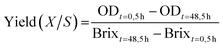Supercritical extraction as an effective first-step in a maize stover biorefinery†
Thomas M. Attarda,
Elke Theeuwesb,
Leonardo D. Gomezc,
Emma Johanssond,
Ioanna Dimitrioue,
Phillip C. Wrighte,
James H. Clarka,
Simon J. McQueen-Masonc and
Andrew J. Hunt*a
aDepartment of Chemistry, The University of York, Heslington, York, YO10 5DD, UK. E-mail: andrew.hunt@york.ac.uk; Fax: +44 (0)1904 432705; Tel: +44 (0)1904 324456
bEcover Co-ordination Center NV, Malle, Belgium
cCNAP, Biology Department, University of York, PO box 374, York, YO10 5DD, UK
dSP Processum AB, SP Technical Research Institute of Sweden Box 70, Örnsköldsvik, SE-891 22, Sweden
eDepartment of Chemical and Biological Engineering, The University of Sheffield, Mappin Street, Sheffield, S1 3JD, UK
First published on 7th May 2015
Abstract
Supercritical carbon dioxide (scCO2) has been investigated for the generation of valuable waxy compounds and as an added-value technology in a holistic maize stover biorefinery. ScCO2 extraction and fractionation was carried out prior to hydrolysis and fermentation of maize stover. Fractionation of the crude extracts by scCO2 resulted in wax extracts having different compositions and melting temperatures, enabling their utilisation in different applications. One such fraction demonstrated significant potential as a renewable defoaming agent in washing machine detergent formulations. Furthermore, scCO2 extraction has been shown to have a positive effect on the downstream processing of the maize stover. Fermentation of the scCO2 extracted maize stover hydrolysates exhibited a higher glucose consumption and greater potential growth for surfactant (in comparison with non-scCO2 treated stover) and ethanol production (a 40% increase in overall ethanol production after scCO2 pre-treatment). This work represents an important development in the extraction of high value components from low value wastes and demonstrates the benefits of using scCO2 extraction as a first-step in biomass processing, including enhancing downstream processing of the biomass for the production of 2nd generation biofuels as part of an integrated holistic biorefinery.
1. Introduction
Ever-increasing world demands are being placed on fossil resources as a feedstock for the manufacture of chemicals and fuels.1 A recent resurgence in the use of renewable feedstocks in holistic integrated biorefineries has aided in reducing the reliance on petroleum-based products.2,3 C4 plants, such as maize, display high photosynthetic activity, high rates of carbon dioxide-fixation, high radiation and high water use efficiencies resulting in rapid growth and excellent productivity.4–8 The abundance of maize stover makes it a promising strategic feedstock for the manufacture of bio-based products as well as for bioenergy.9–11 After the maize grain is harvested, the maize stover is partially incorporated back into the soil, thus leaving a significant amount that can be used as potential feedstock.12A first stage in an integrated maize stover biorefinery could be the extraction of high value waxes from the plant surface prior to the application of destructive technologies.2 A decline in petroleum wax production coupled with the transition to greener based products by consumers has led to an increase in the demand for natural waxes.13,14 Supercritical carbon dioxide (scCO2) has been shown as an effective solvent for the extraction of epicuticular waxes and offers a number of advantages for wax extraction.15,16 The advantage of using scCO2 as a solvent is that the extraction of non-polar compounds can be made selective by fine-tuning the solvent power, which is done by varying the temperature and pressure.17–22 No solvent residue remains when extracting with scCO2 and therefore, unlike conventional extraction methods, the resulting products may be used in food, pharmaceutical and personal care applications. Furthermore, scCO2 is a greener solvent than traditional solvents such as hexane, which is petroleum-based, a hazardous air pollutant (as listed by the US EPA in the Clean Air Act, 1990) and a neurotoxin, having severe adverse effects on the nervous system.23–25 ScCO2 extraction of epicuticular waxes from biomass has been previously reported in literature.3,16,26–29
Herein, the use of scCO2 extraction as a first step in a holistic maize stover biorefinery was investigated whereby the maize stover was subjected to scCO2 extraction prior to hydrolysis and fermentation. The wax extracted from the maize stover was characterised and applications for the compounds found were indicated. Furthermore, the effects of scCO2 extraction on the downstream processing of the maize stover were investigated by comparing the scCO2 extracted maize stover to non-scCO2 treated maize stover.
