Standardized extracts from black bean coats (Phaseolus vulgaris L.) prevent adverse cardiac remodeling in a murine model of non-ischemic cardiomyopathy
Abstract
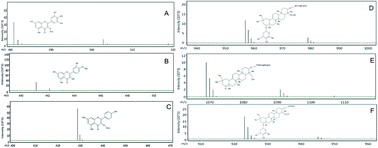
Black bean coats (Phaseolus vulgaris) contain bioactive compounds, including flavonoids and saponins, which have anti-fibrotic effects in which a standardized black bean extract (BBE) has been found to prevent liver fibrosis. Accordingly, the purpose of this study was to test whether BBE prevents remodeling in a murine model of non-ischemic cardiomyopathy. Saponins and flavonols were identified and quantified in BBE. Identification of flavonoids and saponins was confirmed by HPLC-MS-TOF. The cardiomyopathy model was produced by administering angiotensin and oral supplementation of L-NAME. Experimental animals received BBE in their diet at a dose equivalent to 40 mg per kg per day. The cardiomyopathy group (CMP) was characterized by severe maladaptive remodeling, left ventricular (LV) hypertrophy, decreased ejection fraction and increased LV end-diastolic volume. CMP mice treated with BBE had an improvement in ventricular function and reduction in LV mass. In addition, we found 65%, 85% and 83% reductions in interstitial fibrosis, brain natriuretic peptide (BNP) and transforming growth factor (TGFβ) expression, respectively. Consistent with those observations, collagen and TGFβ expression by isolated cardiac fibroblasts was reduced 82% and 70% following administration of BBE. BBE prevents adverse cardiac remodeling by reducing the extent of fibrosis and collagen deposition that occur in cardiomyopathy. These initial studies provide the basis for future research into the therapeutic potential of BBE in heart failure.

 Please wait while we load your content...
Please wait while we load your content...