Highly electrical and thermoelectric properties of a PEDOT:PSS thin-film via direct dilution–filtration†
Jinhua
Xiong‡
,
Fengxing
Jiang‡
,
Weiqiang
Zhou
,
Congcong
Liu
and
Jingkun
Xu
*
Department of Physics, Jiangxi Science and Technology Normal University, Nanchang 330013, China. E-mail: xujingkun@tsinghua.org.cn
First published on 3rd July 2015
Abstract
Herein, a rapid and robust method for a highly conductive PEDOT:PSS thin-film has been developed by direct dilution–filtration with common organic solvents. A large electrical conductivity of 1500 S cm−1 has been achieved and a high thermoelectric figure of merit (ZT ∼ 0.1) makes it a promising organic thermoelectric candidate.
The design of highly conductive organic thin-film materials is of immense scientific interest and has created opportunities for the development of organic electronic materials with a process that is facile, low cost and scalable.1–3 Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) has been regarded as an excellent organic electronic material due to its flexibility, lightweight, transparency and highly conductive properties.4–7 An essential post-treatment with common organic solvents for pristine PEDOT:PSS is required, especially to allow for thin-film fabrication and for an improvement in electrical conductivity. However, these treatment processes are liable to induce film damage leading to a non-uniform thin-film.
Highly conductive PEDOT:PSS have been reported as one of the most promising organic thermoelectric (TE) materials available today. Recent years have witnessed a great increase in the TE property with two orders of magnitude from 10−3 to 10−1 for PEDOT:PSS.4,8,9 The TE property is determined by the dimensionless figure of merit:
 | (1) |
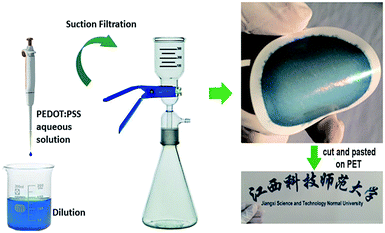
Presently, limited technologies such as spin-coating and printing have been employed to fabricate a highly conductive PEDOT:PSS thin-film with an extra treatment.5,16–18 Here, we integrate PEDOT:PSS thin-film fabrication and solvent treatment by a simple, rapid, robust method using one-step direct dilution–filtration with common organic solvents without any further post-treatment. The PEDOT:PSS film on PVDF was so thin and flexible that it could be cut into any desired form and pasted onto a transparent PET substratum (Fig. 1). The thickness of the thin-film can be controlled by changing the amount of pristine aqueous PEDOT:PSS in common solvents such as methanol (MeOH), ethanol (EtOH), isopropanol (IPA), N,N-dimethylformamide (DMF), dimethylsulfoxide (DMSO), ethylene glycol (EG) and N-methyl-2-pyrrolidone (NMP). This approach which avoids the waste of pristine PEDOT:PSS, use of sophisticated instruments, the limitation of solvent types, and complex processes is expected to produce positive effects on the electrical and TE properties.
It is difficult to prepare a thin-film by the direct filtration of a pristine PEDOT:PSS aqueous solution (Clevios PH1000) owing to its narrow particle size distribution of 20–30 nm. The buildup of large nanoparticles is a necessary requirement to achieve the filtration separation of PEDOT:PSS from solution. The common organic solvents (Table S1, ESI†) were chosen to dilute the pristine PEDOT:PSS. This process led to the agglomeration of PEDOT:PSS nanoparticles over 250 nm due to the partial dissolution of PSS in the diluent (Table S2, ESI†). Therefore, the operation of dilution–filtration succeeded in the separation of the PEDOT:PSS nanoparticles from the solvents. The as-prepared PEDOT:PSS film has an average thickness of about 233 nm (Fig. S1 and Table S2, ESI†) and is denoted as P100.