2. Experimental
2.1 Materials
Maize stover was obtained from plants grown under field conditions near York (UK). The maize was harvested after R6 stage (silage) and the cobs removed. Stover samples were milled to 0.5 cm particles prior to pre-treatment using a hammer mill. The initial weight prior to milling, the final weight, the milling time required as well as the electricity and heat requirements (calculated based on equipment specifications) were recorded.2.2 Supercritical fluid extraction and fractionation of biomass
The supercritical carbon dioxide extractions were carried out using a SFE-500 provided by Thar technologies. Supercritical fluid grade carbon dioxide (99.99%) was used to conduct the extractions. 1.8 kg of maize stover were loaded into 2 × 5 L extraction vessels and connected to the extraction system. The required temperature and pressure were applied. The reaction vessel was heated to 65 °C and 5 minutes were allowed for it to equilibrate. Two internal pumps were used in order to obtain the required pressure (400 bar). The system was run in dynamic mode, in which the carbon dioxide which contained the lipids was allowed to flow into three fractionating vessels, each having a different pressure and temperature (150 bar/50 °C, 85 bar/35 °C and ATM/50 °C). A flow rate of 300 g min−1 of liquid CO2 was applied and the extraction was carried out for 4 hours. The lipids were collected by depressurising the fractionators.2.3 Biomass thermochemical pre-treatment
Biomass pre-treatment was carried out in a pressurised vessel with a 100 L total capacity. 2–3 kg of maize stover were loaded into the vessel together with 20–30 kg of water (pre-treatment media) in a 1/10 solid loading proportion. The pre-treatment was carried out at 160 °C ± 5 °C for 40 minutes, after which the vessel was cooled down to the discharge temperature (80 °C). The solid and liquids were separated after pre-treatment and the solid was stored in a cold room while the liquor was frozen. A number of parameters were recorded including the initial biomass weight, final biomass weight (wet), water consumption, pre-treatment time, electricity and heat requirements (calculated based on equipment specifications).2.4 Hydrolysis
10% dry matter content was used in a total volume of approximately 30 litres to perform the enzymatic hydrolysis of the pre-treated biomass. Prior to hydrolysation at pilot scale, shake flasks experiments were done in order to determine the enzyme dosage and hydrolysation time. The enzyme cocktail used was Cellic CTec2 (Novozymes). Hydrolysis was done over a 24 h period using an enzyme dosage of 10% (based on dry weight of raw material), at 55 °C and 5.2 pH. Analyses of monosaccharides, polysaccharides and common inhibitory substances were carried out after enzymatic hydrolysis.2.5 Fermentation with Saccharomyces cerevisiae Thermosacc
The hydrolysate was subjected to fermentation without filtration. The fermentation organism was Saccharomyces cerevisiae Thermosacc. Temperature, pH and agitation were set to 30 °C, 5.0 and 150 rpm respectively. Fermentations were performed in shake flasks.2.6 Fermentation with Starmerella bombicola
100 mL of hydrolysate was fermented in 500 mL shake flasks, with the addition of 0.25% yeast extract and 0.025% urea (modified from Rau et al.).30 The pH of the medium was adjusted to 3.5 with citric acid. After autoclaving the hydrolysate was inoculated with Starmerella bombicola and grown for 3 days at 25 °C and 120 rpm and the optical density (OD) and the Brix value were measured in the supernatant. In total 4 maize hydrolysate samples were tested, as well as glucose as a reference, in duplicate.The overall yield was calculated as followed:
2.7 Foam measurements (maize stover wax)
Foamability and foam stability measurements were analysed using a Krüss Dynamic Foam analyser DFA 100. A typical sample comprised 0.32 g of commercial available washing powder and 0.01–0.02 g of maize stover wax. A control sample was also prepared. 40 mL of tap water was added to each sample and heated to 50 °C. Air was pumped through at a flow rate of 0.3 L min−1 for 18 seconds. Total height, foam height and liquid height as well as foam decay were measured.2.8 Washing machine tests
A standard formulation used in washing machine detergents was prepared by mixing together the following: 21 g zeolite, 17 g SKS 6, 16 g sodium percarbonate, 17.5 g Ufarol TCT/90A, 9 g sodium bicarbonate, 4 g sodium citrate, 4 g polypeptide Donlar, 5 g fatty acid methyl esters ethoxylates (FAMEE) and 3 g tetraacetylethylenediamine (TAED). The washing machine (Siemens SIWAMAT XLP 1330) was loaded with 10 towels and 10 dish towels along with the formulation, a soil ballast sheet (SBL 2004, 8 g soil piece, wfk-Testegewebe GmbH) and a known amount of the maize stover wax sample (1.5 g, 3 g). A 60 °C programme was set. A webcam was setup to monitor the washing machine run and measure the foam height. The experiment was run in triplicates. A number of blank runs (without the wax sample) were also carried out.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. An initial oven temperature of 60 °C was maintained for 1 minute. The temperature was increased at a ramp rate of 8 °C min−1 until 360 °C and held at this temperature for 30 minutes.
