Coumarin-modified gold nanoprobes for the sensitive detection of caspase-3
Abstract
Caspase-3 has been identified as a key mediator and a well-established cellular marker of apoptosis. To increase the sensitivity, coumarin-functionalized gold nanoparticles (AuNPs) connected to polypeptide chains containing specific sequences (DEVD) were designed and synthesized for the sensing of caspase-3, because there was a large overlap between the emission of coumarin-343 and the absorption of the AuNPs. The fluorescence of coumarin 343 was quenched due to the energy transfer process by the gold nanoparticles. The fluorescence could be restored after the particular polypeptide sequence (DEVD) was cut off by caspase-3. Based on this mechanism, the caspase enzyme activity in vitro could be detected by a fluorescence assay with a high sensitivity. The effect of the different lengths of polypeptide chains on the luminescence quenching efficiency and sensing ability was also studied, which is of great importance in designing FRET-based sensing platforms. This kind of sensitive luminescent functional coumarin 343-modified gold nanoprobe is suitable for caspase-3 sensing in biological applications.
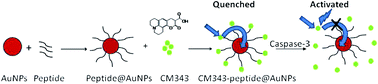

 Please wait while we load your content...
Please wait while we load your content...