To obtain structural and compositional information, XPS was performed and it is shown in Fig. S2.† An evident change between the pristine and diluted PEDOT:PSS thin-films can be observed for the intensity of the S(2p) peaks, which can be assigned to the sulfur signals from the sulfonate of PSS (166–170 eV) and the thiophene of PEDOT (162–166 eV).5,19 The PSS ratio in the PEDOT:PSS thin-films markedly decreases based on the area of S(2p) peaks (Table 1). The largest drop of PSS (53.7%) can be observed for the DMSO-diluted PEDOT:PSS thin-film, which is lower than the result (36%) reported by Kim and co-workers.15 A similar phenomenon was also observed by Pipe et al.5 using dip-treatment in an EG bath and by Ouyang et al.12,13 using the drop-treatment method with acids. The drop-treatment with DMF on P100–H2O yielded a decline of PSS of about 8.37% from XPS (Fig. S2, ESI†). This slight decrease probably originates from the conformation change of PEDOT:PSS since PSS cannot be removed from the solid thin-film during the DMF drop-treatment. Also, it indicates that the diluent-treatment with the solvent is a more effective strategy for the depletion of PSS from PEDOT:PSS when compared to other treatments. Furthermore, the removal of PSS was confirmed by Raman spectroscopy (Fig. S3, ESI†) and UV-Vis-NIR absorption spectroscopy (Fig. S4, ESI†).
| Solvent | PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEDOT ratio/% PEDOT ratio/% |
Carrier concentration/×1022 cm−3 | Mobility/cm2 V−1 s−1 | σ b/S cm−1 | σ c/S cm−1 | T 0 d/K |
|---|---|---|---|---|---|---|
| a Provided by Heraeus Co. b The calculated values are based on σ = neµ. c The measured results. d T 0 is the characteristic temperature based on the VRH model. | ||||||
| Pristinea | 2.55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | — | — | 0.05 | — |
| Water (H2O) | 2.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | — | — | 1.2 | — |
| Methanol (MeOH) | 1.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7.33 | 0.11 | 1290 | 1220 | 43.8 |
| Ethanol (EtOH) | 1.62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5.54 | 0.14 | 1241 | 1369 | 66.3 |
| Isopropyl alcohol (IPA) | 1.91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6.54 | 0.08 | 837 | 838 | 125.8 |
| Dimethyl formamide (DMF) | 1.29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5.73 | 0.15 | 1375 | 1470 | 42.8 |
| DMF drop-treatment | 1.86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.78 | 0.22 | 627 | 1011 | — |
| Dimethylsulfoxide (DMSO) | 1.18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3.11 | 0.30 | 1492 | 1399 | 41.9 |
| Ethylene glycol (EG) | 1.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5.0 | 0.17 | 1360 | 1411 | 38.3 |
| N-Methyl-2-pyrrolidone (NMP) | 1.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3.6 | 0.08 | 461 | 538 | 173.2 |
Fig. 2 and S5 (ESI†) show the atomic force microscopy (AFM) images of P100. One can see that the striking difference is the bright phase on the morphology images which corresponds to the PEDOT-rich grains.15,20 It implies that the diluent-treatment process can induce phase separation between conductive PEDOT and insulating PSS, since the pristine PEDOT:PSS thin-film exhibits a weak phase separation.15,21 The phase separation allows for the aggregation of PEDOT chains and the formation of PEDOT with higher crystallinity surrounded by a hydrophilic PSS shell. Note that the aggregated nanoparticles on P100–DMSO and P100–EtOH are more visible when compared with P100–H2O in the bright regions, indicating more distinct phase boundaries between the conductive PEDOT and the insulating PSS. The AFM images display the surface roughness of films with a root-mean-square (RMS) of 1.039, 1.373, and 2.048 nm for P100–H2O, P100–EtOH, and P100–DMSO, respectively, which are close to the previous values reported by Mengistie et al. with formic acid treatment (RMS = 1.31–1.89 nm).22 A larger RMS value is associated with a higher removal of PSS.23 Takano et al.10 found that EG is a more effective solvent for improving PEDOT crystallinity than water and ethanol. However, we found that using ethanol as the diluent can also induce the aggregation of PEDOT. Unlike them, it is due to a large depletion of PSS by dilution–filtration, leading to the aggregation of PEDOT with increasing crystallinity in PEDOT:PSS solid thin-film, which is likely to accelerate the charge hopping eventually.22
