DOI:
10.1039/C5RA07495A
(Paper)
RSC Adv., 2015,
5, 51549-51558
A water-soluble antimicrobial acrylamide copolymer containing sulfitobetaine for enhanced oil recovery†
Received
24th April 2015
, Accepted 21st May 2015
First published on 22nd May 2015
Abstract
Herein, we report a novel acrylamide copolymer with antimicrobial property as an enhanced oil recovery chemical. The copolymer was synthesized from acrylamide (AM), acrylic acid (AA) and 2-((2-(acryloyloxy)ethyl)dimethylammonio)ethyl sulfite (ADMES) using oxidation–reduction initiation system. Subsequently, the copolymer AM/AA/ADMES was evaluated and characterized on several aspects such as IR, 1H NMR, intrinsic viscosity, and dissolubility. The AM/AA/ADMES solution exerted remarkable thickening ability, salt tolerance ability and viscoelasticity. In addition, the rheological properties, temperature resistance ability and long-term stability of AM/AA/ADMES were investigated systematically in the presence of sulfate-reducing bacteria and relatively low viscosity loss could be obtained compared to partially hydrolyzed polyacrylamide. On the basis of core flooding experiments, AM/AA/ADMES was found to be a valuable prospect with 10.5 resistance factor, 4.6 residual resistance factor and up to 11.0% enhanced oil recovery.
Introduction
Currently, enhanced oil recovery (EOR) technology by means of polymer flooding has been attracting increasing attention in many countries.1–3 In addition, the oil field experiments applying polymer flooding to enhance crude oil recovery in the main oil regions of many countries, such as Daqing oilfield (China), North Burbank oilfield (America) and Sanand oilfield (India), have reported some achievements.4–7 Nevertheless, new problems arise with the use of polymers in high-temperature and high-salinity reservoirs.8 Hydrolysis, degradation, and the coil of molecular chains in brine of commonly used polymers, such as partially hydrolyzed polyacrylamide (HPAM), result in reducing oil recovery efficiency.8,9 Therefore, many different water-soluble polymers have been synthesized and studied, which contributes significantly to providing a theoretical foundation for the application of these polymers utilized in EOR.10
Most research in water-soluble polymers for EOR has focused on water-soluble polymers containing hydrophobic,11,12 rigid ring,13,14 and zwitterionic groups. The introduction of these special functional groups benefits the improvement of the performances on thickening ability, temperature resistance, salt tolerance and anti-shear property.8 Recently, polymers containing zwitterionic groups have been studied most extensively owing to their special solution properties, outstanding antimicrobial properties, as well as providing the capability of further function.15–19 Polyampholytes that were synthesized via conventional free radical polymerization were first reported in 1950s.20–23 Later, McCormick et al. demonstrated that polyampholytes displayed a higher intrinsic viscosity than HPAM at higher ionic strength, which was explained by the probable result of the inherent bulkiness of the polymer unit that restricted the rotational freedom of the polymer chain.24,25 Therefore, polyampholytes are used for many technology processes, such as EOR, papermaking, water treatment, biomedical technology, and so on.26–29
Polymers containing zwitterionic groups, such as sulfobetaine, phosphobetaine and carboxybetaine, have been the subject of considerable interest and extensive research in resisting bacterial growth in aqueous solution as well as on the surface.30–33 As we know, polymers for enhanced oil recovery were dissolved in oilfield water where a large group of sulfate-reducing bacteria (SRB) grow. SRB are a large group of anaerobic microorganisms that obtain their energy for growth using sulfate as the terminal electron acceptor.34–37 SRB have been recognized as the most important contributors to microbiological induced corrosion (MIC) leading to severe industrial problems.38,39 For example, SRB can cause pipeline corrosion and oil well perforation, promote the growth and reproduction of other types of bacteria, and produce large amount of sticky mud blocking pipeline.40 In addition, the presence of SRB could cause the degradation of HPAM, which has a significant influence on the recovery efficiency. Currently, a bactericide commonly used is a non-oxidizing bactericide dosing, dodecyl dimethyl benzyl ammonium chloride (1227), but resistance arises after long-term use. Ma and Guan et al. synthesized a bactericide containing sulfitobetaine groups, which exhibited excellent bactericidal efficiency.41,42
On the basis of these findings and research, the development of antimicrobial polymers is critical for polymer flooding. In this paper, a novel antimicrobial sulfitobetaine-based acrylamide copolymer AM/AA/ADMES was synthesized by the copolymerization of acrylamide (AM), acrylic acid (AA), and 2-((2-(acryloyloxy)ethyl) dimethylammonio)ethyl sulfite (ADMES) as an EOR chemical. The thickening property and salt tolerance ability of AM/AA/ADMES were investigated in deionized water. In addition, the loss in apparent viscosity of AM/AA/ADMES and HPAM was studied in the presence of SRB under different conditions, such as varying copolymers concentration, different pH, changing temperature, and predetermined testing time. To obtain the rheological properties of the copolymers, shear resistance ability and viscoelasticity were researched in the presence of SRB, which provides a theoretical perspective for the EOR ability of the copolymers. The mobility control ability and EOR ability of AM/AA/ADMES were investigated by core flooding experiments, and the copolymer AM/AA/ADMES was expected to have remarkable ability to enhance oil recovery in high-temperature and high-salinity reservoirs.