1. An initial oven temperature of 60 °C was maintained for 1 minute. The temperature was increased at a ramp rate of 8 °C min−1 until 360 °C and held at this temperature for 30 minutes.The data was collected with the PerkinElmer enhanced TurboMass (Ver5.4.2) chemical software and compounds were identified by comparison of mass fragmentation patterns with spectra contained in the NIST library (v. 2.2) and by direct comparison with standard compounds.
3. Results and discussion
3.1 Supercritical extraction of epicuticular waxes from plant surface
The optimisation of the wax extraction from maize leaves was performed following a 2 × 2 factorial experimental design.31 The optimisation was carried out on leaves due to the high wax yields in this part of the plant. However, in industry, all parts of the biomass would be utilised in the extraction (stem, leaves etc.) and therefore the overall %yield for this would be lower than that achieved solely for the leaves. The temperature range that was chosen for this work was in the range of 35–65 °C, while the pressure range was set from 80–400 bar.There is a variation in the crude wax yield with different conditions as well as a compositional change during the course of the extraction (Fig. 1). This is due to a change in the solvation properties with different temperatures and pressures resulting in a change in the solubility of compounds in CO2. The wax content extracted from the maize leaves under the different conditions implemented varied from 0.02–1.76%. Temperature and pressure dictate the dielectric constant and density of CO2. Increasing the pressure above 80 bar significantly increased the %yield of wax extracted. This indicated that the density of CO2 is an important factor in the extraction process (0.33% at 80 bar/65 °C compared to 1.02% at 350 bar/50 °C). Moreover, the highest yields (1.76%) were achieved using a pressure of 400 bar and temperature of 65 °C. The density of CO2 under these conditions is 0.87 g cm−3 and the yields obtained were significantly higher than those obtained at 350 bar and 50 °C (1.02%), which has the highest density in this study of 0.9 g cm−3. This shows that even though density has an important role, there are other factors that dictate the solubility of hydrophobic compounds in CO2. Temperature also plays an important role as higher yields are obtained at higher temperatures (65 °C). The cuticular waxes are semi-crystalline and therefore relatively high thermal energy is required to increase their solubility. The melting range of the maize leaf wax was found to be around 40–65 °C with an endothermic minimum at 54 °C, indicating that temperatures above the melt point of the wax aid in its solubilisation (Fig. 2).
