KOtBu mediated efficient approach for the synthesis of fused heterocycles via intramolecular O-/N-arylations†
Raju Singha,
Atiur Ahmed,
Yasin Nuree,
Munmun Ghosh and
Jayanta K. Ray*
Department of Chemistry, Indian Institute of Technology Kharagpur, Kharagpur 721302, India. E-mail: jkray@chem.iitkgp.ernet.in; Tel: +91 03222283326
First published on 1st June 2015
Abstract
A novel and efficient methodology for the synthesis of 6H-benzo[c]chromenes, 6H-benzo[c]chromen-6-ones, carbazoles, dibenzofurans, dibenzooxepins has been developed. The reaction goes through intramolecular O-/N-arylations with sp2C–Br bonds via typical SNAr pathway in presence of potassium-tert-butoxide base.
Transition metal catalyzed cross coupling reactions to construct carbon–carbon and carbon–heteroatom bonds are the most powerful tool in modern organic synthesis.1 The frequently used transition metal catalysts are based on Pd or Cu and recently Fe, Ru, Rh, Co, Ni, Pt, Ag and Au mediated methodologies have also been developed.1,2 In spite of the remarkable advances in last few decades, some drawbacks remained such as high catalyst loading, expensiveness of metal catalysts with sophisticated ligands and product purification problem. Thus the development of transition metal free protocols for the formation of ‘cross-coupling products’ is highly desirable. Recently few groups reported the transition metal free process for the C–H arylations to construct biaryl frameworks.3
In late 2008, Itami and co-workers first reported the potassium tert-butoxide promoted biaryl coupling of electron deficient nitrogen heterocycles and haloarenes in absence of transition metal catalysts.4 After that few other groups also reported the similar type of phenomena in presence or absence of diamine ligands.5 Recently, Shi research group has reported the KOtBu promoted synthesis of fused rings via intramolecular cross coupling reaction (Scheme 1). However, it has some limitations such as (i) the methodology was not suitable for the substrates having unprotected amine groups, (ii) it gave good yields of 6H-benzo[c]chromenes but 6H-benzo[c]chromen-6-one was not formed at all.6 However, such KOtBu promoted protocols mainly focused on the formation of carbon–carbon bonds and now we are reporting a carbon-O/N bond formation protocol. There are some reports in literature for the carbon-O/N bond formation in presence of KOtBu along with different transition metal catalysts.7 Herein, we are reporting a KOtBu promoted methodology for the synthesis of oxygen and nitrogen containing fused heterocycles via intramolecular O-/N-arylations (Scheme 1) in absence of any transition metal catalyst.
Benzochromens8 and carbazoles9 are important classes of organic compounds present in numerous natural products and bioactive molecules. Considering the importance of these molecules, various methodologies have been reported in literature for their synthesis but most of them are associated with different transition metal catalysts.10,11 To the best of our knowledge, we are the first reporting potassium tert-butoxide promoted synthesis of 6H-benzo[c]chromenes, 6H-benzo[c]chromen-6-ones and carbazoles in absence of transition metal catalysts and ligands.
At first we had taken 1a as the model substrate to optimize the reaction conditions. When the substrate 1a was refluxed in benzene in presence of potassium tert-butoxide, it gave the desired product 6H-benzo[c]chromene in 76% yield. Then we had tried with other solvents (Table 1, entries 1–5) and the best result was obtained with DMSO in 82% of yield. On decreasing the reaction temperature to 80 °C, the starting material remained unreacted even after 6 h. Then we used some other bases (Table 1, entries 7–10). However, no product was obtained even in presence of strong base n-butyllithium. These results imply that the base KOtBu is crucial for this transformation. We had carried out the reaction using 1,10-phenanthroline as ligand but it was observed that the ligand has no significant role for this reaction.
