Enhanced ammonia sensing at room temperature with reduced graphene oxide/tin oxide hybrid films†
Ruma Ghosha,
Arpan Kumar Nayakb,
Sumita Santrac,
Debabrata Pradhanb and
Prasanta Kumar Guha*a
aDepartment of Electronics & Electrical Communication Engineering, Indian Institute of Technology, Kharagpur, 721302, India. E-mail: pkguha@ece.iitkgp.ernet.in; Fax: +91 3222 255303; Tel: +91 3222 283538
bCentre of Material Science, Indian Institute of Technology, Kharagpur, 721302, India
cDepartment of Physics, Indian Institute of Technology, Kharagpur, 721302, India
First published on 21st May 2015
Abstract
Sensitive and selective detection of ammonia at room temperature is required for proper environmental monitoring and also to avoid any health hazards in the industrial areas. The excellent electrical properties of reduced graphene oxide (RGO) and sensing capabilities of SnO2 were combined to achieve enhanced ammonia sensitivity. RGO–SnO2 films were synthesized hydrothermally as well as prepared by mixing different amounts of hydrothermally synthesized SnO2 nanoparticles with graphene oxide (GO). It was observed that the response of the hybrid sensing layer was considerably better than intrinsic RGO or SnO2. However, the best performance was observed in the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (RGO–SnO2) sample. The sample was exposed to nine different concentrations of ammonia in the presence of 20% RH at room temperature. The response of the sensor varied from 1.4 times (25 ppm) to 22 times (2800 ppm) with quick recovery after purging with air. The composite formation was verified by characterizing the samples using field emission scanning electron microscopy (FESEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and high resolution transmission electron microscopy (HRTEM). The results and their significance have been discussed in detail.
8 (RGO–SnO2) sample. The sample was exposed to nine different concentrations of ammonia in the presence of 20% RH at room temperature. The response of the sensor varied from 1.4 times (25 ppm) to 22 times (2800 ppm) with quick recovery after purging with air. The composite formation was verified by characterizing the samples using field emission scanning electron microscopy (FESEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and high resolution transmission electron microscopy (HRTEM). The results and their significance have been discussed in detail.
1. Introduction
Ammonia is a toxic pollutant, which occurs naturally in the environment through human wastes and industries.1 It has a sharp and pungent odor but we can smell it only if the concentration of ammonia is more than 50 ppm (parts per million).2 Therefore, it is quite natural to get exposed to lower levels of ammonia in our day-to-day lives without even knowing. Ammonia can cause severe effects on human body like irritation of the eyes, throat, skin and respiratory system when exposed to concentrations greater than 35 ppm for even just 15 minutes.3 Therefore, it is necessary to develop highly sensitive and selective ammonia sensors that can detect low concentrations of ammonia.Tin dioxide (SnO2) is an n-type semiconducting material. It is highly sensitive towards different chemical analytes.4–6 Different morphologies of SnO2 nanostructures and its composites have already been employed as ammonia sensors in the past few years.7 However, similar to other metal oxides, SnO2 also suffers from two major drawbacks. The first is high temperature operability, i.e. it can sense gases only at elevated temperature (200–500 °C), which increases the power consumption as well as limits its feasibility as a sensor in conditions where high temperature operations are not allowed.8 The second drawback is poor selectivity, i.e. tin oxide shows a similar response towards different gases and thereby demonstrates no specificity towards any particular gas.9
In these regards, graphene and reduced graphene oxide (RGO) gain advantage. Graphene is a 2D carbon nanomaterial with a high aspect ratio and excellent electronic properties, which facilitate sensing gases at room temperature.10 However, graphene in its pure form is not ideal for gas detection, because it is devoid of any functional groups and defect sites, which play a vital role in gas sensing. In addition, graphene is usually synthesized using sophisticated and expensive techniques such as chemical vapor deposition (CVD) and epitaxial methods,11 and one of the cheapest techniques to synthesize graphene is through mechanical exfoliation but that too suffers with scalability issues.12 RGO on the other hand can be synthesized chemically, which is usually a low cost technique, and also RGO contains functional groups and defect sites, which act as active regions for the gas molecules to get attached.13 However, the response of RGO towards gases is not as high as that of metal oxides.
Inspired by the outstanding properties of RGO and SnO2 (RGO can sense gases at room temperature and SnO2 gives a large response), here we have developed RGO–SnO2 hybrid samples for ammonia sensing. RGO, intrinsically a p-type material, was synthesized by reducing graphene oxide (GO) thermally. The n-type SnO2 nanoparticles were synthesized using a hydrothermal technique. The RGO–SnO2 hybrid material was prepared in two ways, which have been discussed in the Experimental section. The sensor featured a very large response (larger than RGO or SnO2 response towards ammonia), good selectivity, fast response and recovery, even at room temperature. The enhanced sensing behavior of the hybrid film is due to the combined nature of the p-type and n-type sensing materials. The response of the sensor was measured in the presence of ammonia (25–2800 ppm) and different VOCs. The sensing results and their mechanism have been explained in detail.