 | ||
| Fig. 2 DSC plot illustrating the melting profiles for each wax fraction. Fraction A (400 bar/50 °C), B (150–80 bar/35 °C) and C (80 bar-ATM/50 °C). | ||
The supercritical extractions gave rise to high-value waxes, containing a wide range of high-value compounds such as n-policosanols, long-chain fatty acids, fatty aldehydes, n-hydrocarbons, sterols, steroid ketones and wax esters (Fig. 3). These compounds could be used in a variety of applications, including nutraceuticals, ingredients for cleaning products, flavours, degreasers, cosmetics and lubricants.32–39
 | ||
| Fig. 3 Composition of groups of compounds for each wax fraction A (400–150 bar/50 °C) B (150–80 bar/35 °C) C (80-ATM/50 °C). | ||
One such application for waxes is their use as defoaming agents in washing machine formulations. Foam control in horizontal axis washing machines is an important issue. Due to mechanical agitation, elevated temperature, and high surfactant concentration, an excess of foam can be generated. Excessive foam has an adverse effect on washing performance due to impaired movement of the laundry itself, and inefficient rinsing and drainage of the machine. Furthermore, the electronic parts of the washing machine may be damaged. Several types of antifoam substances are currently used for foam control. Some of those have been reported as having a negative impact on the environment:40,41 phosphates (eutrophication), nitrogen-containing compounds (possible carcinogenic by-products nitrosamines), organic silicon compounds (persistent) and fluoro-compounds. Waxes represent an environmentally friendly option, but it is crucial that the wax has a melting point range between 30–50 °C and low saponification values.41 Crude plant and mineral waxes generally have melting points that range between 41 and 87 °C.28 Therefore, an advantage of utilising scCO2 over conventional solvents (such as hexane), is the possibility of fractionating the crude epicuticular wax into groups of compounds resulting in wax fractions with different melting point ranges. Successful extraction and fractionation of wax from maize stover has been carried out on a semi-pilot scale. The crude yield of wax obtained was 0.9% using extraction conditions of 400 bar and 65 °C. Fractional separation at three different pressures and temperatures was achieved at: 400–150 bar/50 °C (fraction A); 150–80 bar/35 °C (fraction B); and 80 bar-ATM/50 °C (fraction C) (Fig. 2) resulting in three wax fractions with different compositions, textures and crucially melting point ranges (Fig. 2 and 3), opening doors into multiple applications.
Wax fraction A has the highest melting profile with an endothermic minimum centred at around 74 °C, which could make it suitable for applications such as instrument and automobile polishes which require waxes with higher melting temperatures. This wax fraction has the largest quantities of wax esters (43 mg g−1 of wax). Wax esters are highly sought after because their high molecular weight allows them to be used in a variety of applications ranging from cosmetics to lubricants, plasticisers, coatings and polishes.36 Wax fraction B is predominantly phytosterols (402 mg g−1 of wax) which have significant nutraceutical properties. Phytosterols have been proven to be effective anti-cancer compounds and have shown to play an important role in cholesterol metabolism and treatment of atherosclerosis.37,42 The substantial amount of phytosterols in this wax fraction (42% of wax fraction composition) could allow for easy isolation and purification of these compounds for nutraceutical application of commercial interest. Wax fraction C has a melting point profile ranging from 28–41 °C. GC and IR data (Fig. S1 and S2 – ESI†) indicate a higher abundance of fatty acids and a significantly lower amount of wax esters in this fraction when compared to fractions A and B. The low melting point range of the wax fraction C allowed for defoaming tests to be performed.
Foam measurements can provide important information about the defoaming capacity of waxes. Foam production and foam decay were measured with high resolution optical sensors (LED illumination and light detection). Fig. 4 shows a significant defoaming effect produced by the maize stover wax on foam ability and foam stability. The wax significantly reduced the quantity of foam generated as well as decreased the time taken for the foam to break up. The control represents a sustainable antifoam currently used on the market in washing powder formulations. The commercially available washing powder used contains soap and on addition of tap water (containing calcium and magnesium), salts with poor water solubility are generated and it is these which can act as antifoams. Therefore in Fig. 4, the blue line represents the current technology. It can be observed that addition of waxes gives rise to a substantial improvement in antifoaming over the current strategy. The average foam height (average of 3 runs) measured after 250 s (approximately 42.2 mm) was close to the starting value when using 0.02 g of maize wax. The average foam height obtained for the control was 92.7 mm at the same time. Furthermore, the maize stover wax reduced the foam height faster than the control.
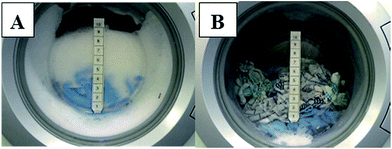
These results illustrate the defoaming performance of the maize wax showing its potential as a defoaming agent. These defoaming characteristics were further investigated by mimicking a real-life situation, whereby the wax was incorporated in washing machine runs in order to see whether the defoaming effects observed during the foam measurements also occurred during a washing run. Washing machine tests were carried out with detergent formulation (containing wax), together with a number of towels, dish towels and soil ballast sheet, on a typical washing machine run programme. A number of runs were carried out in order to ensure that repeatability was achieved.
