occurrence is less noticeable in APAP induced hepatotoxicity,61 as the sustained JNK activation mediated redox effects and ATP exhaustion hinder caspase activation and thus the primary mode of cell death occurs via necrosis.Switching death and survival pathways
The results of in vitro and in vivo studies strongly reveal that APAP triggers TNF-α in hepatocytes through the redox insult.62 TNF-α binds to its hepatocytic receptor and activates both the death and survival pathways of hepatocytes comprising NF-kB activation.63 The activated NF-kB initiates gene transcription for the antioxidant (SOD and ferritin) and signalling proteins to inhibit the JNK activity.61 On inhibition of NF-kB, a sustained JNK activity is seen due to the indirect inhibition of the antioxidant enzyme gene transcription. Mice hepatocytes treated with a sublethal dose of APAP revealed a TNF-α cytotoxic effect which was mediated by a redox insult facilitated NF-kB inhibition.64,65 Thus, as the TNF-α induced NF-kB implies a fleeting effect on JNK, hepatocytes acquire a pro-survival and proliferative response against the cytotoxic effect induced by TNF-α.It was also reported that JNK itself is involved in modulating the function of pro/anti-apoptotic mitochondrial proteins.66,67 In brief, JNK is involved in the up-shift of pro-apoptotic and/or down-shift of anti-apoptotic genes via nucleus signalling. The phosphorylated JNK translocates to the nucleus and activates the AP-1 groups of transcription factors (i.e., c-Jun).68 When the AP-1 group of the genetic factors underwent transcription, a wide array of pro-apoptotic proteins were produced thus resulting in apoptosis.69 JNK activation also leads to the suppression of apoptosis via phosphorylation mediated deactivation of the Bcl-2 family protein, BAD. Thus inhibiting the binding and neutralizing effects of BAD over the Bcl2 and Bcl XL mediated anti-apoptotic activities.70 Despite the fact that the pro/anti-apoptotic role of JNKs is at an initial level of indulgence, it should be noted that the JNK pro-apoptotic activity is habitually regulated by other cellular and molecular influences. These supposedly involve the simultaneous activation of the cell survival/anti-apoptotic pathway and the whole apoptotic signalling strength.
Inspite of JNK, other pro-death/survival proteins exist in the endo and exo-mitochondria to facilitate cell death. The myeloid cell leukemia-1 (Mcl-1), a member of the Bcl2 family, is known to be modulated by GSK-3β and thereby it regulates both the pro- and anti-apoptotic signal mediated mitochondrial death pathways.71 GSK-3β has been reported to be involved in the phosphorylation of Bax, translocation to mitochondria and also the phosphorylation of Mcl-1 in mitochondria, which promotes its degradation.49,72 A recent study revealed that silencing GSK-3β did not influence the Bax translocation to mitochondria, however, it is involved in the reduction of Mcl-1 loss. Thus a GSH independent mechanism was proposed where GSK-3β promotes the Mcl-1 degradation following the APAP treatment and does not regulate Bax activation, thereby inducing cell death.49 But previous studies reported the translocation of GSK-3β to mitochondria, in both their active (p-tyrosine) and dormant (p-serine 9) forms, where either or both forms together regulate the downstream process of GSK-3β resulting in a protective role.73 However, the role of the kinase activated form of GSK-3β (p-tyrosine) and the inactive GSK-3β (p-serine 9) in APAP hepatotoxicity is still to be further assessed.74 Further studies on GSK-3β activation might reveal the exact switch role of GSK-3β over the APAP induced cell death/survival pathway in the liver. These are a few pieces of evidence of the switching mechanisms that modulate directly/indirectly the APAP overdose-induced hepatocyte death/survival pathways.