2. Experimental section
2.1. Material synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 10
3, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 10
4, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 10
5, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and 1
8 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
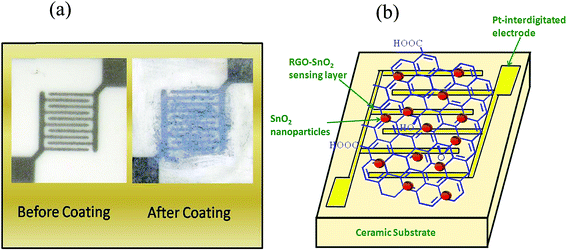
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The GO–SnO2 mixed samples were ultrasonicated for 30 minutes, so as to obtain uniform dispersion and also for proper mixing of GO and SnO2 nanoparticles. The samples were then drop casted on Pt based interdigitated electrodes (Synkera Technologies, length and width of the electrode fingers being 2.54 mm and 100 μm, respectively, and having 100 μm space between two adjacent fingers), dried in air and then heated at 160 °C for 30 minutes to reduce the GO present in the hybrid sensing layer.
1. The GO–SnO2 mixed samples were ultrasonicated for 30 minutes, so as to obtain uniform dispersion and also for proper mixing of GO and SnO2 nanoparticles. The samples were then drop casted on Pt based interdigitated electrodes (Synkera Technologies, length and width of the electrode fingers being 2.54 mm and 100 μm, respectively, and having 100 μm space between two adjacent fingers), dried in air and then heated at 160 °C for 30 minutes to reduce the GO present in the hybrid sensing layer.2.2. Material characterizations
The RGO–SnO2 hybrid sensing layers were characterized using a Zeiss Auriga Compact field emission scanning electron microscope (FESEM) to observe their morphologies as well as their composition. FEI TECNAI G2 high resolution transmission electron microscope (HRTEM) was employed to observe the detailed microstructures of the hybrid sensing layers. The X-ray diffraction (XRD) pattern was observed using a Panalytical X'Pert Pro diffractometer with a conventional X-ray tube (Cu Kα radiation). Moreover, in order to ensure proper reduction of GO to RGO, X-ray photoelectron spectroscopy (XPS) was performed using a PHI 5000 Versa Probe II.2.3. Gas sensing
The gas sensing set-up was assembled in-house. It consists of three mass flow controllers (MFCs) to control the flow rates of the gases, each MFC can allow a maximum of 100 standard cubic centimeters per minute (sccm) of gas to flow through it, an airtight stainless steel chamber to probe the samples, and an Agilent 34972A LXI data acquisition (DAQ) card (which can record the resistance of the samples after every 5 s) to interface the complete system with a computer.3. Results and discussion
3.1. Material characterizations
The SEM images of RGO, SnO2 nanoparticles and the hybrid films are shown in Fig. 1.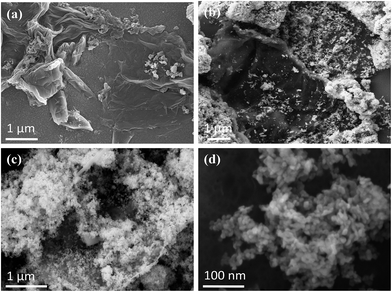 | ||
| Fig. 1 FESEM images of (a) thermally reduced GO, (b and c) the RGO–SnO2 hybrid film prepared by mixing GO and SnO2 and (d) SnO2 nanoparticles. | ||
The RGO film used to carry out ammonia sensing tests was multilayered as there are visible wrinkles in the SEM images of the RGO film, as shown in Fig. 1(a). Fig. 1(b) shows the FESEM image of the RGO–SnO2 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) hybrid film. The SnO2 nanoparticles attached with RGO flakes can be seen in the image. In addition, it was observed that SnO2 nanoparticles got agglomerated over the RGO flakes. Owing to their high surface energy, the SnO2 nanoparticles probably got agglomerated while ultrasonicating the GO and metal oxide mixture in the ethanol medium.1 The amount of SnO2 particles was observed to increase when the proportion of SnO2 was increased (ratio of RGO to SnO2 was 10
3) hybrid film. The SnO2 nanoparticles attached with RGO flakes can be seen in the image. In addition, it was observed that SnO2 nanoparticles got agglomerated over the RGO flakes. Owing to their high surface energy, the SnO2 nanoparticles probably got agglomerated while ultrasonicating the GO and metal oxide mixture in the ethanol medium.1 The amount of SnO2 particles was observed to increase when the proportion of SnO2 was increased (ratio of RGO to SnO2 was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8), as shown in Fig. 1(c). The FESEM image of 10
8), as shown in Fig. 1(c). The FESEM image of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 10
4, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 1
5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 RGO-SnO2 samples is shown in Fig. S1 of the ESI.† The FESEM of SnO2 nanoparticles is shown in Fig. 1(d).
1 RGO-SnO2 samples is shown in Fig. S1 of the ESI.† The FESEM of SnO2 nanoparticles is shown in Fig. 1(d).
In order to ensure the composition of the hybrid samples synthesized both ways, energy dispersive X-ray spectroscopy (EDS) was carried out for all the samples. The EDS results of the samples are shown in Fig. 2.
 | ||
Fig. 2 EDS results of (a) 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (RGO 4 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) hybrid samples and (b) hydrothermally synthesized RGO–SnO2 hybrid samples. SnO2) hybrid samples and (b) hydrothermally synthesized RGO–SnO2 hybrid samples. | ||
The compositional analysis revealed the proportion of C, Sn and O present in the hybrid samples. Fig. 2(a) shows the composition of RGO-SnO2 hybrid samples prepared by mixing already synthesized GO and SnO2, followed by thermal reduction. The EDS result of the hydrothermally synthesized hybrid samples is shown in Fig. 2(b).