Fig. 5 is a representative washing machine run carried out without wax (A), i.e. a blank run and a washing machine run where the wax was added (B). Anti-foaming agents typically constitute 0.8–4% by weight of the total detergent formulation43 and therefore the washing machine tests were carried out using 3 g (≈3%) and 1.5 g (≈1.5%) of maize stover wax. There is a significant difference in the amount of foam generated between the two runs, where there was on-average, a twelve-fold reduction in foam height in the washing machine when 1.5 g of maize stover wax was incorporated in the formulation. This further gives evidence that there is a defoaming effect on the foam by the wax sample. The same results were observed for all the runs (Fig. S3 ESI†). Therefore, these promising results indicate the potential of utilising the wax extracted from maize stover in washing machine detergent formulations as a renewable defoaming agent. For laundry powder, commercial spray dried and granulated anti-foams range from 5–10 € per kg. It has been estimated that the price of the waxes will be cost effective compared to spray-dried or granulated components.
Supercritical pre-treatment of maize stover leads to the extraction of high-value waxes that can be incorporated into a host of applications. However, the extraction of wax from maize stover only utilises around 1% of the total biomass, leaving 99% of the material unutilised. ScCO2 is a non-polar solvent and therefore no sugar is extracted during this process. In order to have a systemic view of a maize stover processing scenario where scCO2 extraction is integrated into a biorefinery, the supercritical extracted maize stover (MA scCO2) was subjected to hydrolysis and fermentation and the results were compared to non-treated maize stover (MA).
3.2 Pre-treatment and hydrolysis
ScCO2 extracted maize stover as well as non-treated maize stover, were subjected to a mild hydrothermal pre-treatment, and the yield of sugars after enzymatic hydrolysis was determined in both cases.Fig. 6 shows the carbohydrate composition of the material, as well as the yield of sugars after enzymatic hydrolysis for both scCO2 extracted and non-treated maize stover. The level of xylan in the scCO2 extracted material was relatively high (149–175 g kg−1 TS−1) (Fig. 6A). This is consistent with a low sugar yield after enzymatic hydrolysis (Fig. 6B). Since the pre-treatment and hydrolysis conditions only partially released the sugar present in the stover, the subsequent fermentation gives an indication of the fermentability of the slurry, and by no means a value of the potential yield in final products from maize stover. ScCO2 extracted stover shows a small but significantly higher proportion of glucan.
3.3 Fermentation
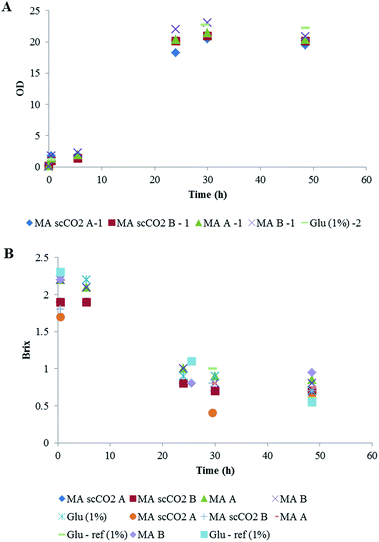
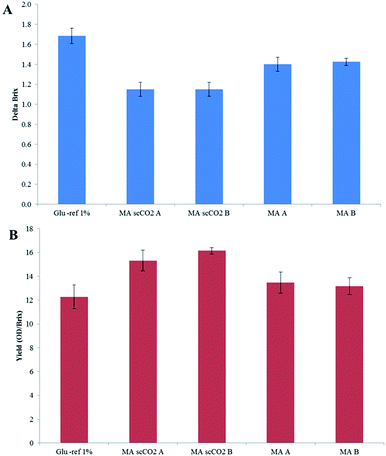
Two types of fermentation were carried out. The first involved the use of S. bombicola for the production of sophorolipid surfactants from maize stover hydrolysate.44 The growth and substrate consumption during shake flask experiments with S. bombicola is shown in Fig. 7. Although a limited number of samples were taken, a lag phase, an exponential phase and stationary phase were observed. Furthermore, a decrease in substrate concentration occurred. No inhibition in growth was observed for any of the samples. In comparison with the reference, the same growth (OD) was reached.The substrate consumption and overall yield are shown in Fig. 8A and B, where 2 repeats were carried out for the scCO2 extracted (MA scCO2 A and MA scCO2 B) and non-treated maize stover (MA A and MA B). The values of sugar consumption were carried out using Brix. Although Brix is not a precise measurement for sugar, it gives an indication of sugar consumption in order to compare the scCO2-extracted stover with the non-treated stover. Fig. 8A shows that there is on average, a 19% increase in glucose consumption with the scCO2 extracted maize stover when compared to the non-treated maize stover. Furthermore, Fig. 8B indicates that, on average, substrate growth increased by 18% (Fig. 8B) with the scCO2 extraction of maize stover. Therefore it can be seen that scCO2 has an overall positive effect on fermentation for the production of sophorolipid surfactants in comparison with the non-scCO2 extracted hydrolysates.
