Coordination versatility of phosphine derivatives of fluoroquinolones. New CuI and CuII complexes and their interactions with DNA†
Abstract
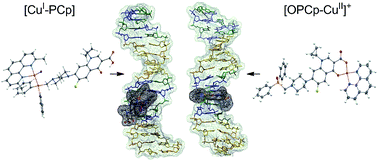
In this paper, new copper(I) and copper(II) complexes with phosphine derivatives of two fluoroquinolones (ciprofloxacin and norfloxacin) are presented. The synthesized compounds ([CuI-PCp], [CuI-PNr], [OPCp-CuII]+ and [OPNr-CuII]+) were characterized by elemental analysis and MS as well as by the NMR, EPR and IR spectroscopies. X-ray techniques were used to determine the crystal and molecular structures of [CuI-PCp]·CH2Cl2·CH3CN and OPCp-CuII]NO3·3H2O. For all the studied compounds, the ability to interact with DNA was determined using three different methods. The results of gel electrophoresis revealed that in the presence or absence of H2O2, the copper(I) complexes caused only single-stranded cleavage of the sugar–phosphate backbone of DNA. In turn, the copper(II) complexes damaged the plasmid exclusively in the presence of the oxidant. The addition of H2O2 caused distinct changes in the plasmid structure, resulting in a complete disappearance of its native form. Forms II and III arising from single- and double-strand cleavage were detected. Studies of the interactions with calf thymus DNA in the presence of ethidium bromide (EB) showed that the tested complexes and phosphines interact with DNA in a partial intercalation mode, contrary to unmodified antibiotic and oxide derivatives, which do not displace EB from the system. Molecular docking (AutoDock Vina program) was performed using the synthetic double-stranded hexadecanucleotide (sequence: ATATCGCGATATCGCG). Data analysis showed that a majority of the compounds preferably bound to the minor or major grooves, however most of them were also able to intercalate with the DNA double helix.

 Please wait while we load your content...
Please wait while we load your content...