Fig. 3a shows the in-plane σ of P100 with different organic diluents at room temperature. The σ has no remarkable change for P100–H2O (1.2 S cm−1) when compared to the pristine PEDOT:PSS thin-film formed by spin-coating (0.2 S cm−1). When the diluent of water is replaced by the organic solvents, the σ of P100 experiences dramatic changes with four orders of magnitude enhancement from 10−1 to 103 S cm−1. When DMF, DMSO, and EtOH are the diluent, they show a more positive effect on the σ of PEDOT:PSS which is close to 1500 S cm−1. It can be found, based on the results of the AFM, that the PEDOT-rich particles with a larger size (40–60 nm) agglomerate randomly on the surface of the P100–DMSO and P100–EtOH solid thin-films. It is due to the fact that the PSS depletion leads to a thinner thickness of the PSS layer, separating PEDOT-rich grains, and inevitably the consequent alignment of the PEDOT grains.24 The temperature dependent σ can be described by the quasi one dimensional variable range hopping (VRH) model (Fig. S6, ESI†),
 | (2) |
The T0 values (Table 1) suggest that the P100 thin-films have a different energy barrier between the localized states for charge transport. A lower T0 value implies that the charge hopping among the PEDOT chains is easier and the degree of disorder in the disordered regions of PEDOT:PSS is smaller due to improved intrachain and interchain packing of the PEDOT-rich nanoparticles.25–27
For comparison, the dip-treatment of P100–H2O was performed in different organic solvents. Unfortunately, the as-prepared P100–H2O film was so thin that it broke up in the organic solvent bath. After that, we tried to use the drop-treatment method with pure organic solvents on the surface of the solid P100–H2O thin films. We found that the pure low boiling point solvent had little effect on the electrical conductivity of the P100–H2O film. These results are consistent to those reported by Ouyang et al.28 They also found that a co-solvent of a common low boiling point organic solvent (EtOH, IPA, etc.) and water slightly enhanced the σ of the PEDOT:PSS thin-film by drop-treatment (72.7 S cm−1), but no striking effects could be observed for the pure organic solvent. Our experimental results found only the co-solvent of EtOH and H2O (80%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20%, v/v) showed a positive effect (122 S cm−1), since no obvious enhancement was obtained by the drop-treatment with a pure low boiling point organic solvent which could not effectively solvate PSS after the conformational changes of PEDOT:PSS and the phase separation of PSS from PEDOT:PSS. Therefore, the low boiling point organic solvents cannot give rise to a significant enhancement for the σ of the PEDOT:PSS thin-film. In contrast, striking changes are achieved just with the high boiling point solvents such as DMF, DMSO, EG, and NMP (Fig. S7, ESI†). However, the σ is still lower than that of the diluted P100 with the corresponding organic solvents, which is further confirmed by σ = neµ with tested carrier concentration (n) and mobility (µ) in Table 1 and S3 (ESI†). Furthermore, the P100 thin-films were immersed in different organic solvents, yet the films were too thin and so they were destroyed. Note that the strategy by the diluent-treatment for increasing σ is not limited to the high boiling point solvents. Surprisingly, the low boiling point solvents (MeOH, EtOH, and IPA) show similar effects, especially for EtOH (1369 S cm−1).
20%, v/v) showed a positive effect (122 S cm−1), since no obvious enhancement was obtained by the drop-treatment with a pure low boiling point organic solvent which could not effectively solvate PSS after the conformational changes of PEDOT:PSS and the phase separation of PSS from PEDOT:PSS. Therefore, the low boiling point organic solvents cannot give rise to a significant enhancement for the σ of the PEDOT:PSS thin-film. In contrast, striking changes are achieved just with the high boiling point solvents such as DMF, DMSO, EG, and NMP (Fig. S7, ESI†). However, the σ is still lower than that of the diluted P100 with the corresponding organic solvents, which is further confirmed by σ = neµ with tested carrier concentration (n) and mobility (µ) in Table 1 and S3 (ESI†). Furthermore, the P100 thin-films were immersed in different organic solvents, yet the films were too thin and so they were destroyed. Note that the strategy by the diluent-treatment for increasing σ is not limited to the high boiling point solvents. Surprisingly, the low boiling point solvents (MeOH, EtOH, and IPA) show similar effects, especially for EtOH (1369 S cm−1).