The second example of fermentation was carried out using Saccharomyces cerevisiae Thermosacc for ethanol production.
Extraction of stover using scCO2 leads to a 40% increase in ethanol production, when compared to the non-extracted maize stover (Fig. 9). Fig. 6 shows that the total sugars is higher in the scCO2 extracted maize (higher glucan content) and therefore fermentation can occur at a higher rate due to more available substrate. These results are consistent with observations found in the literature that indicate enhanced hydrolysis of biomass post-treatment in a supercritical reactor (in static mode).45,46 It is possible that the removal of waxy lipid layers from the plant improves the effectiveness of the hydrothermal pre-treatment and enzyme access to polysaccharides during downstream processing of the biomass. It has been shown that cuticular waxes of C4 biomass are critical inhibitors of fermentation.47 Crucially, within this current study it has been demonstrated that by conducting the scCO2 treatment as an extraction (in dynamic flow rather than in static mode) there is an enhanced downstream effect on hydrolysis and fermentation, whilst also providing a source of valuable waxes.
The techno-economic assessment of the holistic maize stover biorefinery shows that with the integration of scCO2 extraction the production costs of ethanol are 35% lower when compared with the non-scCO2 extracted maize stover. Even though equipment costs and thus the total capital investment of the biorefinery are increased with the inclusion of the scCO2 pre-treatment, these are outweighed by the increased product rates which result in lower manufacturing costs. Generally, wax sales decrease overall production costs by 1–1.5%.
Thus scCO2 would be an effective extraction step in a maize stover biorefinery leading to the extraction of high-value waxes as well as enhancing the downstream processing of the maize stover biomass. The following schematic is proposed for the development of a holistic maize stover biorefinery, which leads to the production of waxes, surfactants and fuels (ethanol and solid fuels for power generation). From a systemic viewpoint, it could also be possible to produce silicates solutions and recovery metals from the ashes generated in combusting any biomass residues (Fig. 10).48–50
4. Conclusions
This work illustrates the numerous benefits of integrating supercritical extraction for maize stover in a holistic biorefinery. Waxes, containing a wide range of high-value compounds such as n-policosanols, long-chain fatty acids, aldehydes, hydrocarbons sterols and wax esters were successfully extracted. ScCO2 separation of the wax resulted in fractions containing different compositions and melting points enabling their utilisation in different applications. One of the fractions has been shown to be an effective defoaming substance which can be implemented in detergents in place of non-renewable defoaming agents. Furthermore, it has been shown that scCO2 extraction has a positive effect on downstream processing of the maize stover biomass. Two types of fermentation were carried out; the first type for surfactant production, the second type for ethanol production. In the first fermentation to produce surfactants, results show that there was a higher glucose consumption and greater growth for the scCO2 extracted maize stover. In the second type of fermentation, there is a 40% increase in overall ethanol production for the scCO2 extracted maize stover when compared to the non-treated stover. Additionally, the techno-economic assessment of the maize stover biorefinery shows that the integration of scCO2 extraction decreases ethanol production costs by 35%. This indicates that scCO2 is an ideal solvent for the generation of valuable waxy compounds in a holistic biorefinery and also leads to enhanced fermentation processes and increased economic viability of the biorefinery.Acknowledgements
We gratefully acknowledge funding through the European Commission's Directorate-General for Research within the 7th Framework Program (FP7/2007–2013) under the grant agreement no. 251132 (SUNLIBB).Notes and references
- P. S. Shuttleworth, M. De bruyn, H. L. Parker, A. J. Hunt, V. L. Budarin, A. S. Matharu and J. H. Clark, Green Chem., 2014, 16, 573–584 RSC.