| Entry | Solvent | Base | Temp. (°C) | Time (h) | Yielda (%) |
|---|---|---|---|---|---|
| a Isolated yield.b Standard reaction condition: the substrate 1a (0.3 mmol), KOtBu (1.5 mmol), DMSO (3 mL), 100 °C, 3 h.c 10 mol% of 1,10-phenanthroline was used. | |||||
| 1 | Benzene | KOtBu | 100 | 3 | 76 |
| 2 | Toluene | KOtBu | 100 | 3 | 69 |
| 3 | DMF | KOtBu | 100 | 3 | 73 |
| 4 | DMSO | KOtBu | 100 | 3 | 82b |
| 5 | THF | KOtBu | 65 | 6 | 43 |
| 6 | DMS | KOtBu | 80 | 6 | 71 |
| 7 | DMSO | K2CO3 | 100 | 6 | 00 |
| 8 | DMSO | Na2CO3 | 100 | 6 | 00 |
| 9 | DMSO | Et3N | 100 | 6 | 00 |
| 10 | THF | BuLi | 65 | 6 | 00 |
| 11 | DMSO | KOtBu | 100 | 3 | 80c |
Once we got the optimized reaction condition, we applied it on different substrates to examine its versatility and the results are summarized in Table 2. The electron rich substrates gave the corresponding 6H-benzo[c]chromenes in good to excellent yields (Table 2, entries 2a–e) while the electron poor substrates gave lower yields (Table 2, entries 2f–g). The yield of 2d is lower probably due to the steric hindrance with the adjacent aryl ring. The yield of 2g is poor probably due to the decomposition of the nitro substrate as no unreacted starting material was observed after completion of the reaction.
After getting success in the synthesis of a series of 6H-benzo[c]chromenes, we applied our methodology on the substrate 3a for the construction of 6H-benzo[c]chromen-6-ones and the results are shown in Table 3. Similar to Table 2, the electron rich substrates gave higher yields (Table 3, entries 4c–e) than electron deficient substrate (entry 4b). The reaction temperature was higher than that of Table 2 probably due to the lower nucleophilicity of carboxylic group than alkoxide group. However, the overall yield of the reaction was good to excellent.
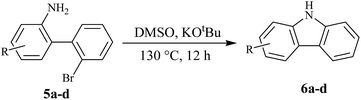
After successfully synthesizing different 6H-benzo[c]chromenes and 6H-benzo[c]chromen-6-ones, we applied our methodology on the substrate 2′-bromo-biphenyl-2-amines for the synthesis of carbazoles (Table 4). Different carbazole derivatives were obtained in good yields where the substrate took longer time for the completion of the reaction. It was noticed that the electron rich biaryl amine substrate gave lower yield (6c) than the electron poor substrate (6b).
It is worth mentioning that the acyl protected aniline and phenol precursors gave the corresponding deacylated followed by cyclized product carbazole or dibenzofuran (Scheme 2) because of the deacylation of amine or phenol in presence of strong base potassium tert-butoxide at higher temperature.
According to the literature reports, three types of mechanisms are possible for such transformations such as (i) through benzyne intermediate, (ii) radical pathway, or (iii) aromatic nucleophilic substitution (SNAr) pathway. To gain the deeper mechanistic understanding, we have done some controlled experiments (Scheme 3). The substrate 1a gave very trace amount of product 2a in presence of strong base nBuLi in THF or DMSO solvent. Then we did the reaction with the meta-chloro substituted substrate 10. However, the starting material remained unreacted. These results rule out the mechanism via the formation of benzyne intermediate. When we performed the reaction in presence of radical scavenger, the yield of the product was slightly reduced and this indicates that a small portion of the reaction goes through the radical pathway. Then we did the reaction with different halo substituted substrates (Scheme 3, eqn (4)) and we observed that for the fluoro substituted substrate, the reaction was complete within 30 minutes. The Cl and Br substituted substrates took almost similar time 3 h and the iodo substrate took 1 h for completion of the reaction. In presence of TEMPO (3 equiv.) the iodo-substrate gave lower yield probably due to the formation of radical intermediate. Thus the reactivity order with different halogens was F > I > Br, Cl. Although all the halogens are effective for such transformations, the halogen other than ortho-position remained intact throughout the reaction (Table 4, entry 6b or Scheme 3, compound 10) and these results also exclude both benzyne and radical pathways. From the above results we can conclude that for the iodo substrate, a significant portion of the reaction went through radical pathway and the other substrates followed the typical SNAr mechanism.
After studying the mechanistic details we tried to apply our methodology for the synthesis of higher ring sized heterocycles and we successfully synthesized 5H-dibenzo oxepin in 73% of yield (Scheme 4).
Conclusions
In conclusion, we have developed a potassium tert-butoxide promoted novel and efficient methodology for the synthesis of 6H-benzo[c]chromenes, 6H-benzo[c]chromen-6-ones and carbazoles. The methodology is also applicable for the synthesis of other fused heterocycles such as dibenzofurans and dibenzooxepins. The reaction goes through intramolecular O-/N-arylations in 2′-bromobiphenyl-2-methanol/carboxylic acid/amine via typical SNAr mechanism. This methodology will be very much useful in organic synthesis because of its transition metal free mild reaction conditions, easily synthesizable starting materials, intactness of halogens other than ortho-positions and finally the good to excellent yields of the reactions.Notes and references
-
(a) X. Chen, K. M. Engle, D. H. Wang and J. Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed
; (b) L. M. Xu, B. J. Li, Z. Yang and Z. J. Shi, Chem. Soc. Rev., 2010, 39, 712 RSC
; (c) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed
; (d) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS PubMed
; (e) J. W. Delord, T. Droge, F. Liu and F. Glorius, Chem. Soc. Rev., 2011, 40, 4740 RSC
.