On the other hand, Fig. 3a presents a little difference in S among the P100 samples (14.6–18.6 µV K−1) and it is similar to the value of the pristine spin-coated PEDOT:PSS film (15.2 µV K−1). A slightly higher S was observed for P100–H2O (20.5 µV K−1) and P100–DMSO (18.6 µV K−1) when H2O and DMSO were used as the diluent. During the dilution process, the oxidation state of the PEDOT:PSS thin-films does not change according to the UV-Vis-NIR spectroscopy (Fig. S4, ESI†), indicating that the slight change of S cannot be attributed to the doping levels of P100 based on the Mott relation.29,30 However, an enhanced S is not observed for P100 with the depletion of PSS, which is in agreement with the results of Stöcker et al.31 Importantly, the significant increase of σ does not lead to a striking decrease of S. Moreover, both σ (Fig. S7, ESI†) and S (Fig. S8, ESI†) of P100 still achieved 90% of the initial value after 30 days in an ambient atmosphere, indicating good environmental stability. The TE performance of P100 was accessed using the power factor (σS2) and ZT values in Fig. 3b and S9 (ESI†). The highest σS2 is 48.3 µW m−1 K−2, and the largest ZT value is 0.1 for the P100–DMSO thin-film (κ = 0.18 W m−1 K−1, ESI†) which is higher than those reported by Yoo et al. (1.37 × 10−3)3 and Liu et al. (0.065).2 A greater enhancement for the TE property of PEDOT:PSS can thus be expected if the Seebeck coefficient is enhanced further without sacrificing its highly electrical conductivity.
Conclusions
In conclusion, a highly flexible and conductive PEDOT:PSS TE thin-film has been fabricated using direct dilution–filtration technology with common organic solvents. The diluting process leads to significant aggregation of the PEDOT-rich nanoparticles due to the partial dissolution of PSS in the organic solvents. The resulting PEDOT:PSS thin-films show a high σ of about 1500 S cm−1 not only for the high boiling point solvents, but also for the low boiling point ones. This facile treatment method simplifies the operation and combines the positive effects of drop-treatment and dip-treatment with organic solvents for the enhancement of σ. The S of the PEDOT:PSS thin-films remains fairly constant between 14.5 and 20.5 µV K−1 despite the depletion of PSS. Both σ and S of the PEDOT:PSS thin-films have good long-term stability in an ambient atmosphere. The large power factor (48.3 µW m−1 K−2) and ZT value (0.1) have been achieved with a facile treatment, which is great progress when compared to most other previous reports (∼10−2) as a prospective organic TE material. The enhancement of S will be explored to further obtain a high ZT value for the organic TE devices. This technology and experimental studies can also give new insights into the design of organic electronic thin-film devices.Acknowledgements
We gratefully acknowledge the financial support of National Natural Science Foundation of China (no. 51463008, 51402134, and 51303073), the Ganpo Outstanding Talents 555 projects, and the Youth Outstanding Talent Project of Jiangxi Science and Technology Normal University (2014QNBJRC001).Notes and references
- R. Venkatasubramanian, E. Siivola, T. Colpitts and B. O’Quinm, Nature, 2001, 413, 597–602 CrossRef CAS PubMed.
- S. Liu, H. Deng, Y. Zhao, S. Ren and Q. Fu, RSC Adv., 2015, 5, 1910–1917 RSC.
- D. Yoo, W. Son, S. Kim, J. J. Lee, S. H. Lee, H. H. Choi and J. H. Kim, RSC Adv., 2014, 4, 58924–58929 RSC.
- O. Bubnova, Z. U. Khan, A. Malti, S. Braun, M. Fahlman, M. Berggren and X. Crispin, Nat. Mater., 2011, 10, 429–433 CrossRef CAS PubMed.
- G. H. Kim, L. Shao, K. Zhang and K. P. Pipe, Nat. Mater., 2013, 12, 719–723 CrossRef CAS PubMed.
- O. Bubnova, Z. U. Khan, H. Wang, S. Braun, D. R. Evans, M. Fabretto, P. Hojati-Talemi, D. Dagnelund, J. B. Arlin, Y. H. Geerts, S. Desbief, D. W. Breiby, J. W. Andreasen, R. Lazzaroni, W. M. Chen, I. Zozoulenko, M. Fahlman, P. J. Murphy, M. Berggren and X. Crispin, Nat. Mater., 2014, 13, 190–194 CrossRef CAS PubMed.
- R. Yue and J. Xu, Synth. Met., 2012, 162, 912–917 CrossRef CAS PubMed.