- J. H. Clark, V. Budarin, F. E. I. Deswarte, J. J. E. Hardy, F. M. Kerton, A. J. Hunt, R. Luque, D. J. Macquarrie, K. Milkowski, A. Rodriguez, O. Samuel, S. J. Tavener, R. J. White and A. J. Wilson, Green Chem., 2006, 8, 853–860 RSC.
- V. L. Budarin, P. S. Shuttleworth, J. R. Dodson, A. J. Hunt, B. Lanigan, R. Marriott, K. J. Milkowski, A. J. Wilson, S. W. Breeden, J. Fan, E. H. K. Sin and J. H. Clark, Energy Environ. Sci., 2011, 4, 471–479 CAS.
- J. M. Greef and M. Deuter, Angew. Bot., 1993, 67, 87–90 Search PubMed.
- H. W. Zub and M. Brancourt-Hulmel, Agron. Sustainable Dev., 2010, 30, 201–214 CrossRef PubMed.
- C. V. Beale and S. P. Long, Plant, Cell Environ., 1995, 18, 641–650 CrossRef PubMed.
- J. C. Clifton-Brown and I. Lewandowski, Ann. Bot., 2000, 86, 191–200 CrossRef.
- I. Linde-Laursen, Hereditas, 1993, 119, 297–300 CrossRef PubMed.
- S. Sokhansanj, A. Thurhollow, K. Kadam and J. McMillan, Resource, 2001, 8, 11–12 Search PubMed.
- Corn stover for bioethanol—your new cash crop?, http://www.nrel.gov/docs/fy01osti/29691.pdf, accessed 26th June 2014.
- J. Hettenhaus, R. Wooley and A. Wiselogel, Biomass commercialization prospects in the next 2–5 years, NREL, Golden, CO, 2000 Search PubMed.
- K. S. Dhugga, Crop Sci., 2007, 47, 2211–2227 CrossRef CAS.
- D. W. Lemke and M. C. Thiede, US Pat., 0024281, 2010.
- Kline, Global wax industry 2010: market analysis and opportunities, Kline & Company Inc., 2011 Search PubMed.
- A. J. Hunt, E. H. K. Sin, R. Marriott and J. H. Clark, ChemSusChem, 2010, 3, 306–322 CrossRef CAS PubMed.
- E. H. K. Sin, R. Marriott, A. J. Hunt and J. H. Clark, C. R. Chim., 2014, 17, 293–300 CrossRef CAS PubMed.
- M. A. McHugh and V. J. Krukonis, Supercritical fluid extraction: principles and practice, Butterworth-Heinemann, 1994 Search PubMed.
- A. Özcan and A. S. Özcan, Talanta, 2004, 64, 491–495 CrossRef PubMed.
- M. Zougagh, M. Valcárcel and A. Ríos, TrAC, Trends Anal. Chem., 2004, 23, 399–405 CrossRef CAS.
- Q. Lang and C. M. Wai, Talanta, 2001, 53, 771–782 CrossRef CAS.
- J. H. Y. Vilegas, E. de Marchi and F. M. Lancas, Phytochem. Anal., 1997, 8, 266–270 CrossRef CAS.
- F. W. Jones, B. O. Bateup, D. R. Dixon and S. R. Gray, J. Supercrit. Fluids, 1997, 10, 105–111 CrossRef CAS.
- J. M. DeSimone, Science, 2002, 297, 799–803 CrossRef CAS PubMed.
- H. H. Schaumburg and P. S. Spencer, Brain, 1976, 99, 183–192 CrossRef CAS PubMed.