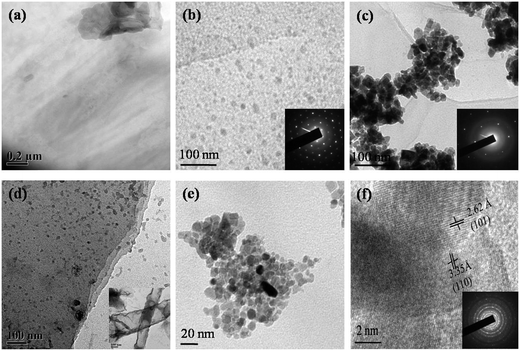
TEM was performed to observe the crystalline nature and microstructural properties of the hybrid sensing materials prepared hydrothermally as well as by mixing GO and SnO2 in different proportions. The TEM and HRTEM images of the samples are shown in Fig. 3.
The TEM image (Fig. 3(a)) of thermally reduced GO appears to be transparent and few layered. Fig. 3(b) shows the imprints of uniformly distributed SnO2 nanoparticles (diameter = ∼10 nm) over RGO flakes in the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (RGO
3 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) hybrid sample. The inset shows the hexagonal rings of the RGO. Fig. 3(c) demonstrates the presence of an increased amount of SnO2 nanoparticles in the 10
SnO2) hybrid sample. The inset shows the hexagonal rings of the RGO. Fig. 3(c) demonstrates the presence of an increased amount of SnO2 nanoparticles in the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (RGO
8 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) hybrid sample. The agglomeration of nanoparticles is clearly visible in the TEM image and also the same was observed in the FESEM image. The inset of Fig. 3(c) also shows the hexagonal ring of the C-atoms. Fig. 3(d) shows uniformly distributed SnO2 nanoparticles over multilayered RGO flakes in the hydrothermally synthesized RGO–SnO2 hybrid sample. RGO in the hydrothermal sample attained a different folded structure due to high temperature treatment. One such hollow tubular RGO structure found in the hydrothermally synthesized sample is shown in the inset of Fig. 3(d). The particle size of the SnO2 nanoparticles was around 10 nm as can be seen in Fig. 3(e). Fig. 3(f) shows the HRTEM image of the 10
SnO2) hybrid sample. The agglomeration of nanoparticles is clearly visible in the TEM image and also the same was observed in the FESEM image. The inset of Fig. 3(c) also shows the hexagonal ring of the C-atoms. Fig. 3(d) shows uniformly distributed SnO2 nanoparticles over multilayered RGO flakes in the hydrothermally synthesized RGO–SnO2 hybrid sample. RGO in the hydrothermal sample attained a different folded structure due to high temperature treatment. One such hollow tubular RGO structure found in the hydrothermally synthesized sample is shown in the inset of Fig. 3(d). The particle size of the SnO2 nanoparticles was around 10 nm as can be seen in Fig. 3(e). Fig. 3(f) shows the HRTEM image of the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (RGO
8 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) sample. It shows the presence of (101) and (110) SnO2 planes in the hybrid sample, which are perpendicular to each other. The result agrees well with the XRD data of SnO2 nanoparticles, as shown in Fig. 4. Moreover, the SAED pattern of the SnO2 nanoparticles was found to be polycrystalline, as is shown in the inset of Fig. 3(f).
SnO2) sample. It shows the presence of (101) and (110) SnO2 planes in the hybrid sample, which are perpendicular to each other. The result agrees well with the XRD data of SnO2 nanoparticles, as shown in Fig. 4. Moreover, the SAED pattern of the SnO2 nanoparticles was found to be polycrystalline, as is shown in the inset of Fig. 3(f).
A broad peak at around 15° was observed in the 30 minutes thermally reduced GO sample, as is shown in Fig. 4. For SnO2 nanoparticles, sharp peaks were observed, which signify its highly crystalline nature. Our observed SnO2 XRD result matched well with the tetragonal structure of SnO2 with lattice constants of a = 4.7552, b = 4.7552, and c = 3.1992 (JCPDS no. 01-077-0452). For the hybrid samples, the XRD of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (RGO
8 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) before and after reducing it thermally were carried out. Although no significant peak shifting corresponding to the reduction of GO was observed, peaks of both RGO and SnO2 nanoparticles are clearly visible in GO–SnO2 and the RGO–SnO2 samples.
SnO2) before and after reducing it thermally were carried out. Although no significant peak shifting corresponding to the reduction of GO was observed, peaks of both RGO and SnO2 nanoparticles are clearly visible in GO–SnO2 and the RGO–SnO2 samples.