-
(a) Metal-Catalyzed Cross-Coupling Reactions; Second, Completely Revised and Enlarged Edition; Volume 1; Edited by A. de Meijere and F. Diederich, 2008; DOI: 10.1002/9783527619535;
(b) R. Jana, T. P. Pathak and M. S. Sigman, Chem. Rev., 2011, 111, 1417 CrossRef CAS PubMed
; (c) G. Cahiez and A. Moyeux, Chem. Rev., 2010, 110, 1435 CrossRef CAS PubMed
.
- C. L. Sun and Z. J. Shi, Chem. Rev., 2014, 114, 9219 CrossRef CAS PubMed
and the references there in.
- S. Yanagisawa, K. Ueda, T. Taniguchi and K. Itami, Org. Lett., 2008, 10, 4673 CrossRef CAS PubMed
.
-
(a) E. Shirakawa, K. I. Itoh, T. Higashino and T. Hayashi, J. Am. Chem. Soc., 2010, 132, 15537 CrossRef CAS PubMed
; (b) A. Studer and D. P. Curran, Angew. Chem., Int. Ed., 2011, 50, 5018 CrossRef CAS PubMed
; (c) C. L. Sun, Y. F. Gu, B. Wang and Z. J. Shi, Chem.–Eur. J., 2011, 17, 10844 CrossRef CAS PubMed
; (d) C. L. Sun, Y. F. Gu, W. P. Huang and Z. J. Shi, Chem. Commun., 2011, 47, 9813 RSC
; (e) S. Yanagisawa and K. Itami, ChemCatChem, 2011, 3, 827 CrossRef CAS PubMed
; (f) M. Rueping, M. Leiendecker, A. Das, T. Poisson and L. Bui, Chem. Commun., 2011, 47, 10629 RSC
; (g) D. S. Roman, Y. Takahashi and A. B. Charette, Org. Lett., 2011, 13, 3242 CrossRef CAS PubMed
.
- C. L. Sun, Y. F. Gu, W. P. Huang and Z. J. Shi, Chem. Commun., 2011, 9813 RSC
.
-
(a) H. L. Aalten, G. V. Koten, D. M. Grove, T. Kuilman, O. G. Pickstra, L. A. Hulshof and R. A. Sheldon, Tetrahedron, 1989, 45, 5565 CrossRef CAS
; (b) L. Chen, G. Yu, F. Li, X. Zhu, B. Zhang, R. Guo, X. Li, Q. Yang, S. Jin, C. Liu and S. H. Liu, J. Organomet. Chem., 2010, 695, 1768 CrossRef CAS PubMed
; (c) A. S. Gajare, K. Toyota, M. Yoshifuji and F. Ozawa, Chem. Commun., 2004, 1994 RSC
; (d) N. Kataoka, Q. Shelby, J. P. Stambuli and J. F. Hartwig, J. Org. Chem., 2002, 67, 5553 CrossRef CAS PubMed
; (e) X. Xie, T. Y. Zhang and Z. Zhang, J. Org. Chem., 2006, 71, 6522 CrossRef CAS PubMed
.
-
(a) K. Koch, J. Podlech, E. Pfeiffer and M. Metzler, J. Org. Chem., 2005, 70, 3275 CrossRef CAS PubMed
; (b) H. Abe, K. Nishioka, S. Takeda, M. Arai, Y. Takeuchi and T. Harayama, Tetrahedron Lett., 2005, 46, 3197 CrossRef CAS PubMed
; (c) C. Garino, F. Bihel, N. Pietrancosta, Y. Laras, G. Quelever, I. Woo, P. Klein, J. Bain, J. L. Boucher and J. L. Kraus, Bioorg. Med. Chem. Lett., 2005, 15, 135 CrossRef CAS PubMed
; (d) W. Sun, L. D. Cama, E. T. Birzin, S. Warrier, L. Locco, R. Mosley, M. L. Hammond and S. P. Rohrer, Bioorg. Med. Chem. Lett., 2006, 16, 1468 CrossRef CAS PubMed
; (e) R. W. Pero and D. Harvan, Tetrahedron Lett., 1973, 14, 945 CrossRef
; (f) W. T. L. Sidwell, H. Fritz and C. Tamm, Helv. Chim. Acta, 1971, 54, 207 CrossRef CAS PubMed
; (g) H. Raistrick, C. E. Stilkings and R. Thomas, Biochemistry, 1953, 55, 421 CAS
.