Possible molecular targets – APAP hepatoxicity
A wide array of molecular targets can be culled on understanding the pathological mechanism of the APAP induced hepatotoxicity. The cell signalling modulations induced by APAP induced hepatotoxicity include various molecular events. A few possible molecular targets have been elucidated as follows.NAPQI/CYP2E1 suppression with elevated SOD activity
APAP-induced ROS activates superoxide activity, where the Cu/Zn-SOD (super oxide dismutase) catalyses the dismutation of superoxide. A study on the SOD activity in mice revealed a protective effect of Cu/Zn-SOD in APAP induced hepatotoxicity allied with a lack of JNK activation.75 This was further clarified by an additional study on Cu/Zn-SOD knockout mice, which showed that Cu/Zn-SOD induces CYP2E1 down regulation, which effectively reduces the production of NAPQI, upregulates the GSH level, and also hinders the JNK activation.76 In addition, a study revealed that decarboxylated S-adenosyl-L-methionine actively protects APAP-induced hepatotoxicity through escalating the cytosolic Cu/Zn-SOD activity and mitochondrial Mn-SOD activity.77 This compilation of research findings identifies the NAPQI/CYP2E1 suppression and GSH/SOD upsurge activity as a potential molecular target for further clinical evaluation.GSK-3β/ASK-1/JNK-interplay
It was proposed, that apart from ASK-1 mediated sustained JNK activation, the glycogen synthase kinase-3 beta (GSK-3β), also induces parallel activation of JNK and consequently subsides the level of the glutamate cysteine ligase catalytic subunit (GCL-c), a key enzyme in GSH production. However the mechanism of activation is not well established. In spite of the JNK activation, APAP treatment facilitates the mitochondrial translocation of GSK-3β and eradicates the Mcl-1 available in the mitochondrial membranes of hepatocytes. On silencing the GSK-3β, the hepatic GCL-c loss was significantly decreased and promotes the restoration of the GSH level in APAP administered livers and also inhibits JNK activation by reducing both its early phase (within 2 h) and late phase (>4 h) activation, thereby constraining the APAP mediated JNK translocation to the mitochondria.49 A very recent study revealed that the protein tyrosine phosphatase 1B (PT1B), a down regulator of tyrosine kinase signaling, plays a significant role in the APAP mediated signaling pathway by dual modulation of the Nrf-2/ARE through GSK3β and survival signaling through the Akt signaling pathway, thereby silencing PT1B which might stand as an efficient therapeutic strategy against APAP-hepatotoxicity.78This evidence suggests that the candidates may facilitate the inhibition of GSK-3β, ASK-1 and JNK and stabilization of Mcl-1, constrain the loss of GCL-c and the protein tyrosine phosphatase 1B (PT1B) and promote the GSH level, which will be more therapeutically valuable.
MPT activity
NAPQI also induces redox modification in the mitochondria through mitochondrial GSH depletion and covalent binding. JNK restrain mitochondrial respiration by inducing MPT and this mitochondrial translocation of JNK also plays a substantial role in apoptosis/necrosis, which can be inhibited by the MPT inhibitor (i.e. cyclosporin A).53 The identification of active candidates to inhibit MPT or enhance the cyclosporin A activity will be helpful for further preventive/therapeutic interventions.Hepatocyte gap junction
In general, the adjacent cells tend to swap cytosolic elements among each other through hexameric hemichannels known as gap junctions. They play a key role in intracellular communication of secondary messengers such as hormone and neural signals and their mediated responses. The gap junctions were specifically classified as connexin 26 and connexin 32 in hepatocytes, and connexin 43 in bile duct epithelia. These connexions are involved in the regulation of specific liver functions such as bile secretion, glucose transportation, hepatic regeneration and tumor annihilation.79Recent studies on APAP-hepatotoxic mice projected that gap junction communication can be targeted as a novel pharmacological approach to defend the augmentation of APAP-induced hepatotoxicity. The author revealed that being a gap junction protein of hepatocytes, connexin 32 (Cx32) plays a major role as a key mediator involved in exacerbation of drug induced liver impairment, which was evidently shown as the Cx32 deficient mice exhibited a protective effect against hepatotoxic drug-induced acute inflammation, liver impairment and cell death.80 In accordance, a recent study reported that APAP inflicts hepatic necrosis through gap junctions (Cx26 and 32) and the knockout model of these junctions relatively expressed low necrosis compared to the wild type.81 However another study claims that inhibition of Cx32 might interrupt the glutathione (GSH) and inorganic ion transmission to neighbouring cells which can be considered an undesirable effect.82 Though deep investigative work in this area is required, this may raise a new molecular platform for targeting potential active inhibitor candidates.