The elemental and compositional information of the hybrid samples were further investigated using XPS. The XPS results of the RGO–SnO2 samples are shown in Fig. 5.
 | ||
| Fig. 5 XPS results of (a) comparative survey scan of RGO and RGO–SnO2, (b) Sn 3d spectra in the RGO–SnO2 hybrid sample, (c) C 1s peak of GO and (d) C 1s peak of the RGO–SnO2 hybrid sample. | ||
The surface spectrum of the RGO–SnO2 sample (Fig. 5(a)) clearly shows the presence of carbon, oxygen and tin. No other peaks corresponding to the precursors of the samples (GO or SnO2) were observed, hence signifying that the samples were highly pure. Two Sn 3d peaks were observed at 486.4 and 494.8 eV, which correspond to Sn 3d5/2 and Sn 3d3/2, respectively, as shown in Fig. 5(b).15 These peaks are attributed to the +4 oxidation state of Sn in RGO–SnO2 hybrid samples. The C 1s peak of GO can be de-convoluted into three peaks, 284.5, 286.6 and 288.3 eV, corresponding to C–C, C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.16 The peak intensities corresponding to C–O and C
O.16 The peak intensities corresponding to C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O got reduced significantly after thermally reducing the RGO–SnO2 sample, as is evident from Fig. 5(d), thereby signifying that the GO got properly reduced. One interesting observation that was made during the XPS characterization was that the intensities of the peaks corresponding to C–O and C
O got reduced significantly after thermally reducing the RGO–SnO2 sample, as is evident from Fig. 5(d), thereby signifying that the GO got properly reduced. One interesting observation that was made during the XPS characterization was that the intensities of the peaks corresponding to C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in GO–SnO2 were significantly reduced and were almost similar to that of the 30 minutes reduced GO–SnO2 sample. This signifies that the SnO2 nanoparticles assisted in the reduction of GO. Similar results were observed in XRD pattern, where no considerable peak shift was observed, as shown in Fig. 4. However, no measurable resistance could be measured at room temperature in the GO–SnO2 samples unless they had been thermally reduced for 30 minutes.
O in GO–SnO2 were significantly reduced and were almost similar to that of the 30 minutes reduced GO–SnO2 sample. This signifies that the SnO2 nanoparticles assisted in the reduction of GO. Similar results were observed in XRD pattern, where no considerable peak shift was observed, as shown in Fig. 4. However, no measurable resistance could be measured at room temperature in the GO–SnO2 samples unless they had been thermally reduced for 30 minutes.
The samples coated on IDEs were probed inside the stainless steel chamber and purged with dry air for 20 minutes to stabilize the baseline resistances of the samples at room temperature. After that, the target gas, ammonia, in the presence of 20% relative humidity (RH), was allowed into the chamber for 5 minutes followed by dry air purging. The response of all the samples was calculated as follows:
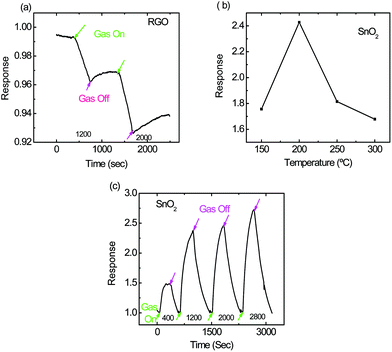
The response of thermally reduced GO towards ammonia at room temperature is shown in Fig. 7(a). The resistance of the RGO sensor increased when exposed to ammonia as RGO is inherently a p-type material and ammonia is a donor molecule, as already been discussed in our previously reported work.17 SnO2 is a semiconducting material, which senses analytes at higher temperature. Thus, the SnO2 nanoparticle based sensor was exposed to 1200 ppm ammonia at different temperatures (150–300 °C) to find out the temperature of its optimum response. The temperature profile and the response of the SnO2 based sensor towards different concentrations of ammonia at its optimum temperature (200 °C) are shown in Fig. 7(b) and (c), respectively.
The basic mechanism of sensing gases using a metal oxide (e.g. tin oxide) has already been discussed in the literature.18 Briefly, when air comes into contact with metal oxide, oxygen molecules trap electrons from the metal oxide surface, thereby increasing the sensing layer resistance. These adsorbed oxygen species act as reaction sites when gas molecules (e.g. NH3) come in contact with them, thus releasing electrons back to the conduction band of tin oxide.
| 4NH3 + 3O2(ads)− → 2N2 + 6H2O + 3e− |
Hence, the resistance of the SnO2 nanoparticle based sensor decreased when exposed to ammonia as can be seen in Fig. 7(c), thereby depicting n-type behavior of the SnO2 nanoparticle based sensor. The response was found to be 2.4 times in the presence of 1200 ppm NH3, which is considerably larger than what we obtained from RGO sensors, but the temperature of sensing was higher than room temperature i.e. 200 °C.
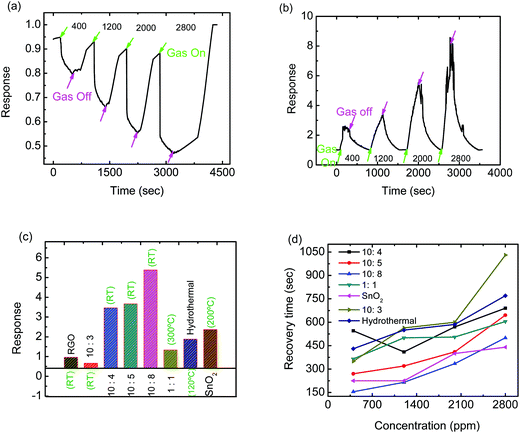
In order to integrate the higher sensitivity of the SnO2 based sensor and the ability of RGO to detect gases at room temperature (which helps in lowering the power dissipation), RGO–SnO2 hybrid sensing layers were prepared by mixing GO and SnO2 in different wt%. The sample prepared after mixing GO to SnO2 in the ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and then thermally reducing it demonstrated enhanced sensitivity, even at room temperature, but the resistance of the hybrid sensing layer increased when exposed to ammonia, thus showing an effective p-type behavior. The response of the hybrid sensor towards four different concentrations of ammonia is shown in Fig. 8(a). The 10
3 and then thermally reducing it demonstrated enhanced sensitivity, even at room temperature, but the resistance of the hybrid sensing layer increased when exposed to ammonia, thus showing an effective p-type behavior. The response of the hybrid sensor towards four different concentrations of ammonia is shown in Fig. 8(a). The 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (RGO
3 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) exhibited a response of 0.665 times against 1200 ppm of ammonia. Again, when the amount of SnO2 was increased to 10
SnO2) exhibited a response of 0.665 times against 1200 ppm of ammonia. Again, when the amount of SnO2 was increased to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 wt% (RGO
4 wt% (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2), the resistance of the hybrid sensor was found to decrease at room temperature when ammonia was introduced to the test chamber, thus showing an effective n-type behavior. A response of around 3.4 times was observed against 1200 ppm ammonia for the 10
SnO2), the resistance of the hybrid sensor was found to decrease at room temperature when ammonia was introduced to the test chamber, thus showing an effective n-type behavior. A response of around 3.4 times was observed against 1200 ppm ammonia for the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (RGO
4 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) sample, as shown in Fig. 8(b).