Experimental
Materials
Acrylamide (AM), acrylic acid (AA), NaHSO3, (NH4)2S2O8, NaOH, NaCl, MgCl2·6H2O, CaCl2, thionyl chloride (SOCl2), ethylene glycol, ethanol and 1,4-dioxane were purchased from Chengdu Kelong Chemical Reagent Company (Sichuan, China). 2-(Dimethylamino)ethyl acrylate (DMAEA) was purchased from Shanghai Jingchun Scientifical Co., Ltd. The viscosity–average molecular weight of partially hydrolyzed polyacrylamide (HPAM, Daqing Refining and Chemical Company) was 8.0 × 106. Ethylene sulfite (ES) was synthesized according to the reported method.43
Synthesis of compound ADMES
2-((2-(Acryloyloxy)ethyl)dimethylammonio)ethyl sulfite (ADMES) was synthesized by referring to the reported methods, as shown in Scheme 1a.43 Briefly, DMAEA was dissolved in 1,4-dioxane and excess ES was added slowly at 90–105 °C. The reaction of DMAEA and ES was carried out under reflux for 2 h and TLC analysis using petroleum ether–ethyl acetate mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) confirmed the product purity. After the reaction was complete, the reaction product was allowed to cool to room temperature and concentrated on a rotary evaporator. The final product was purified further by a recrystallisation method with acetonitrile. The white powdery solid ADMES were obtained. ADMES: 1H NMR (400 MHz, D2O, δ): 3.16 (m, 10H, –C(O)OCH2CH2N+(CH3)CH2–), 3.54 (t, J = 5.0 Hz, 2H, –C(O)OCH2CH2–), 4.01 (t, J = 2.4 Hz, 10H, –CH2CH2–OSO2−), 4.54 (t, J = 2.3 Hz, 2H, –C(O)OCH2CH2–), 5.71 (m, 1H, H(H)C
3 v/v) confirmed the product purity. After the reaction was complete, the reaction product was allowed to cool to room temperature and concentrated on a rotary evaporator. The final product was purified further by a recrystallisation method with acetonitrile. The white powdery solid ADMES were obtained. ADMES: 1H NMR (400 MHz, D2O, δ): 3.16 (m, 10H, –C(O)OCH2CH2N+(CH3)CH2–), 3.54 (t, J = 5.0 Hz, 2H, –C(O)OCH2CH2–), 4.01 (t, J = 2.4 Hz, 10H, –CH2CH2–OSO2−), 4.54 (t, J = 2.3 Hz, 2H, –C(O)OCH2CH2–), 5.71 (m, 1H, H(H)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 5.96 (m, 1H, CH2
CH–), 5.96 (m, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 6.37 (m, 1H, H(H)C
CH–), 6.37 (m, 1H, H(H)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–). 13C NMR (100 MHz, D2O, δ): 52.07, 55.37, 58.56, 63.32, 66.21, 127.38, 131.94, 172.60.
CH–). 13C NMR (100 MHz, D2O, δ): 52.07, 55.37, 58.56, 63.32, 66.21, 127.38, 131.94, 172.60.
 |
| | Scheme 1 The synthesis routes of ADMES (a) and AM/AA/ADMES (b). | |
Synthesis of AM/AA/ADMES
AM/AA/ADMES was synthesized via aqueous solution copolymerization, and the synthesis route is shown in Scheme 1b. A certain amount of AM, AA and ADMES was dissolved in deionized water in a 100 mL three neck round-bottom flask under a nitrogen atmosphere. The pH of the reaction was adjusted by adding NaOH and the solution was kept in a water bath heater at a constant temperature. The indicated loading of the initiator [n((NH4)2S2O8)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(NaHSO3) = 1
n(NaHSO3) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] was added slowly to the copolymerization system. After that, copolymerization was carried out at indicated temperature under a nitrogen atmosphere for 8–10 h. The final product was precipitated with ethanol and dried overnight at 45 °C in a vacuum oven to obtain the corresponding copolymer, AM/AA/ADMES. The copolymerization conditions are presented in Table 1 and the optimal copolymerization conditions are provide in ESI.†
1] was added slowly to the copolymerization system. After that, copolymerization was carried out at indicated temperature under a nitrogen atmosphere for 8–10 h. The final product was precipitated with ethanol and dried overnight at 45 °C in a vacuum oven to obtain the corresponding copolymer, AM/AA/ADMES. The copolymerization conditions are presented in Table 1 and the optimal copolymerization conditions are provide in ESI.†
Table 1 Copolymerization conditions of AM/AA/ADMES
| Copolymer |
pH |
Initiator (wt%) |
Temperature (°C) |
Concentration (wt%) |
Monomer (wt%) |
| AM |
AA |
ADMES |
| AM/AA/ADMES |
8 |
0.2 |
35 |
20 |
50 |
48 |
2 |
Characterization
IR. The IR spectra of the samples was measured with KBr pellets using a WQF-520A IR Spectrophotometer (Beijing Rayleigh Analytical Instrument Company) in the optical range, 4500–400 cm−1, by averaging 32 scans at a resolution of 4 cm−1.
1HNMR. The 1H NMR spectra of the monomer ADMES and copolymer AM/AA/ADMES were recorded on a BRUKER AM 400 MHz Nuclear Magnetic Resonance Spectrometer (Bruker Co., Switzerland) in D2O.
Intrinsic viscosity measurement. All solutions were prepared by dissolving a certain amount of copolymer in brine (1 mol L−1 NaCl), and the polymer concentration (C = 1.0, 0.67, 0.50, 0.33 and 0.25 mg L−1) was adjusted by adding a salt solution to the flask in a constant temperature bath at 30 °C. The specific viscosity (ηsp) was calculated using the following eqn (1). The [η] of AM/AA/ADMES was the y-axis intercept of the ηsp/C versus C relationship line according to eqn (2).| |
 | (1) |
| |
 | (2) |
where ηsp is the specific viscosity of the polymer; t0 is flux time of 1 mol L−1 NaCl brine, s; and t is flux time of the polymer brine solution, s.Fig. S1 (see ESI†) shows that the intrinsic viscosity ([η]) of the copolymer, AM/AA/ADMES, was 1162 mL g−1.
Cultivation of SRB
Seed SRB bacteria were isolated from oilfield injection water collected from the Tuha oil field in China. The culture was prepared with 4.0 mL sodium lactate, 1.0 g yeast extract, 0.5 g K2HPO4 in 1.0 L simulated formation water with the total dissolved solid (TDS) of 9753 mg L−1 (Table 2). The culture solution was autoclaved at 121 °C for 30 min prior to use. SRB were cultivated in ready-prepared cultures on a shaking platform at 120 rpm for 7 days at 30 °C. SRB were counted using the most probable number (MPN) method.