Immunological targets
As discussed earlier, cytokines and chemokines play a major role in the advancement of APAP-induced acute liver damage. Though many inflammatory markers have been under the process of evaluation, it was recently reported that IFN-γ KO mice revealed receding effects against APAP-induced hepatotoxicity through suppression of leukocyte infiltration and the downregulation of the genetic expression of inflammatory markers such as cytokines, chemokines and inducible nitric oxide synthase (iNOS) compared with the wild type mice.It was further confirmed that the anti-IFN-γ antibody significantly receded the effects of APAP-induced hepatotoxicity even 2 or 8 h after APAP administration.25 It has also been recently reported that the Toll-like receptors (TLR) play a key role in APAP-induced mitochondrial damage inflammation and result in aggravation of APAP hepatotoxicity in conjunction with TNF-α.83 The neutralizing Abs administered against mice TLR3, exhibited substantial hepatoprotective activity in TLR3+/+ wild type mice, and further in vitro studies revealed the existence of cooperation between TNFα and TLR3 agonists which leads to hepatic cell death.83 These findings give a way to determine potential candidates that can immunoneutralize IFN-γ and TLR3 against APAP-induced liver injury.
Conclusion and future perspectives
The liver, being the largest organ in our body, characteristically executes various pivotal roles. In view of this fact, the liver is implicated with an array of interlinked biochemical processes; it is no marvel that they are subjected to various underlying pathological factors. This review mainly focused on various phases and prevailing sources of APAP-induced liver impairment and its molecular mechanism. APAP hepatotoxicity occurs frequently, can be life-threatening and impersonates all forms of liver disorders. The configuration of the reactive toxic metabolite, covalent binding, imbalance of ROS/RNS level, interplay on signalling pathways, and mitochondrial impairment are elucidated to be the key phases of APAP-induced hepatotoxicity. Though earlier it was conceptually accepted that APAP causes hepatotoxicity, the underlying mechanism is still under the process of exploration. These claims highlight the importance of drafting/proposing an outline of APAP-induced hepatotoxicity mediated by various intrinsic and extrinsic factors. Also, few molecular targets have been identified from understanding the mechanism, to resolve the clinical challenge of APAP-induced hepatotoxicity. This may facilitate researchers worldwide to manifest novel and effective systematic therapeutic interventions for alleviating drug-induced liver injury and associated complications.Declaration of interest
The authors do not have any conflict of interest with the content of the manuscript.Acknowledgements
This review work was supported by a research grant from the Ministry of Science, Technology and Innovation of Malaysia under E-science Project number 02-01-04-SF1144.References
- A. Sendensky and J.-F. Dufour, in Chronic Liver Failure, ed. P. Ginès, P. S. Kamath and V. Arroyo, Humana Press, 2011, pp. 33–45 Search PubMed.
- R. Ramachandran and S. Kakar, J. Clin. Pathol., 2009, 62, 481–492 CrossRef CAS PubMed.
- N. Kaplowitz, Clin. Infect. Dis., 2004, 38(suppl. 2), S44–S48 CrossRef PubMed.
- J. D. P. Gerald, Acetaminophen: Background and Overview, 2009, available in http://www.fda.gov/SiteIndex/default.htm Search PubMed.