SnO2) sample, as shown in Fig. 8(b).
In order to further investigate the role of the amount of SnO2 in the hybrid film, the wt% of SnO2 was gradually increased and ammonia tests were carried out. It was observed that with increase in the amount of SnO2 in the RGO–SnO2 hybrid film, the response of the sensors got enhanced towards ammonia (at room temperature). However, when RGO and SnO2 were mixed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, the samples did not show any measurable resistance up to 290 °C. Such an increment of resistance of the RGO–SnO2 hybrid sample is associated with the depletion of electrons due to the formation of a p–n junction in the hybrid sample, as reported in the literature.19 Therefore, the ammonia test was carried out at 300 °C for this 1
1 ratio, the samples did not show any measurable resistance up to 290 °C. Such an increment of resistance of the RGO–SnO2 hybrid sample is associated with the depletion of electrons due to the formation of a p–n junction in the hybrid sample, as reported in the literature.19 Therefore, the ammonia test was carried out at 300 °C for this 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio sample, but we observed that the response got reduced. For example, the response of this sample towards 1200 ppm ammonia was found to be ∼1.3 times only. This response from a 1
1 ratio sample, but we observed that the response got reduced. For example, the response of this sample towards 1200 ppm ammonia was found to be ∼1.3 times only. This response from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (RGO
1 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) ratio sample was found to be similar to the response of pristine SnO2 nanoparticles at 300 °C, as shown in Fig. 7(b). This suggests that SnO2 nanoparticles were playing the predominant role for ammonia sensing in the 1
SnO2) ratio sample was found to be similar to the response of pristine SnO2 nanoparticles at 300 °C, as shown in Fig. 7(b). This suggests that SnO2 nanoparticles were playing the predominant role for ammonia sensing in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hybrid sample.
1 hybrid sample.
The hydrothermally synthesized hybrid sample also did not show any measurable resistance at room temperature. This could be explained as follows: RGO can sense gases at room temperature due to its planar 2D structure. However, when the hybrid sample was synthesized hydrothermally, the RGO flakes got folded and formed multilayered structures, as is evident in Fig. 3(d), due to which the 2D structure no longer existed and thus no resistance could be measured at room temperature. Therefore, the ammonia test was carried out at 120 °C. Moreover, the response of the hydrothermally synthesized sample was found to be poorer than the samples prepared by mixing GO and SnO2. For example, the response of the hydrothermally synthesized sample towards 1200 ppm ammonia was found to be ∼1.9 times. The 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (RGO
8 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) hybrid sample showed the maximum response towards (measurements carried out at 1200 ppm) ammonia, as can be seen in Fig. 8(c). Moreover, it was observed that the sensors recovered faster with increase in the amount of SnO2 nanoparticles in hybrid samples, as shown in Fig. 8(d). The recovery time of the 10
SnO2) hybrid sample showed the maximum response towards (measurements carried out at 1200 ppm) ammonia, as can be seen in Fig. 8(c). Moreover, it was observed that the sensors recovered faster with increase in the amount of SnO2 nanoparticles in hybrid samples, as shown in Fig. 8(d). The recovery time of the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (RGO
8 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) hybrid sensor was found to be the shortest among all the sensors. The recovery times of the RGO based ammonia sensor was not included in Fig. 8(d) because the recovery time is comparatively longer as is evident from the response plot of RGO in Fig. 7(a). The response times of the RGO–SnO2 hybrid samples were found to be comparable with that of intrinsic SnO2 nanoparticles but were to be found considerably faster than that of intrinsic RGO. For example, the response time of the 10
SnO2) hybrid sensor was found to be the shortest among all the sensors. The recovery times of the RGO based ammonia sensor was not included in Fig. 8(d) because the recovery time is comparatively longer as is evident from the response plot of RGO in Fig. 7(a). The response times of the RGO–SnO2 hybrid samples were found to be comparable with that of intrinsic SnO2 nanoparticles but were to be found considerably faster than that of intrinsic RGO. For example, the response time of the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (RGO
8 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) sample was found to be around 210 s against 1200 ppm ammonia, whereas the response times of RGO and SnO2 nanoparticles against 1200 ppm ammonia were 450 and 210 s, respectively. A comparative plot of response times of RGO and SnO2 hybrid samples for different concentrations of ammonia is shown in Fig. S2 of the ESI.† The response of the best sample (10
SnO2) sample was found to be around 210 s against 1200 ppm ammonia, whereas the response times of RGO and SnO2 nanoparticles against 1200 ppm ammonia were 450 and 210 s, respectively. A comparative plot of response times of RGO and SnO2 hybrid samples for different concentrations of ammonia is shown in Fig. S2 of the ESI.† The response of the best sample (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (RGO
8 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2)) to different concentrations of ammonia is shown in Fig. 9.
