Improve piezoelectricity and elasticity of Ce-doped BaTiO3 nanofibers — towards energy harvesting application†
Abstract
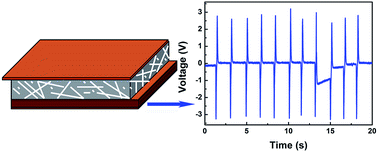
Ce-doped BaTiO3 (BTO) nanofibers were prepared by sol–gel combined with electrospinning method. The influence of cerium ions concentration on BTO crystal phase, microstructure, piezoelectricity, and elasticity were investigated. The energy harvesting properties of the device based on Ce-doped BTO nanofibers were tested. The piezoelectric property of BTO nanofibers were improved after cerium doping. The elastic property of the fibers changed little after cerium doping. The elastic modulus of the pure BTO nanofibers was about 3.37 GPa. When the Ce/Ba atomic ratio was 0.6%, the elastic modulus dropped to 1.18 GPa. Our analysis of the temperature range of the energy harvesting device indicates that an effective operation can be obtained when the working temperature of the device is below 110 °C. The largest delivered power of the energy harvesting device was 14.37 μW with a load resistance of 100 MΩ, when the Ce/Ba atomic ratio was 0.6%.

 Please wait while we load your content...
Please wait while we load your content...