Table 2 Ions composition of TDS
| Inorganic ions |
Na+ |
Mg2+ |
Ca2+ |
SO42− |
HCO3− |
CO32− |
Cl− |
TDS |
| Content (mg L−1) |
3452 |
165 |
260 |
69 |
305 |
15 |
5487 |
9753 |
Antimicrobial activity test
The monomer ADMES and 1227 (40 mg L−1) were added into the medium containing SRB, respectively. The medium solution was incubated for 24 h at 30 °C. The medium without fungicide (blank sample) was placed at less than 5 °C for 24 h. SRB was then counted by the MPN method. The antibacterial rate (AR) was calculated using the following eqn (3).| | |
AR = (1 − N1/N0) × 100%
| (3) |
where N1 is the colony forming units at 30 °C; and N0 is the colony forming units at less than 5 °C.
Water solubility test
The dissolution behaviour of the copolymer (16–20 meshes) was studied by measuring the conductivity of the polymer solution at different times using a DDS-11A conductivity meter (Shanghai Rex Xinjing instruments Co. Ltd, China). The relationship curve between electrical conductivity and the dissolution time of the polymer solution was measured at 30 °C to study the water solubility of the copolymer.
Thickening ability and salt tolerance test
The thickening ability of the copolymer was explored by measuring the apparent viscosity at different concentrations of polymers solution, and the salt tolerance of 2000 mg L−1 copolymer solution was investigated by NaCl, CaCl2 and MgCl2 with different concentrations. The apparent viscosity of the polymers solution was measured at 30 °C using a Brookfield DV-III Programmable Rheometer (Brookfield Co., America) with a 00# (6 rpm) or 62# (18.8 rpm) rotor.
Concentration test
To examine the effects of the copolymer concentration, SRB were seeded into a 100 mL culture solution with different amounts of copolymer. The culture (3.0 × 103 CFU mL−1 SRB) was incubated for 7 days at 30 °C. Then the apparent viscosity of the culture solution with different concentrations of copolymer was measured at 30 °C using a Brookfield DV-III Programmable Rheometer (Brookfield Co., America) with a 00# (6 rpm) or 62# (18.8 rpm) rotor.
pH test
To assess the effect of pH, the cultures with 2000 mg L−1 copolymer and SRB (3.0 × 103 CFU mL−1) at different pH were cultivated at 30 °C for 7 days. The culture solution was measured at 30 °C to obtain the apparent viscosity.
Temperature test
To evaluate the effect of SRB at different temperature on the apparent viscosity, the cultures with 2000 mg L−1 copolymer and SRB (3.0 × 103 CFU mL−1) were incubated at different temperatures for 7 days, and the apparent viscosity of the copolymer was then determined using a HAAKE MARS III Rheometer (Haake Technik Co., Germany) at a shear rate of 7.34 s−1.
Rheology experiments and viscoelasticity
SRB (3.0 × 103 CFU mL−1) in the culture solution with 2000 mg L−1 copolymer was incubated at 30 °C for 7 days. The apparent viscosity was measured using a HAAKE MARS III Rheometer (Haake Technik Co., Germany) in the range of shear rates, 7.34 to 1000 s−1, at 30 °C. The elastic modulus (G′) and viscous modulus (G′′) were determined using HAAKE MARS III Rheometer to obtain the viscoelasticity property of the copolymer.
Long-term stability
SRB (3.0 × 103 CFU mL−1) in the culture with 2000 mg L−1 copolymer were cultivated at 60 °C under sealed conditions for 30 days to obtain the long-term stability behavior. The apparent viscosity of the copolymer was measured for different times using Brookfield DV-III Programmable Rheometer (Brookfield Co., America) with a 00#(6 rpm) or 62# (18.8 rpm) rotor.
Mobility control ability test
The mobility control ability of the polymer solutions was characterized by the resistance factor (RF) and the residual resistance factor (RRF).44 The core used in the core flooding tests was stainless steel (500 mm in length and 25 mm in inner diameter) and packed with quartz sand (80–100 mesh, porosity: 22.8%, penetration: 820 × 10−3 μm2). The sand was washed with an 18% hydrochloric acid solution and then with a massive amount of water until the pH reached 7. The copolymer solution was dissolved in simulated formation water with TDS of 9753 mg L−1 (Table 2). The brine and polymers solutions prepared with the brine were injected at 2.0 mL min−1 using a ISCO 260D syringe pump and the injection pressure was recorded by a pressure sensor. The experiments were carried out at 85 °C. The RF was defined by eqn (4), and the RRF was calculated using eqn (5):| |
 | (4) |
| |
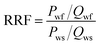 | (5) |
where Pp is stable pressure when injecting polymer solution, MPa; Qp is injecting rate when injecting polymer solution, mL min−1; Pws is stable pressure when saturating water, MPa; and Qws is the injecting rate when saturating water, mL min−1; Pwf is stable pressure when water flooding, MPa; and Qwf is the injecting rate when water flooding, mL min−1.
Enhanced oil recovery test
The EOR ability of these polymers solutions (2000 mg L−1) prepared with simulated formation water (Table 2) was studied. Each polymer flooding test was preceded by water flooding and followed by subsequent water flooding at 85 °C. The water flooding was conducted with brine at 3 mL min−1 until the water content exceeded 98%. The polymer flooding and subsequent water flooding were conducted at 1 mL min−1. The value for enhanced oil recovery (EOR) was determined using the following eqn (6):where H1 is the oil recovery of polymer flooding and H2 is the oil recovery of water flooding.