SnO2)) to different concentrations of ammonia is shown in Fig. 9.
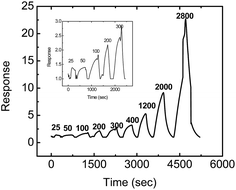
The 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (RGO
8 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) sensor was also exposed to nine different concentrations of ammonia (25–2800 ppm) in the presence of 20% RH, in order to ensure its performance as a practical ammonia sensor. The response of the 10
SnO2) sensor was also exposed to nine different concentrations of ammonia (25–2800 ppm) in the presence of 20% RH, in order to ensure its performance as a practical ammonia sensor. The response of the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (RGO
8 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) sensor was found to be excellent, as shown in Fig. 9, and varied from 1.4 times against 25 ppm ammonia to 22 times against 2800 ppm ammonia. In addition, the recovery of the sensor was very fast. It took merely 150 seconds to recover to its baseline resistance after exposure to 400 ppm of ammonia (but in the case of RGO, the recovery was found to be more than 650 s, which is very slow). The response of all the other hybrid samples against different concentrations of ammonia is shown in the ESI (Fig. S3–S5†). The responses of the sensors are also highly reproducible and repeatable. The reproducible response of our best sample is shown in Fig. 10(a).
SnO2) sensor was found to be excellent, as shown in Fig. 9, and varied from 1.4 times against 25 ppm ammonia to 22 times against 2800 ppm ammonia. In addition, the recovery of the sensor was very fast. It took merely 150 seconds to recover to its baseline resistance after exposure to 400 ppm of ammonia (but in the case of RGO, the recovery was found to be more than 650 s, which is very slow). The response of all the other hybrid samples against different concentrations of ammonia is shown in the ESI (Fig. S3–S5†). The responses of the sensors are also highly reproducible and repeatable. The reproducible response of our best sample is shown in Fig. 10(a).
The 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (RGO
8 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) hybrid sensor device was prepared twice and the response of both the sensors towards ammonia was found to be similar, as shown in Fig. 10(a). However, it was observed that the recovery of the first sample (Sample 1) was faster than the second sample (Sample 2). The reason is not yet known and needs further investigation.
SnO2) hybrid sensor device was prepared twice and the response of both the sensors towards ammonia was found to be similar, as shown in Fig. 10(a). However, it was observed that the recovery of the first sample (Sample 1) was faster than the second sample (Sample 2). The reason is not yet known and needs further investigation.
Recent reports on RGO–SnO2 based hybrid gas and volatile organic compound (VOC) sensors are available in the literature.20–23 However, in most of the cases, the detection temperature was high. A comparison of our achieved response with recently reported literature values on RGO–SnO2 based ammonia sensor is shown in Fig. 10(b) (only the work reported by Ghaddab et al. is on ammonia sensing by a SWNT/SnO2 hybrid).24–28 The temperature of sensing for all the reported works have been indicated in the plot itself. For our results, the temperature of sensing is room temperature. It was found that our response is better than reported literature values against respective concentrations of ammonia. Only the response of the sensors reported by Zhang et al. was better than our result, but their working temperature was very high (260 °C). The response of the hybrid ammonia sensor in air ambience reported by Lin et al. was found to be poorer than the response (in N2 ambience) that has been plotted in Fig. 10(b). They achieved a response of around 7% i.e. 0.9347 times against 50 ppm of ammonia in air ambience, while in our case all the tests were done in air only.
Metal oxide based sensors are supposed to have very poor selectivity. Therefore, in order to ensure the selectivity of our sensor, the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (RGO
8 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) hybrid film was exposed to 1000 ppm of different volatile organic compounds (VOCs) and also to 30% RH.
SnO2) hybrid film was exposed to 1000 ppm of different volatile organic compounds (VOCs) and also to 30% RH.
The response of the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (RGO
8 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) hybrid sensor towards 1000 ppm of different VOCs was found to be poorer (∼1.25 times) than that of 1000 ppm of ammonia (5.6 times), as is evident from Fig. 11. Moreover, as the ammonia tests were carried out at 20% RH; therefore, the sensor was also exposed to 30% RH to ensure that there was no considerable contribution of RH to the response of the sensors towards ammonia. It was observed that the sensor did not respond well against 30% RH. A mere response of 1.08 times was observed against RH. Hence, the hybrid sensor was found highly selective towards ammonia.
SnO2) hybrid sensor towards 1000 ppm of different VOCs was found to be poorer (∼1.25 times) than that of 1000 ppm of ammonia (5.6 times), as is evident from Fig. 11. Moreover, as the ammonia tests were carried out at 20% RH; therefore, the sensor was also exposed to 30% RH to ensure that there was no considerable contribution of RH to the response of the sensors towards ammonia. It was observed that the sensor did not respond well against 30% RH. A mere response of 1.08 times was observed against RH. Hence, the hybrid sensor was found highly selective towards ammonia.