- J. L. Woodhead, B. A. Howell, Y. Yang, A. H. Harrill, H. J. Clewell 3rd, M. E. Andersen, S. Q. Siler and P. B. Watkins, J. Pharmacol. Exp. Ther., 2012, 342, 529–540 CrossRef CAS PubMed.
- E. Bjornsson and R. Olsson, Dig. Liver Dis., 2006, 38, 33–38 CrossRef CAS PubMed.
- American Liver Foundation, Liver Disease Information Center, 2011, available in http://www.liverfoundation.org/aboutthelive,r/info/.
- A.-R. Marzilawati, Y.-Y. Ngau and S. Mahadeva, BMC Pharmacol. Toxicol., 2012, 13, 8 CrossRef CAS PubMed.
- R. Clark, J. E. Fisher, I. S. Sketris and G. M. Johnston, BMC Clin. Pharmacol., 2012, 12, 11 CrossRef CAS PubMed.
- M. S. Wolf, J. King, K. Jacobson, L. Di Francesco, S. C. Bailey, R. Mullen, D. McCarthy, M. Serper, T. C. Davis and R. M. Parker, J. Gen. Intern. Med., 2012, 27, 1587–1593 CrossRef PubMed.
- C. Wang, E. R. Blough, R. Arvapalli, X. Dai, S. Paturi, N. Manne, H. Addagarla, W. E. Triest, O. Olajide and M. Wu, Free Radical Biol. Med., 2013, 65, 1417–1426 CrossRef CAS PubMed.
- H. G. Shertzer, E. L. Kendig, H. A. Nasrallah, E. Johansson and M. B. Genter, Int. J. Obes., 2010, 34, 970–979 CrossRef CAS PubMed.
- K. Kon, K. Ikejima, K. Okumura, K. Arai, T. Aoyama and S. Watanabe, Diabetic KK-Ay mice are highly susceptible to oxidative hepatocellular damage induced by acetaminophen, Am. J. Physiol.: Gastrointest. Liver Physiol., 2010, 299, G329–G337 CrossRef CAS PubMed.
- O. Kucera, H. Lotkova, P. Stankova, M. Podhola, T. Rousar, V. Mezera and Z. Cervinkova, Int. J. Exp. Pathol., 2011, 92, 281–289 CrossRef CAS PubMed.
- J. Aubert, K. Begriche, M. Delannoy, I. Morel, J. Pajaud, C. Ribault, S. Lepage, M. R. McGill, C. Lucas-Clerc, B. Turlin, M. A. Robin, H. Jaeschke and B. Fromenty, J. Pharmacol. Exp. Ther., 2012, 342, 676–687 CrossRef CAS PubMed.
- G. Graham, R. Day, A. Graudins and A. Mohamudally, Inflammopharmacology, 2010, 18, 47–55 CrossRef CAS PubMed.
- Leon, “This American Life” report on acetaminophen sensationalistic and inaccurate, 2013, available at http://www.thepoisonreview.com/2013/09/26/this-american-life-report-on-acetaminophen-sensationalistic-and-inaccurate/.
- D. J. Antoine, A. H. Harrill, P. B. Watkins and B. K. Park, Toxicol. Res., 2014, 3, 75–85 RSC.
- J. Janssen and S. Singh-Saluja, Can. Fam. Physician, 2015, 61, 347–349 Search PubMed.
- D. H. Jang, M. D. Weaver and A. F. Pizon, J. Med. Toxicol., 2013, 9, 49–53 CrossRef CAS PubMed.
- D. C. Dahlin, G. T. Miwa, A. Y. Lu and S. D. Nelson, Proc. Natl. Acad. Sci. U. S. A., 1984, 81, 1327–1331 CrossRef CAS.
- J. A. Hinson, A. B. Reid, S. S. McCullough and L. P. James, Drug Metab. Rev., 2004, 36, 805–822 CrossRef CAS PubMed.
- V. Racanelli and B. Rehermann, Hepatology, 2006, 43, S54–S62 CrossRef CAS PubMed.