Results and discussion
IR
IR spectra of ADMES and AM/AA/ADMES are shown in Fig. 1a. The IR spectra of ADMES exhibited the following characteristic peaks. The peaks at 2969 cm−1 and 2887 cm−1 were due to the stretching vibration of C–H in the methyl and methylene groups. The peak at 1733 cm−1 was assigned to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The characteristic absorption peak at 1651 cm−1 was the result of the stretching vibration of C
O. The characteristic absorption peak at 1651 cm−1 was the result of the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C. In addition, the peaks at 1479 cm−1 and 1399 cm−1 were the bending vibration peaks of C–H. The characteristic peaks at 1180 cm−1 and 1039 cm−1 indicated the presence of S
C. In addition, the peaks at 1479 cm−1 and 1399 cm−1 were the bending vibration peaks of C–H. The characteristic peaks at 1180 cm−1 and 1039 cm−1 indicated the presence of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The IR spectra of AM/AA/ADMES showed the expected characteristic peaks. The peak at 3413 cm−1 was attributed to the stretching vibration peak of N–H. The 2923 cm−1 and 2849 cm−1 peaks were assigned to the stretching vibration of C–H of methyl and methylene, respectively. The peak at 1660 cm−1 was the characteristic peaks of C
O. The IR spectra of AM/AA/ADMES showed the expected characteristic peaks. The peak at 3413 cm−1 was attributed to the stretching vibration peak of N–H. The 2923 cm−1 and 2849 cm−1 peaks were assigned to the stretching vibration of C–H of methyl and methylene, respectively. The peak at 1660 cm−1 was the characteristic peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The characteristic absorption peaks at 1176 cm−1 and 1079 cm−1 corresponded to the stretching vibration of S
O. The characteristic absorption peaks at 1176 cm−1 and 1079 cm−1 corresponded to the stretching vibration of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. As expected, the IR spectra indicated the successful copolymerization of AM, AA and ADMES.
O. As expected, the IR spectra indicated the successful copolymerization of AM, AA and ADMES.
 |
| | Fig. 1 (a) IR spectra of ADMES and AM/AA/ADMES, (b) 1H NMR spectra of AM/AA/ADMES. | |
1H NMR
The 1H NMR spectra of AM/AA/ADMES are displayed in Fig. 1b. In the 1H NMR spectra of the copolymer AM/AA/ADMES, the chemical shift at 1.55–1.65 ppm was assigned to the signal of protons of –CH2– groups in the polymeric main chain. The signal of protons at 2.01–2.23 ppm was due to –CH– groups in the polymeric main chain. In the expanded region, The peak at 3.14–3.18 ppm was related to the signal of protons of –CH2–N+(CH3)2–CH2–OSO2− groups of the ADMES functional monomer in the copolymer. The relevant signal of protons with –N+(CH3)2CH2–CH2–OSO2− groups in the copolymer appeared at 3.26 ppm. The signal at 3.68–3.70 ppm indicated the existence of –COO–CH2–CH2–N+(CH3)2CH2– groups. The chemical shift of protons at 3.80 ppm was due to –CH2–OSO2− groups of the ADMES functional monomer in the copolymer. The signal of protons at 4.01–4.02 ppm showed the presence of –COO–CH2– groups. Therefore, the structure of the copolymer AM/AA/ADMES was consistent with the original design by 1HNMR analysis.
Antimicrobial activity of monomer ADMES
The antimicrobial activity results were shown in Table 3. The antibacterial rate of ADMES (92.5%) was lower than that of 1227 (99.9%). The result showed that ADMES exerted obvious antimicrobial activity. The introduction of ADMES could contribute to resistance of degradation of the copolymer by SRB, which was due to that the adhesion of cationic charge groups to the microbe cell resulted in the death of microbes.45
Table 3 Antimicrobial activity of the monomer ADMES
| Compounda |
Colony forming unitsb (CFU mL−1) |
Antibacterial rate (%) |
| Cultivation conditions: reagent dosages = 40 mg L−1, time = 24 h. Colony forming units was measured after 24 h. |
| Blank sample |
1.0 × 103 |
— |
| ADMES |
7.5 × 101 |
92.5 |
| 1227 |
0.4 × 100 |
99.9 |
Water solubility
The dissolution behaviour of the polymer was studied using conductometric technique. The polymer solution possessed certain power of conduct electricity when polymer containing ion groups was dissolved in deionized water.46 The results were illustrated in Fig. 2a. The conductivity of these polymer solutions was small in the early stage, and then with time increasing, the conductivity of the polymer increased due to the increasing of the dissolved quantity. Finally the conductivity tended to be constant and the conductivity of the polymer AM/AA/ADMES and HPAM when they were dissolved completely in deionized water was 0.317 mS and 0.285 mS, respectively. The dissolving time of AM/AA/ADMES was 32 min, which was shorter than that of HPAM (50 min).
 |
| | Fig. 2 (a) Dissolution time of AM/AA/ADMES and HPAM, (b) thickening ability of AM/AA/ADMES and HPAM. | |
Thickening ability
To investigate the relationship between the apparent viscosity and concentration, the apparent viscosity as a function of the concentration in deionized water was determined for the polymers AM/AA/ADMES and HPAM, as shown in Fig. 2b. For AM/AA/ADMES, as the concentration increased, a dramatic increase in the apparent viscosity was observed. The apparent viscosity of 546.5 mPa s could have been obtained under the concentration of an AM/AA/ADMES solution with 2000 mg L−1, while the apparent viscosity of 2000 mg L−1 HPAM solution was 320.2 mPa s. The electrostatic attraction between the different polymer molecular chains could contribute to the apparent viscosity of AM/AA/ADMES being higher than that of HPAM. The copolymer AM/AA/ADMES showed better thickening ability compared to HPAM.