- P. S. Spencer and H. H. Schaumburg, Proc. R. Soc. Med., 1977, 70, 37 CAS.
- F. E. I. Deswarte, J. H. Clark, J. J. E. Hardy and P. M. Rose, Green Chem., 2006, 8, 39–42 RSC.
- Y. Athukorala, G. Mazza and B. D. Oomah, Eur. J. Lipid Sci. Technol., 2009, 111, 705–714 CrossRef CAS PubMed.
- Y. Athukorala and G. Mazza, Ind. Crops Prod., 2010, 31, 550–556 CrossRef CAS PubMed.
- Y. Choi, J. Kim, M. Noh, E. Park and K.-P. Yoo, Korean J. Chem. Eng., 1996, 13, 216–219 CrossRef CAS.
- U. Rau, C. Manzke and F. Wagner, Biotechnol. Lett., 1996, 18, 149–154 CrossRef CAS.
- M. Arshadi, A. J. Hunt and J. H. Clark, RSC Adv., 2012, 2, 1806–1809 RSC.
- E. Sjöström, Biomass Bioenergy, 1991, 1, 61–64 CrossRef.
- I. Gill and R. Valivety, Trends Biotechnol., 1997, 15, 401–409 CrossRef CAS.
- K. Hill, Pure Appl. Chem., 2000, 72, 1255–1264 CrossRef CAS.
- F. P. Schiestl, M. Ayasse, H. F. Paulus, C. Löfstedt, B. S. Hansson, F. Ibarra and W. Francke, Nature, 1999, 399, 421 CrossRef CAS.
- E. R. Gunawan, M. Basri, M. B. A. Rahman, A. B. Salleh and R. N. Z. A. Rahman, Enzyme Microb. Technol., 2005, 37, 739–744 CrossRef CAS PubMed.
- P. G. Bradford and A. B. Awad, Mol. Nutr. Food Res., 2007, 51, 161–170 CAS.
- M. Majeed, G. K. Gangadharan and S. Prakash, US Pat., 20070196507A1, 2007.
- C. P. F. Marinangeli, P. J. H. Jones, A. N. Kassis and M. N. A. Eskin, Crit. Rev. Food Sci. Nutr., 2010, 50, 259–267 CrossRef CAS PubMed.
- P. R. Garrett, Defoaming: Theory and Industrial Applications, Taylor & Francis, 1992 Search PubMed.
- Detergents Ingredients Database, http://ec.europa.eu/environment/ecolabel/documents/did_list/didlist_part_a_en.pdf, accessed 30th June 2014.
- M. H. Moghadasian and J. J. Frohlich, Am. J. Med., 1999, 107, 588–594 CrossRef CAS.
- G. Wagner, Waschmittel - Chemie, Umwelt, Nachhaltigkeit, Wiley, 2011 Search PubMed.
- D. W. G. Develter and S. J. J. Fleurackers, in Surfactants from Renewable Resources, John Wiley & Sons, Ltd, 2010, pp. 213–238 Search PubMed.
- K. H. Kim and J. Hong, Bioresour. Technol., 2001, 77, 139–144 CrossRef CAS.
- N. Narayanaswamy, A. Faik, D. J. Goetz and T. Gu, Bioresour. Technol., 2011, 102, 6995–7000 CrossRef CAS PubMed.
- D. Cai, Z. Chang, C. Wang, W. Ren, Z. Wang, P. Qin and T. Tan, Bioresour. Technol., 2013, 149, 470–473 CrossRef CAS PubMed.
- J. R. Dodson, E. C. Cooper, A. J. Hunt, A. Matharu, J. Cole, A. Minihan, J. H. Clark and D. J. Macquarrie, Green Chem., 2013, 15, 1203–1210 RSC.
- J. R. Dodson, A. J. Hunt, V. L. Budarin, A. S. Matharu and J. H. Clark, RSC Adv., 2011, 1, 523–530 RSC.
- J. R. Dodson, A. J. Hunt, H. L. Parker, Y. Yang and J. H. Clark, Chemical Engineering and Processing: Process Intensification, 2012, vol. 51, pp. 69–78 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra07485a |
| This journal is © The Royal Society of Chemistry 2015 |