- Z. Tu, A. Bozorgzadeh, I. N. Crispe and M. S. Orloff, Clin. Exp. Immunol., 2007, 149, 186–193 CrossRef CAS PubMed.
- Y. Ishida, T. Kondo, T. Ohshima, H. Fujiwara, Y. Iwakura and N. Mukaida, FASEB J., 2002, 16, 1227–1236 CrossRef CAS PubMed.
- Z. X. Liu, S. Govindarajan and N. Kaplowitz, Gastroenterology, 2004, 127, 1760–1774 CrossRef CAS PubMed.
- J. Zhang, S. Song, Q. Pang, R. Zhang, L. Zhou, S. Liu, F. Meng, Q. Wu and C. Liu, Sci. Rep., 2015, 5, 8098 CrossRef CAS PubMed.
- K. Kon, K. Ikejima, K. Okumura, T. Aoyama, K. Arai, Y. Takei, J. J. Lemasters and N. Sato, J. Gastroenterol. Hepatol., 2007, 22(suppl. 1), S49–S52 CrossRef CAS PubMed.
- H. Malhi, G. J. Gores and J. J. Lemasters, Hepatology, 2006, 43, S31–S44 CrossRef CAS PubMed.
- M. Bourdi, Y. Masubuchi, T. P. Reilly, H. R. Amouzadeh, J. L. Martin, J. W. George, A. G. Shah and L. R. Pohl, Hepatology, 2002, 35, 289–298 CrossRef CAS PubMed.
- H. Tsutsui and S. Nishiguchi, Int. J. Mol. Sci., 2014, 15, 7711–7730 CrossRef CAS PubMed.
- V. A. Fadok, D. L. Bratton, A. Konowal, P. W. Freed, J. Y. Westcott and P. M. Henson, J. Clin. Invest., 1998, 101, 890–898 CrossRef CAS PubMed.
- S. Goerdt and C. E. Orfanos, Immunity, 1999, 10, 137–142 CrossRef CAS.
- M. P. Holt, L. Cheng and C. Ju, J. Leukocyte Biol., 2008, 84, 1410–1421 CrossRef CAS PubMed.
- D. H. Adams, C. Ju, S. K. Ramaiah, J. Uetrecht and H. Jaeschke, Toxicol. Sci., 2010, 115, 307–321 CrossRef CAS PubMed.
- P. J. Winwood and M. J. Arthur, Semin. Liver Dis., 1993, 13, 50–59 CrossRef CAS PubMed.
- M. J. Masson, L. D. Carpenter, M. L. Graf and L. R. Pohl, Hepatology, 2008, 48, 889–897 CrossRef CAS PubMed.
- Z.-X. Liu, S. Govindarajan and N. Kaplowitz, Gastroenterology, 2004, 127, 1760–1774 CrossRef CAS PubMed.
- H. Jaeschke, Hepatology, 2008, 48, 699–701 CrossRef PubMed.
- C. M. Hogaboam, C. L. Bone-Larson, M. L. Steinhauser, N. W. Lukacs, L. M. Colletti, K. J. Simpson, R. M. Strieter and S. L. Kunkel, FASEB J., 1999, 13, 1565–1574 CAS.
- S. L. Michael, N. R. Pumford, P. R. Mayeux, M. R. Niesman and J. A. Hinson, Hepatology, 1999, 30, 186–195 CrossRef CAS PubMed.
- C. L. Bone-Larson, K. J. Simpson, L. M. Colletti, N. W. Lukacs, S.-C. Chen, S. Lira, S. L. Kunkel and C. M. Hogaboam, Immunol. Rev., 2000, 177, 8–20 CAS.
- C. M. Hogaboam, K. J. Simpson, S. W. Chensue, M. L. Steinhauser, N. W. Lukacs, J. Gauldie, R. M. Strieter and S. L. Kunkel, Gene Ther., 1999, 6, 573–584 CrossRef CAS PubMed.