Salt resistance
The influence of salt on the copolymer AM/AA/ADMES and HPAM solution (2000 mg L−1) was measured under different salt concentrations. The change trend of the apparent viscosity to salt concentration is shown in Fig. 3. It was obvious that the apparent viscosity of the copolymer solution decreased with increasing salt concentration. At the first stage, a large decrease in the apparent viscosity was observed. The influence, reduction of intramolecular electrostatic repulsion with the addition of salt, resulted in the weakening of shrinkage degree of the polymer chain. The addition of small molecular electrolyte can weaken intra-anionic electrostatic repulsion, which led to the coil of the polymer molecular chain. As the salt concentration was increased further, a slight decrease in apparent solution viscosity was observed. For the copolymer AM/AA/ADMES, a viscosity retention rate of 25.3% (Fig. 3a), 20.9% and 18.4% (Fig. 3b) was obtained at a concentration of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1 NaCl, 2000 mg L−1 CaCl2 and 2000 mg L−1 MgCl2, respectively. Compared to HPAM, AM/AA/ADMES possessed better salt resistance, which might be because the interaction between zwitterionic groups and salts could weaken the influence of salts on shrinkage degree of the copolymer chain.
000 mg L−1 NaCl, 2000 mg L−1 CaCl2 and 2000 mg L−1 MgCl2, respectively. Compared to HPAM, AM/AA/ADMES possessed better salt resistance, which might be because the interaction between zwitterionic groups and salts could weaken the influence of salts on shrinkage degree of the copolymer chain.
 |
| | Fig. 3 Salt resistance of AM/AA/ADMES and HPAM, (a) effect of NaCl, (b) effect of CaCl2 and MgCl2. | |
Effect of concentration and SRB
The effect of SRB on the apparent viscosity of the copolymer solution with different concentration solutions was also investigated. When the copolymer solution was seeded by SRB, the apparent viscosity of the copolymer solution displayed a declining trend. SRB could use the copolymer as the sole carbon source to grow, which promoted copolymer degradation leading to a decreasing viscosity.47 As shown in Fig. 4a, the apparent viscosity of HPAM decreased obviously at concentrations from 300 to 1000 mg L−1 because the SRB could grow under these concentrations. The decreased viscosity loss was attributed to the reduced growth of SRB with increasing concentration.48 The obtained maximum loss in apparent viscosity was up to 30.9% at 500 mg L−1. On the contrary, the changes in apparent viscosity of the AM/AA/ADMES solution were extremely small. The only the viscosity loss of 13.2% was obtained under the concentration of 400 mg L−1 and the AM/AA/ADMES solution with 2000 mg L−1 had little loss of the apparent viscosity. The extraordinary antibacterial property was attributed to the presence of the betaine groups in the copolymer.30
 |
| | Fig. 4 (a) Effect of the concentration and SRB on the apparent viscosity of AM/AA/ADMES and HPAM solution, (b) effect of pH and SRB on the apparent viscosity of the AM/AA/ADMES and HPAM solution. | |
Effect of pH and SRB
The effect of pH on the apparent viscosity of 2000 mg L−1 copolymer solution was studied to obtain the results shown in Fig. 4b. HPAM and AM/AA/ADMES were insoluble under the condition of the relatively low pH due to the uncharged nature.49 As the pH changed to 5 from 8, the degree of ionization of the carboxylic acid in the polymer was reduced. The extension of the polymer chain was limited, which caused the low apparent viscosity. With further increases in pH, a sharp decrease was observed in the apparent viscosity of the two polymers. At relatively high pH, intermolecular repulsion led to a decrease in apparent viscosity with increasing ionic strength.
As shown in Fig. 4b, the loss of the apparent viscosity was relatively lower under acidic or alkaline conditions because the copolymer solutions at pH of 7 and 8 were suitable for the growth of SRB. Nevertheless, AM/AA/ADMES solution at pH of 7 had little loss of the apparent viscosity, which was attributed to the excellent antibacterial ability of AM/AA/ADMES.30
Effect of temperature and SRB
The effect of temperature on the apparent viscosity of AM/AA/ADMES and HPAM solutions was investigated from 20 to 120 °C at 7.34 s−1, and the results are shown in Fig. 5. It was clear that the apparent viscosity of the copolymer decreased marginally under a temperature of 20 to 100 °C and decreased significantly within the range of 100 to 120 °C. The obtained viscosity retention rate of AM/AA/ADMES was up to 27.3% at 120 °C, which was much better than that of HPAM (retention rate 9.5%).
 |
| | Fig. 5 Effect of temperature and SRB on apparent viscosity of AM/AA/ADMES and HPAM. | |
An investigation of the effect of SRB on the apparent viscosity from 20 to 60 °C is displayed in Fig. 5. The apparent viscosity of two copolymers both decreased compared to the copolymer solution without being seeded with SRB at the same temperature. The HPAM solution displayed relatively high loss of 35.7% in apparent viscosity at 35 °C, while the change in apparent viscosity for AM/AA/ADMES was not obvious. The loss in apparent viscosity reached the maximum, which was only up to 4.7%. The obtained small changes in the apparent viscosity were attributed to the antibacterial property of AM/AA/ADMES.
Effect of shear rate
The shear resistance ability of the polymers was investigated, and the results are shown in Fig. 6. The apparent viscosity of the copolymer solution decreased sharply under a shear rate of 7.3–200 s−1 and slightly reduced under a higher shear rate of 500–1000 s−1, suggesting a slight shear-thinning behavior. Compared to the polymer HPAM (viscosity retention rate: 3.3%), the copolymer AM/AA/ADMES had a better viscosity retention rate (6.7%) at 1000 s−1. These results suggest that the copolymer AM/AA/ADMES possessed a good shear resistance ability.
 |
| | Fig. 6 Effect of the shear rate on the apparent viscosity of the AM/AA/ADMES and HPAM solution. Viscoelasticity curves of the AM/AA/ADMES and HPAM solution. | |
When the SRB were seeded into the copolymers solutions, the loss rate in the apparent viscosity of AM/AA/ADMES solution was relatively low at the same shear rate compared to the HPAM solution. It was obvious that the apparent viscosity of HPAM solution exhibited a sharp reduction as a result of degradation by SRB. A lower loss rate (18.3%) in the apparent viscosity of AM/AA/ADMES was observed compared to HPAM (27.1%) under a shear rate of 200 s−1.