3.2. Sensing mechanism
The basic sensing mechanisms of RGO (predominantly p type due to an abundant amount of holes) and tin oxide (adsorbed oxygen species from air act as reaction sites at the metal oxide surface) are already discussed in the earlier section. However, the hybrid samples exhibited enhanced response towards ammonia compared to that of intrinsic RGO and SnO2. This is because of the formation of hetero and homo junctions at the interface of the materials, (i) RGO–SnO2 and (ii) SnO2–SnO2, as is shown in Fig. 12. In the case of hetero junctions (p-type RGO and n-type SnO2), SnO2 donates electrons to RGO, which recombine with the holes of RGO, thereby shifting the Fermi level and thus a depletion region forms. This depletion region is an additional site for the target gases and attracts electrons from the donor molecules (here ammonia) and results in an increase in conductivity. Along with the RGO–SnO2 depletion zone, SnO2–SnO2 depletion region and also the functional groups attached with RGO are active sites for the analytes to react and get attached. | ||
Fig. 12 Schematic (not to scale) representation of sensing mechanism in (a) the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (RGO 3 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) sample showing p-type behavior and (b) the 10 SnO2) sample showing p-type behavior and (b) the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (RGO 8 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) sample showing n-type behavior. SnO2) sample showing n-type behavior. | ||
In addition to superior response, our sensors also showed both p-type and n-type behavior, so the sensing mechanism was dominated by the material proportion present in the hybrid film. For example, the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (RGO
3 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) sample demonstrated a p-type behavior towards ammonia, which is similar to that shown by intrinsic RGO, but an enhanced response was observed in the hybrid sample. This means the sensing was dominated by RGO being available in abundance, but the enhancement of response was observed due to the presence of SnO2 nanoparticles, which gives rise to different potential barriers at the junctions as mentioned above. The sensing mechanism of the 10
SnO2) sample demonstrated a p-type behavior towards ammonia, which is similar to that shown by intrinsic RGO, but an enhanced response was observed in the hybrid sample. This means the sensing was dominated by RGO being available in abundance, but the enhancement of response was observed due to the presence of SnO2 nanoparticles, which gives rise to different potential barriers at the junctions as mentioned above. The sensing mechanism of the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (RGO
3 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) sample is shown in Fig. 12(a).
SnO2) sample is shown in Fig. 12(a).
On the other hand, the sensing phenomenon of the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (RGO
8 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) sample is different than that of the 10
SnO2) sample is different than that of the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (RGO
3 (RGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) hybrid sample. Here RGO, acted primarily as a conducting network, which resulted in a measurable conductivity of the hybrid sample at room temperature, even though there is a large proportion of tin oxide particles, as is also evident from the FESEM image. Here the response of the hybrid sample is predominantly due to the SnO2–SnO2 depletion region and the oxide sites presence at the surface of the tin oxide particles, as can be seen in Fig. 12(b). This is also evident from the effective n-type behavior of the hybrid sensing layer. In addition, there is a contribution of potential barrier presence at the RGO–SnO2 hetero junction. Thus, the response of the hybrid sample for ammonia was found to be considerably higher than that observed in intrinsic SnO2 nanoparticles and RGO.
SnO2) hybrid sample. Here RGO, acted primarily as a conducting network, which resulted in a measurable conductivity of the hybrid sample at room temperature, even though there is a large proportion of tin oxide particles, as is also evident from the FESEM image. Here the response of the hybrid sample is predominantly due to the SnO2–SnO2 depletion region and the oxide sites presence at the surface of the tin oxide particles, as can be seen in Fig. 12(b). This is also evident from the effective n-type behavior of the hybrid sensing layer. In addition, there is a contribution of potential barrier presence at the RGO–SnO2 hetero junction. Thus, the response of the hybrid sample for ammonia was found to be considerably higher than that observed in intrinsic SnO2 nanoparticles and RGO.
The sensing phenomena of all the n-type behaving samples (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 10
4, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and hydrothermal) is similar to that of 10
1 and hydrothermal) is similar to that of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 samples as explained above. The increase in sensitivity towards ammonia with the amount of SnO2 is due to an increase in SnO2–SnO2 homojunctions in the sensing layers.
8 samples as explained above. The increase in sensitivity towards ammonia with the amount of SnO2 is due to an increase in SnO2–SnO2 homojunctions in the sensing layers.
4. Conclusions
RGO–SnO2 hybrid ammonia sensors were fabricated. The sensing layers were synthesized by varying the concentration of SnO2 in RGO and their performances as ammonia detectors at room temperature were observed. Not only an enhanced response over intrinsic RGO and SnO2 was achieved, but also the sensors recovered faster with high selectivity towards ammonia. The sensing performance of in situ hydrothermally synthesized RGO–SnO2 hybrid sample was found to be poorer than that of the samples prepared by mixing SnO2 nanoparticles and GO (synthesized separately). Moreover, the hybrid sensor could sense analytes at room temperature, thereby reducing the power consumption of the sensors.Acknowledgements
S. Santra acknowledges Department of Science and Technology (DST), India for supporting the work partially (project no SR/S2/RJN-104/2011). D. Pradhan and P. K. Guha acknowledge SRIC, IIT Kharagpur for the ISIRD grant to partially support this work. The authors acknowledge DST-FIST Lab (in Department of Physics, IIT Kharagpur) and Central Research Facility, IIT Kharagpur to let us use the XPS facility and FESEM, HRTEM and XRD respectively.References
- B. Timmer, W. Olthuis and A. van den Berg, Sens. Actuators, B, 2005, 107, 666–677 CrossRef CAS PubMed.