- F. X. Jiang, J. K. Xu, B. Y. Lu, Y. Xie, R. J. Huang and L. F. Li, Chin. Phys. Lett., 2008, 25, 2202–2205 CrossRef CAS.
- C. Liu, F. Jiang, M. Huang, R. Yue, B. Lu, J. Xu and G. Liu, J. Electron. Mater., 2011, 40, 648–651 CrossRef CAS PubMed.
- T. Takano, H. Masunaga, A. Fujiwara, H. Okuzaki and T. Sasaki, Macromolecules, 2012, 45, 3859–3865 CrossRef CAS.
- Q. Wei, M. Mukaida, Y. Naitoh and T. Ishida, Adv. Mater., 2013, 25, 2831–2836 CrossRef CAS PubMed.
- J. Ouyang, ACS Appl. Mater. Interfaces, 2013, 5, 13082–13088 CAS.
- Y. Xia, K. Sun and J. Ouyang, Adv. Mater., 2012, 24, 2436–2440 CrossRef CAS PubMed.
- D. Alemu, H. Y. Wei, K. C. Ho and C. W. Chu, Energy Environ. Sci., 2012, 5, 9662–9671 CAS.
- Y. H. Kim, C. Sachse, M. L. Machala, C. May, L. Müller-Meskamp and K. Leo, Adv. Funct. Mater., 2011, 21, 1076–1081 CrossRef CAS PubMed.
- J. Ouyang, Q. Xu, C. W. Chu, Y. Yang, G. Li and J. Shinar, Polymer, 2004, 45, 8443–8450 CrossRef CAS PubMed.
- Y. Xia and J. Ouyang, Macromolecules, 2009, 42, 4141–4147 CrossRef CAS.
- Q. Wei, M. Mukaida, K. Kirihara, Y. Naitoh and T. Ishida, RSC Adv., 2014, 4, 28802–28806 RSC.
- X. Crispin, F. L. E. Jakobsson, A. Crispin, P. C. M. Grim, P. Andersson, A. Volodin, C. V. Haesendonck, M. V. D. Auweraer, W. R. Salaneck and M. Berggren, Chem. Mater., 2006, 18, 4354–4360 CrossRef CAS.
- C. Badre, L. Marquant, A. M. Alsayed and L. A. Hough, Adv. Funct. Mater., 2012, 22, 2723–2727 CrossRef CAS PubMed.
- J. Luo, D. Billep, T. Waechtler, T. Otto, M. Toader, O. Gordan, E. Sheremet, J. Martin, M. Hietschold, D. R. T. Zahn and T. Gessner, J. Mater. Chem. A, 2013, 1, 7576–7583 CAS.
- D. A. Mengistie, M. A. Ibrahem, P. C. Wang and C. W. Chu, ACS Appl. Mater. Interfaces, 2014, 6, 2292–2299 CAS.
- J. Y. Ouyang, Displays, 2013, 34, 423–436 CrossRef CAS PubMed.
- A. M. Nardes, R. A. J. Janssen and M. Kemerink, Adv. Funct. Mater., 2008, 18, 865–871 CrossRef CAS PubMed.
- Y. Xia and J. Ouyang, ACS Appl. Mater. Interfaces, 2012, 4, 4131–4140 CAS.
- J. Joo, S. M. Long, J. P. Pouget, E. J. Oh, A. G. MacDiarmid and A. J. Epstein, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 9567–9580 CrossRef CAS.
- O. Bubnova and X. Crispin, Energy Environ. Sci., 2012, 5, 9345–9362 CAS.
- Y. Xia and J. Ouyang, J. Mater. Chem., 2011, 21, 4927–4936 RSC.
- H. Park, S. H. Lee, F. S. Kim, H. H. Choi, I. W. Cheong and J. H. Kim, J. Mater. Chem. A, 2014, 2, 6532–6539 CAS.
- N. Massonnet, A. Carella, O. Jaudouin, P. Rannou, G. Laval, C. Celle and J. P. Simonato, J. Mater. Chem. C, 2014, 2, 1278–1283 RSC.
- T. Stöcker, A. Köhler and R. Moos, J. Polym. Sci., Part B: Polym. Phys., 2012, 50, 976–983 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental, characterization, Fig. S1–S9 and Tables S1–S3. See DOI: 10.1039/c5ra07820b |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |