Unzipped carbon nanotubes: analytical and binding applications of semisynthetic phlebotropic flavonoid, diosmin†
Abstract
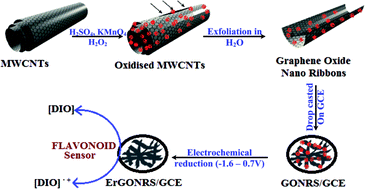
The present study describes the utility of unzipped carbon nanotubes as electrochemical sensing platforms for the determination of diosmin (DIO) in pharmaceutical formulations and for the investigation of its binding to the drug transport protein, human serum albumin (HSA). Graphene oxide nanoribbons (GONRs) were prepared by unzipping of multiwalled carbon nanotubes and characterized by employing powder XRD, FTIR, absorption, Raman, AFM, SEM and electrochemical impedance spectroscopic methods. The suspension of GONRs was drop cast onto a glassy carbon electrode (GCE) and then was subjected to electrochemical reduction (Er) in the potential range of 0.8 to −1.6 V to obtain ErGONRs/GCE. DIO showed a redox peak (Epa = 0.587 V and Epc = 0.548 V) and an irreversible oxidation peak at 0.845 V on ErGONRs/GCE in a phosphate buffer of pH 3. DIO exhibited an enhanced electrochemical response (∼39-fold increment in the peak current) at ErGONRs/GCE when compared to that at bare GCE. Linearity between the peak current and concentration of DIO was noticed in the range of 51.01–39.21 μM and 25–3.48 μM for differential pulse and square wave voltammetric methods, respectively. The practical utility of the proposed sensor was established by determining DIO in pharmaceutical formulations. Furthermore, the sensor was used for understanding the binding mechanism of the DIO–HSA system. The binding constant and binding ratio between HSA and DIO were calculated to be 2.58 × 104 M−1 and 1 : 1, respectively.

 Please wait while we load your content...
Please wait while we load your content...