Viscoelasticity
The viscoelasticity behavior of AM/AA/ADMES and HPAM is quite important for EOR, as displayed in Fig. 6. It is obvious that both elastic modulus (G′) and viscous modulus (G′′) of AM/AA/ADMES were higher than that of HPAM. This suggested that the better viscoelasticity for AM/AA/ADMES could be obtained under a concentration of 2000 mg L−1. The frequency of flow in the elasticity domination state was lower than that of HPAM. At a relatively high frequency, G′ of AM/AA/ADMES was obviously larger than G′′, which showed that AM/AA/ADMES had better elasticity. The viscoelasticity of the AM/AA/ADMES solution with being seeded by SRB was visibly superior to that of the HPAM solution containing SRB. Therefore, the increasing elasticity could contribute to improving the microscopic swept efficiency of the displacing fluid, which could be favorable for enhancing oil recovery.
Long-term stability
Long-term stability is also important for a polymer for EOR due to long-term flow of the displacing fluid in the formation. The apparent viscosity was measured at different times after aging at the indicated temperature, as shown in Fig. 7. The viscosity retention rate of 2000 mg L−1 AM/AA/ADMES solution could reach 81.1% after aging for 30 days, while the viscosity retention rate of HPAM was 71.8%. After the solution seeded with SRB was aged, the HPAM solution exerted a larger loss rate in apparent viscosity. The viscosity loss rate increased with each passing day and the loss rate was 36.1% after being aged for 30 days compared to the HPAM solution without being seeded with SRB. This phenomenon might be due to the growth of SRB promoting the degradation of HPAM.47 In contrast, the AM/AA/ADMES solution seeded with SRB displayed a higher retention rate, which was attributed to the excellent antibacterial ability of AM/AA/ADMES. After being aged for 30 days, compared to the solution without being seeded with SRB, the AM/AA/ADMES solution had little loss in apparent viscosity.
 |
| | Fig. 7 The long-term stability of AM/AA/ADMES and HPAM solution. | |
Mobility control ability
The flow characteristic curves of AM/AA/ADMES and HPAM in porous media are shown in Fig. 8a. The stable injection pressure of three stages was recorded in Table 4. Compared to the HPAM (9.38 and 2.95) solution, the AM/AA/ADMES solution could reach a much higher RF (15.30) and RRF (5.37) under the same conditions. The results indicated that the AM/AA/ADMES solution had stronger mobility control ability that was favourable to enhancing oil recovery.
 |
| | Fig. 8 (a) Flow characteristic curves of the HPAM and AM/AA/ADMES solution, (b) EOR ability for AM/AA/ADMES and HPAM. | |
Table 4 RF and RRF of AM/AA/ADMES and HPAM
| Polymer |
Water saturation pressure (10−3 MPa) |
Polymer solution pressure (10−3 MPa) |
Water flooding pressure (10−3 MPa) |
RF |
RRF |
| AM/AA/ADMES |
22.76 |
340.20 |
122.25 |
15.30 |
5.37 |
| HPAM |
24.81 |
232.74 |
73.15 |
9.38 |
2.95 |
EOR ability
The core flooding test results for enhanced oil recovery of the copolymer AM/AA/ADMES and HPAM are illustrated in Fig. 8b. Compared to water flooding, the copolymer AM/AA/ADMES solution could enhance the oil recovery significantly up to 11.0%, which might be explained by the excellent mobility control ability. Therefore, a conclusion could be drawn that the ability of the copolymer AM/AA/ADMES for enhanced oil recovery was better than that of HPAM (5.5%).
Conclusions
In this paper, a novel copolymer AM/AA/ADMES was synthesized as an EOR chemical. The rheological properties and stability of the AM/AA/ADMES solution were investigated. The copolymer AM/AA/ADMES solution under concentration of 2000 mg L−1 could significantly increase apparent viscosity up to 546.5 mPa s. In addition, the copolymer, AM/AA/ADMES, showed excellent antimicrobial ability, temperature resistance, salt tolerance, long-term stability, shear resistance, and viscoelasticity. A higher enhanced recovery efficiency of 11.0% could be obtained compared to HPAM (5.5%). Therefore, AM/AA/ADMES has certain potential applications for enhanced oil recovery. A further investigation of the applications of other similar copolymer for EOR is in progress.
Acknowledgements
This work was supported by the Key Programs of Educational Commission of Sichuan Province of China (Natural Science, contract grant number 15ZA0051), the Scientific Research Starting Project of Southwest Petroleum University (contract grant number 2014PYZ008), the Foundation of Youth Science and Technology Innovation Team of Sichuan Province (contract grant number 2015TD0007).
References
- B. S. Shiran and A. Skauge, Energy Fuels, 2013, 27, 1223 CrossRef.
- S. Lago, H. Rodríguez, M. K. Khoshkbarchi, A. Soto and A. Arce, RSC Adv., 2012, 2, 9392 RSC.
- T. Sochi, J. Polym. Sci., Part B: Polym. Phys., 2010, 48, 2437 CrossRef CAS PubMed.
- R. Tiwari, R. Marathe, N. Patel, K. P. Ramachandran, C. Maurya and P. Tewari, in Society of Petroleum Engineers Revealed, Proceedings of SPE Asia Pacific Oil and Gas Conference, Perth, Australia, 20–22 October 2008 Search PubMed.
- D. M. Wang, R. S. Seright, Z. B. Shao and J. M. Wang, Soc. Pet. Eng. J., 2008, 11, 1117 CAS.
- L. Koottungal, Oil Gas J., 2008, 106, 47 Search PubMed.
- V. Alvarado and E. Manrique, Energies, 2010, 3, 1529 CrossRef PubMed.
- D. A. Z. Wever, F. Picchioni and A. A. Broekhuis, Prog. Polym. Sci., 2011, 36, 1558 CrossRef CAS PubMed.