- C. Ju, T. P. Reilly, M. Bourdi, M. F. Radonovich, J. N. Brady, J. W. George and L. R. Pohl, Chem. Res. Toxicol., 2002, 15, 1504–1513 CrossRef CAS PubMed.
- P. C. Burcham and A. W. Harman, J. Biol. Chem., 1991, 266, 5049–5054 CAS.
- D. Han, R. Canali, D. Rettori and N. Kaplowitz, Mol. Pharmacol., 2003, 64, 1136–1144 CrossRef CAS PubMed.
- R. Mishra, M. K. Barthwal, G. Sondarva, B. Rana, L. Wong, M. Chatterjee, J. R. Woodgett and A. Rana, J. Biol. Chem., 2007, 282, 30393–30405 CrossRef CAS PubMed.
- M. Sharma, V. Gadang and A. Jaeschke, Mol. Pharmacol., 2012, 82, 1001–1007 CrossRef CAS PubMed.
- M. Shinohara, M. D. Ybanez, S. Win, T. A. Than, S. Jain, W. A. Gaarde, D. Han and N. Kaplowitz, J. Biol. Chem., 2010, 285, 8244–8255 CrossRef CAS PubMed.
- H. Ichijo, E. Nishida, K. Irie, P. Ten Dijke, M. Saitoh, T. Moriguchi, M. Takagi, K. Matsumoto, K. Miyazono and Y. Gotoh, Science, 1997, 275, 90–94 CrossRef CAS.
- H. Nakagawa, S. Maeda, Y. Hikiba, T. Ohmae, W. Shibata, A. Yanai, K. Sakamoto, K. Ogura, T. Noguchi, M. Karin, H. Ichijo and M. Omata, Gastroenterology, 2008, 135, 1311–1321 CrossRef CAS PubMed.
- K. Tobiume, A. Matsuzawa, T. Takahashi, H. Nishitoh, K. Morita, K. Takeda, O. Minowa, K. Miyazono, T. Noda and H. Ichijo, EMBO Rep., 2001, 2, 222–228 CrossRef CAS PubMed.
- N. Hanawa, M. Shinohara, B. Saberi, W. A. Gaarde, D. Han and N. Kaplowitz, J. Biol. Chem., 2008, 283, 13565–13577 CrossRef CAS PubMed.
- P. Donnelly, R. Walker and W. Racz, Arch. Toxicol., 1994, 68, 110–118 CrossRef CAS.
- M. Holt and C. Ju, AAPS J., 2006, 8, E48–E54 CrossRef CAS PubMed.
- Y. Liu, G. Yin, J. Surapisitchat, B. C. Berk and W. Min, J. Clin. Invest., 2001, 107, 917–923 CrossRef CAS PubMed.
- E. H. Goldman, L. Chen and H. Fu, J. Biol. Chem., 2004, 279, 10442–10449 CrossRef CAS PubMed.
- R. Zhang, X. He, W. Liu, M. Lu, J. T. Hsieh and W. Min, J. Clin. Invest., 2003, 111, 1933–1943 CrossRef CAS.
- C. Wiltshire, M. Matsushita, S. Tsukada, D. A. Gillespie and G. H. May, Biochem. J., 2002, 367, 577–585 CrossRef CAS PubMed.
- J. W. Chambers and P. V. LoGrasso, J. Biol. Chem., 2011, 286, 16052–16062 CrossRef CAS PubMed.
- A. Wullaert, K. Heyninck and R. Beyaert, Biochem. Pharmacol., 2006, 72, 1090–1101 CrossRef CAS PubMed.
- D. Han, N. Hanawa, B. Saberi and N. Kaplowitz, Am. J. Physiol.: Gastrointest. Liver Physiol., 2006, 291, G1–G7 CrossRef CAS PubMed.
- K. Sinha, J. Das, P. Pal and P. Sil, Arch. Toxicol., 2013, 87, 1157–1180 CrossRef CAS PubMed.