- M. A. M. Smeets, P. J. Bulsing, S. van Rooden, R. Steinmann, J. A. de Ru, N. W. M. Ogink, C. van Thriel and P. H. Dalton, Chem. Senses, 2007, 32, 11–20 CrossRef CAS PubMed.
- Y. Zhou, K. B. Zheng, J. D. Grunwaldt, T. Fox, L. L. Gu, X. L. Mo, G. R. Chen and G. R. Patzke, J. Phys. Chem. C, 2011, 115, 1134–1142 CAS.
- W. Tan, Q. Yu, X. Ruan and X. Huang, Sens. Actuators, B, 2015, 212, 47–54 CrossRef CAS PubMed.
- H. Wang, Y. Qu, H. Chen, Z. Lin and K. Dai, Sens. Actuators, B, 2014, 201, 153–159 CrossRef CAS PubMed.
- N. D. Chinh, N. Van Toan, V. Van Quang, N. Van Duy, N. D. Hoa and N. Van Hieu, Sens. Actuators, B, 2014, 201, 7–12 CrossRef CAS PubMed.
- C. A. Betty, S. Choudhury and K. G. Girija, Sens. Actuators, B, 2014, 193, 484–491 CrossRef CAS PubMed.
- N. Barsan, D. Koziej and U. Weimar, Sens. Actuators, B, 2007, 121, 18–35 CrossRef CAS PubMed.
- C. Pijolat, B. Riviere, M. Kamionka, J. P. Viricelle and P. Breuil, J. Mater. Sci., 2003, 38, 4333–4346 CrossRef CAS.
- F. Schedin, A. K. Geim, S. V. Morozov, E. W. Hill, P. Blake, M. I. Katsnelson and K. S. Novoselov, Nat. Mater., 2007, 6, 652–655 CrossRef CAS PubMed.
- N. Krane, Selected topics in physics: physics of nanoscale carbon, 2011 Search PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- G. Lu, S. Park, K. Yu, R. S. Ruoff, L. E. Ocola, D. Rosenmann and J. Chen, ACS Nano, 2011, 5, 1154–1164 CrossRef CAS PubMed.
- R. Ghosh, A. Midya, S. Santra, S. K. Ray and P. K. Guha, ACS Appl. Mater. Interfaces, 2013, 5, 7599–7603 CAS.
- S. Bazargan, N. F. Heinig, D. Pradhan and K. T. Leung, Cryst. Growth Des., 2011, 11, 247–255 CAS.
- X. Gao and X. Tang, Carbon, 2014, 76, 133–140 CrossRef CAS PubMed.
- R. Ghosh, A. Singh, S. Santra, S. K. Ray, A. Chandra and P. K. Guha, Sens. Actuators, B, 2014, 205, 67–73 CrossRef CAS PubMed.
- K. Wetchakun, T. Samerjai, N. Tamaekong, C. Liewhiran, C. Siriwong, V. Kruefu, A. Wisitsoraat, A. Tuantranont and S. Phanichphant, Sens. Actuators, B, 2011, 160, 580–591 CrossRef CAS PubMed.
- H. Zhang, J. Feng, T. Fei, S. Liu and T. Zhang, Sens. Actuators, B, 2014, 190, 472–478 CrossRef CAS PubMed.
- G. Neri, S. G. Leonardi, M. Latino, N. Donato, S. Baek, D. E. Conte, P. A. Russo and N. Pinna, Sens. Actuators, B, 2013, 179, 61–68 CrossRef CAS PubMed.
- L. Yin, D. Chen, X. Cui, L. Ge, J. Yang, L. Yu, B. Zhang, R. Zhang and G. Shao, Nanoscale, 2014, 6, 13690–13700 RSC.
- S. J. Choi, B. H. Jang, S. J. Lee, B. K. Min, A. Rothschild and D. Kim, ACS Appl. Mater. Interfaces, 2014, 6, 2588–2597 CAS.
- D. Zhang, A. Liu, H. Chang and B. Xia, RSC Adv., 2015, 5, 3016–3022 RSC.
- B. Ghaddab, J. B. Sanchez, C. Mavon, M. Paillet, R. Parret, A. A. Zahab, J.-L. Bantignies, V. Flaud, E. Beche and F. Berger, Sens. Actuators, B, 2012, 170, 67–74 CrossRef CAS PubMed.
- Z. Zhang, R. Zou, G. Song, L. Yu, Z. Chen and J. Hu, J. Mater. Chem. C, 2011, 21, 17360–17365 RSC.
- Q. Lin, Y. Li and M. Yang, Sens. Actuators, B, 2012, 173, 139–146 CrossRef CAS PubMed.
- S. Mao, S. Cui, G. Lu, K. Yu, Z. Wen and J. Chen, J. Mater. Chem. C, 2012, 22, 11009–11013 RSC.
- Y. Cao, Y. Li, D. Jia and J. Xie, RSC Adv., 2014, 4, 46179–46186 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra06696d |
| This journal is © The Royal Society of Chemistry 2015 |