- A. Sabhapondit, A. Borthakur and I. Haque, J. Appl. Polym. Sci., 2003, 87, 1869 CrossRef CAS PubMed.
- D. K. Han, C. Z. Yang, Z. Q. Zhang, Z. H. Lou and Y. Chang, J. Pet. Sci. Eng., 1999, 22, 181 CrossRef CAS.
- P. Kujawa, A. Audibert-Hayet, J. Selb and F. Candau, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 1640 CrossRef CAS PubMed.
- Q. B. Yang, C. L. Song, Q. Chen, P. P. Zhang and P. X. Wang, J. Polym. Sci., Part B: Polym. Phys., 2008, 46, 2465 CrossRef CAS PubMed.
- B. Wei, L. Romero-Zerón and D. Rodrigue, Adv. Polym. Technol., 2014, 33 DOI:10.1002/adv.21413.
- C. L. Dai, G. Zhao, Q. You and M. W. Zhao, J. Appl. Polym. Sci., 2014, 131 DOI:10.1002/app.39462.
- A. B. Lowe and C. L. McCormick, Chem. Rev., 2002, 102, 4177 CrossRef CAS PubMed.
- Q. Shao and S. Y. Jiang, Adv. Mater., 2014, 27, 15 CrossRef PubMed.
- B. Cao, Q. Tang and G. Cheng, J. Biomater. Sci., Polym. Ed., 2014, 25, 1502 CrossRef CAS PubMed.
- J. de Grooth, W. Ogieglo, W. M. de Vos, M. Gironès, K. Nijmeijer and N. E. Benes, Eur. Polym. J., 2014, 55, 57 CrossRef CAS PubMed.
- Q. Shao, L. Mi, X. Han, T. Bai, S. J. Liu, Y. T. Li and S. Y. Jiang, J. Phys. Chem. B, 2014, 118, 6956 CrossRef CAS PubMed.
- T. Alfrey Jr, H. Morawetz, E. B. Fitzgerald and R. M. Fuoss, J. Am. Chem. Soc., 1950, 72, 1864 CrossRef.
- T. Alfrey Jr, R. M. Fuoss, H. Morawetz and H. Pinner, J. Am. Chem. Soc., 1952, 74, 438 CrossRef.
- T. Alfrey and S. H. Pinner, J. Polym. Sci., 1957, 23, 533 CrossRef CAS PubMed.
- J. Mazur, A. Silberberg and A. Katchalsky, J. Polym. Sci., 1959, 35, 43 CrossRef CAS PubMed.
- E. E. L. Kathmann, D. A. D. Davis and C. L. McCormick, Macromolecules, 1994, 27, 3156 CrossRef CAS.
- E. E. Kathmann and C. L. McCormick, J. Polym. Sci., Part A: Polym. Chem., 1997, 35, 231 CrossRef CAS.
- A. Rabiee, A. Ershad-Langroudi and H. Jamshidi, Rev. Chem. Eng., 2014, 30, 501 CAS.
- K. M. Johnson, M. J. Fevola, R. Y. Lochhead and C. L. McCormick, J. Appl. Polym. Sci., 2004, 92, 658 CrossRef CAS PubMed.
- L. M. Zhang, J. F. Zhou and P. S. Hui, Colloids Surf., A, 2005, 259, 189 CrossRef CAS PubMed.
- M. Gao, K. Gawel and B. T. Stokke, Eur. Polym. J., 2014, 53, 65 CrossRef CAS PubMed.
- M. Charnley, M. Textor and C. Acikgoz, React. Funct. Polym., 2011, 71, 329 CrossRef CAS PubMed.
- R. Lalani and L. Y. Liu, Biomacromolecules, 2012, 13, 1853 CrossRef CAS PubMed.
- S. Chattopadhyay, E. Heine, H. Keul and M. Moeller, Polymers, 2014, 6, 1618 CrossRef CAS PubMed.
- G. Cheng, G. Z. Li, H. Xue, S. F. Chen, J. D. Bryers and S. Y. Jiang, Biomaterials, 2009, 30, 5234 CrossRef CAS PubMed.
- F. Carbonero and H. R. Gaskins, in Encyclopedia of Metagenomics, Springer, 2014, pp. 1–3 Search PubMed.
- P. Qi, D. Zhang and Y. Wan, Talanta, 2014, 118, 333 CrossRef CAS PubMed.
- K. G. Hu, Q. L. Wang, G. Q. Tao, A. H. Wang and D. X. Ding, Procedia Earth Planet. Sci., 2011, 2, 150 CrossRef CAS PubMed.
- A. J. H. Janssen, P. N. L. Lens, A. J. M. Stams, C. M. Plugge, D. Y. Sorokin, G. Muyzer, H. Dijkman, E. Van Zessen, P. Luimes and C. J. Buisman, Sci. Total Environ., 2009, 407, 1333 CrossRef CAS PubMed.
- P. Angell and K. Urbanic, Corros. Sci., 2000, 42, 897 CrossRef CAS.
- D. Enning, H. Venzlaff, J. Garrelfs, H. T. Dinh, V. Meyer, K. Mayrhofer, A. W. Hassel, M. Stratmann and F. Widdel, Environ. Microbiol., 2012, 14, 1772 CrossRef CAS PubMed.
- S. Daumas, Y. Massiani and J. Crousier, Corros. Sci., 1988, 28, 1041 CrossRef CAS.
- J. N. Guan and H. B. Wang, Ind. Water Treat., 2001, 21, 7 CAS.
- B. X. Ji, H. H. Li, X. H. Li and C. L. Tian, Technol. Water Treat., 2008, 8, 34 Search PubMed.
- H. Distler and R. Widder, U. S. Patent 3,652,633, 1972.
- D. A. Z. Wever, F. Picchioni and A. A. Broekhuis, Ind. Eng. Chem. Res., 2013, 52, 16352 CrossRef CAS.
- A. C. Engler, J. P. K. Tan, Z. Y. Ong, D. J. Coady, V. W. Ng, Y. Y. Yang and J. L. Hedrick, Biomacromolecules, 2013, 14, 4331 CrossRef CAS PubMed.