- D. Han, N. Hanawa, B. Saberi and N. Kaplowitz, Free Radical Biol. Med., 2006, 41, 627–639 CrossRef CAS PubMed.
- H. Lou and N. Kaplowitz, J. Biol. Chem., 2007, 282, 29470–29481 CrossRef CAS PubMed.
- H. Schroeter, C. S. Boyd, R. Ahmed, J. P. Spencer, R. F. Duncan, C. Rice-Evans and E. Cadenas, Biochem. J., 2003, 372, 359–369 CrossRef CAS PubMed.
- S. D. Ray and N. Jena, Arch. Toxicol., 2000, 73, 594–606 CrossRef CAS.
- L. Chang and M. Karin, Nature, 2001, 410, 37–40 CrossRef CAS PubMed.
- M. Raman, W. Chen and M. H. Cobb, Oncogene, 2007, 26, 3100–3112 CrossRef CAS PubMed.
- C. Yu, Y. Minemoto, J. Zhang, J. Liu, F. Tang, T. N. Bui, J. Xiang and A. Lin, Mol. Cell, 2004, 13, 329–340 CrossRef CAS.
- R. S. Jope and G. V. Johnson, Trends Biochem. Sci., 2004, 29, 95–102 CrossRef CAS PubMed.
- U. Maurer, C. Charvet, A. S. Wagman, E. Dejardin and D. R. Green, Mol. Cell, 2006, 21, 749–760 CrossRef CAS PubMed.
- H. Eldar-Finkelman, Trends Mol. Med., 2002, 8, 126–132 CrossRef CAS.
- A. Takashima, J. Pharmacol. Sci., 2009, 109, 174–178 CrossRef CAS.
- J. H. Zhu, X. Zhang, J. P. McClung and X. G. Lei, Exp. Biol. Med., 2006, 231, 1726–1732 CAS.
- X. G. Lei, J. H. Zhu, J. P. McClung, M. Aregullin and C. A. Roneker, Biochem. J., 2006, 399, 455–461 CrossRef CAS PubMed.
- J. Brown and A. Michael, Mechanistic Study of S-Adenosyl-L-Methionine Protection Against Acetaminophen Hepatotoxicity, theses, Dissertations and Capstones, 2012, p. 347.
- M. A. Mobasher, A. Gonzalez-Rodriguez, B. Santamaria, S. Ramos, M. A. Martin, L. Goya, P. Rada, L. Letzig, L. P. James, A. Cuadrado, J. Martin-Perez, K. J. Simpson, J. Muntane and A. M. Valverde, Cell Death Dis., 2013, 4, e626 CrossRef CAS PubMed.
- W. Echevarría and M. H. Nathanson, Gap Junctions in the Liver, Mol. Pathog. Cholestasis, 2003 Search PubMed.
- S. J. Patel, J. M. Milwid, K. R. King, S. Bohr, A. Iracheta-Velle, M. Li, A. Vitalo, B. Parekkadan, R. Jindal and M. L. Yarmush, Nat. Biotechnol., 2012, 30, 179–183 CrossRef CAS PubMed.
- Saito, K. Shinzawa and Y. Tsujimoto, Sci. Rep., 2014, 4, 5169 CAS.
- I. Igarashi, T. Maejima, K. Kai, S. Arakawa, M. Teranishi and A. Sanbuissho, Exp. Toxicol. Pathol., 2014, 66, 103–110 CrossRef CAS PubMed.
- K. A. Cavassani, A. P. Moreira, D. Habiel, T. Ito, A. L. Coelho, R. M. Allen, B. Hu, J. Raphelson, W. F. t. Carson, M. A. Schaller, N. W. Lukacs, M. B. Omary, C. M. Hogaboam and S. L. Kunkel, PLoS One, 2013, 8, e65899 CAS.
| This journal is © The Royal Society of Chemistry 2015 |