-
(a) R. S. Kapil, in The Alkaloids, ed. R. H. F. Manske, Academic Press, New York, 1971, vol. 13, p. 273 Search PubMed
; (b) H. P. Husson, in The Alkaloids, ed. A. Brossi, Academic Press, New York, 1985, vol 26, p. 1 Search PubMed
; (c) D. P. Chakraborty, in The Alkaloids, ed. A. Brossi, Academic Press, New York, 1993, vol. 44, p. 257 Search PubMed
; (d) H. J. Knolker, Advances in Nitrogen Heterocycles, ed. C. J. Moody, JAI, Greenwich, 1995, vol. 1, p. 173 Search PubMed
; (e) J. K. R. Thomas, J. T. Lin, Y. T. Tao and C. W. Ko, J. Am. Chem. Soc., 2001, 123, 9404 CrossRef PubMed
; (f) M. Haussler, J. Liu, R. Zheng, J. W. Y. Lam, A. Qin and B. Z. Tang, Macromolecules, 2007, 40, 1914 CrossRef
.
-
(a) Q. J. Zhou, K. Worm and R. E. Dolle, J. Org. Chem., 2004, 69, 5147 CrossRef CAS PubMed
; (b) G. J. Kemperman, B. T. Horst, D. Van de Goor, T. Roeters, J. Bergwerff, R. van der Eem and J. Basten, Eur. J. Org. Chem., 2006, 3169 CrossRef CAS PubMed
; (c) N. Thasana, R. Worayuthakarn, P. Kradanrat, E. Hohn, L. Young and S. Ruchirawat, J. Org. Chem., 2007, 72, 9379 CrossRef CAS PubMed
; (d) S. Furuyama and H. Togo, Synlett, 2010, 2325 CAS
; (e) Y. Li, Y. J. Ding, J. Y. Wang, Y. M. Su and X. S. Wang, Org. Lett., 2013, 15, 2574 CrossRef CAS PubMed
; (f) Q. J. Zhou, K. Worm and R. E. Dolle, J. Org. Chem., 2004, 69, 5147 CrossRef CAS PubMed
; (g) G. J. Kemperman, B. Ter Horst, D. van de Goor, T. Roeters, J. Bergwerff, R. van der Eem and J. Basten, Eur. J. Org. Chem., 2006, 3169 CrossRef CAS PubMed
; (h) W. Zhang, B. I. Wilke, J. Zhan, K. Watanabe, C. N. Boddy and Y. J. Tang, J. Am. Chem. Soc., 2007, 129, 9304 CrossRef CAS PubMed
; (i) J. Luo, Y. Lu, S. Liu, J. Liu and G. J. Deng, Adv. Synth. Catal., 2011, 353, 2604 CrossRef CAS PubMed
; (j) R. Singha, S. Roy, S. Nandi, P. Ray and J. K. Ray, Tetrahedron Lett., 2013, 54, 657 CrossRef CAS PubMed
.
-
(a) L. Ackermann, A. Althammer and P. Mayer, Synthesis, 2009, 3493 CrossRef CAS PubMed
; (b) B. J. Stokes, B. Jovanovic, H. Dong, K. J. Richert, R. D. Riell and T. G. Driver, J. Org. Chem., 2009, 74, 3225 CrossRef CAS PubMed
; (c) J. A. Jordan-Hore, C. C. C. Johansson, M. Gulias, E. M. Beck and M. J. Gaunt, J. Am. Chem. Soc., 2008, 130, 16184 CrossRef CAS PubMed
; (d) B. Liegault, D. Lee, M. P. Huestis, D. R. Stuart and K. Fagnou, J. Org. Chem., 2008, 73, 5022 CrossRef CAS PubMed
; (e) W. C. P. Tsang, N. Zheng and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 14560 CrossRef CAS PubMed
; (f) Z. Liu and R. C. Larock, Org. Lett., 2004, 6, 3739 CrossRef CAS PubMed
; (g) Y. Qiu, W. Kong, C. Fu and S. Ma, Org. Lett., 2012, 14, 6198 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra07532g |
| This journal is © The Royal Society of Chemistry 2015 |