- M. Siddiq, K. C. Tam and R. D. Jenkins, Colloid Polym. Sci., 1999, 277, 1172 CAS.
- X. G. Lu and K. Zhang, Chin. J. Chem., 2008, 26, 770 CrossRef CAS PubMed.
- Q. X. Wen, Z. Q. Chen, Y. Zhao, H. C. Zhang and Y. J. Feng, J. Polym. Environ., 2011, 19, 125 CrossRef CAS PubMed.
- E. Kumacheva, Y. Rharbi, M. A. Winnik, L. Guo, K. C. Tam and R. D. Jenkins, Langmuir, 1997, 13, 182 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Optimum of copolymerization conditions; intrinsic viscosity of copolymer. See DOI: 10.1039/c5ra07495a |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) confirmed the product purity. After the reaction was complete, the reaction product was allowed to cool to room temperature and concentrated on a rotary evaporator. The final product was purified further by a recrystallisation method with acetonitrile. The white powdery solid ADMES were obtained. ADMES: 1H NMR (400 MHz, D2O, δ): 3.16 (m, 10H, –C(O)OCH2CH2N+(CH3)CH2–), 3.54 (t, J = 5.0 Hz, 2H, –C(O)OCH2CH2–), 4.01 (t, J = 2.4 Hz, 10H, –CH2CH2–OSO2−), 4.54 (t, J = 2.3 Hz, 2H, –C(O)OCH2CH2–), 5.71 (m, 1H, H(H)C
3 v/v) confirmed the product purity. After the reaction was complete, the reaction product was allowed to cool to room temperature and concentrated on a rotary evaporator. The final product was purified further by a recrystallisation method with acetonitrile. The white powdery solid ADMES were obtained. ADMES: 1H NMR (400 MHz, D2O, δ): 3.16 (m, 10H, –C(O)OCH2CH2N+(CH3)CH2–), 3.54 (t, J = 5.0 Hz, 2H, –C(O)OCH2CH2–), 4.01 (t, J = 2.4 Hz, 10H, –CH2CH2–OSO2−), 4.54 (t, J = 2.3 Hz, 2H, –C(O)OCH2CH2–), 5.71 (m, 1H, H(H)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 5.96 (m, 1H, CH2
CH–), 5.96 (m, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 6.37 (m, 1H, H(H)C
CH–), 6.37 (m, 1H, H(H)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–). 13C NMR (100 MHz, D2O, δ): 52.07, 55.37, 58.56, 63.32, 66.21, 127.38, 131.94, 172.60.
CH–). 13C NMR (100 MHz, D2O, δ): 52.07, 55.37, 58.56, 63.32, 66.21, 127.38, 131.94, 172.60.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(NaHSO3) = 1
n(NaHSO3) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] was added slowly to the copolymerization system. After that, copolymerization was carried out at indicated temperature under a nitrogen atmosphere for 8–10 h. The final product was precipitated with ethanol and dried overnight at 45 °C in a vacuum oven to obtain the corresponding copolymer, AM/AA/ADMES. The copolymerization conditions are presented in Table 1 and the optimal copolymerization conditions are provide in ESI.†
1] was added slowly to the copolymerization system. After that, copolymerization was carried out at indicated temperature under a nitrogen atmosphere for 8–10 h. The final product was precipitated with ethanol and dried overnight at 45 °C in a vacuum oven to obtain the corresponding copolymer, AM/AA/ADMES. The copolymerization conditions are presented in Table 1 and the optimal copolymerization conditions are provide in ESI.†



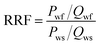
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The characteristic absorption peak at 1651 cm−1 was the result of the stretching vibration of C
O. The characteristic absorption peak at 1651 cm−1 was the result of the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C. In addition, the peaks at 1479 cm−1 and 1399 cm−1 were the bending vibration peaks of C–H. The characteristic peaks at 1180 cm−1 and 1039 cm−1 indicated the presence of S
C. In addition, the peaks at 1479 cm−1 and 1399 cm−1 were the bending vibration peaks of C–H. The characteristic peaks at 1180 cm−1 and 1039 cm−1 indicated the presence of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The IR spectra of AM/AA/ADMES showed the expected characteristic peaks. The peak at 3413 cm−1 was attributed to the stretching vibration peak of N–H. The 2923 cm−1 and 2849 cm−1 peaks were assigned to the stretching vibration of C–H of methyl and methylene, respectively. The peak at 1660 cm−1 was the characteristic peaks of C
O. The IR spectra of AM/AA/ADMES showed the expected characteristic peaks. The peak at 3413 cm−1 was attributed to the stretching vibration peak of N–H. The 2923 cm−1 and 2849 cm−1 peaks were assigned to the stretching vibration of C–H of methyl and methylene, respectively. The peak at 1660 cm−1 was the characteristic peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The characteristic absorption peaks at 1176 cm−1 and 1079 cm−1 corresponded to the stretching vibration of S
O. The characteristic absorption peaks at 1176 cm−1 and 1079 cm−1 corresponded to the stretching vibration of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. As expected, the IR spectra indicated the successful copolymerization of AM, AA and ADMES.
O. As expected, the IR spectra indicated the successful copolymerization of AM, AA and ADMES.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1 NaCl, 2000 mg L−1 CaCl2 and 2000 mg L−1 MgCl2, respectively. Compared to HPAM, AM/AA/ADMES possessed better salt resistance, which might be because the interaction between zwitterionic groups and salts could weaken the influence of salts on shrinkage degree of the copolymer chain.
000 mg L−1 NaCl, 2000 mg L−1 CaCl2 and 2000 mg L−1 MgCl2, respectively. Compared to HPAM, AM/AA/ADMES possessed better salt resistance, which might be because the interaction between zwitterionic groups and salts could weaken the influence of salts on shrinkage degree of the copolymer chain.








