Red up-conversion emission in α-KYb3F10:Er3+ films made by electrodeposition†
Xiaowen Wu,
Linlin Tian,
Qinyan Lu and
Run Liu*
Department of Chemistry, Zhejiang University, Hangzhou, 310027, China. E-mail: runliu@zju.edu.cn
First published on 2nd June 2015
Abstract
Er3+ ions doped α-KYb3F10 thin films were obtained from aqueous solutions through electrodeposition near room temperature. The annealed films show almost single band red emission excited by a 980 nm laser and a two photon up conversion mechanism is involved.
Recently, there has been considerable interest in lanthanide-doped up conversion (UC) phosphors owing to their applications in color 3D displays, solid-state lasers, bioimaging, and photovoltaics.1–10 The UC phosphors normally exhibit multipeak emissions since lanthanide ions generally have more than one excited state.11 To improve the chromatic purity of optical devices and reduce the absorption of tissue, a red-emitting up conversion phosphor with high color purity is vitally needed. Thus, preparation of up conversion phosphors with single red emission is of technological importance and challenge.
For proceeding efficient up conversion, Yb3+ ions are doped in crystals as sensitizers due to the large absorption cross section of Yb3+ ions matched to 980 nm laser excitation sources and the efficient energy transition from Yb3+ to Er3+, Tm3+ and Ho3+ activators.1 An increased amount of Yb3+ content would be likely to enhance the luminescence efficiency and also red-to-green emission ratio in Yb/Er co-doped fluoride crystals.12,13 Therefore, it is worth to explore the up conversion properties of Er3+ ions doped Yb-base fluorides. Moreover, for the device applications, a film of the UC based phosphors is requisite, and preferably made through a low-cost processing. Electrochemical deposition is a well-known solution process to prepare a functional film in an aqueous or non-aqueous solution.14–20 This method has advantages of low temperature and low-cost in controlling the composition and structure of the deposited films.
In this work, we showed that Er3+ ions doped α-KYb3F10 thin films can be obtained by a facile anode electrodeposition process from aqueous solutions near room temperature. The annealed α-KYb3F10:Er3+ thin films exhibited almost single band strong red emissions upon excited with a 980 nm laser. Our results indicate that α-KYb3F10:Er3+ thin films may be used in new optical display devices and bioimaging.
The Er3+ doped α-KYb3F10:Er3+ films were electrodeposited on ITO electrode in aqueous solutions containing lanthanide ions–EDTA complexes and ascorbic acid. Under applied anode potentials (experimental details were provided in ESI†), ascorbic acids are oxidized to generate H+ ions on the surface of ITO electrode.20,22 The released H+ ions will reduce the pH on the surface of the ITO electrode where the lanthanide ions can be set free from lanthanide ions–EDTA complex and then react with K+ and F− ions to form KYb3F10:Er3+ deposits. The possible mechanism for anodic electrodeposition of α-KYb3F10:Er3+is following:
| RE3+ + EDTA → [RE(EDTA)]− | (1) |
| C6H6O6 + H+ + 2e → C6H7O6− | (2) |
| [RE(EDTA)]− + xH+ → [RE1−x(HxEDTA)]x−1 + xRE3+ | (3) |
| K+ + 10F− + 3RE3+ → KRE3F10 | (4) |
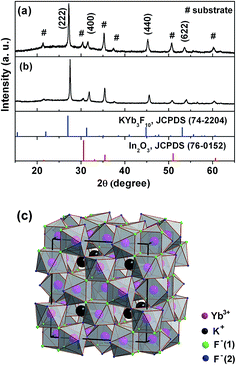
Fig. 1a shows X-ray diffraction results of the as electrodeposited film containing 2 mol% Er3+ ions and the same film annealed at 300 °C in air for two hours. Both XRD patterns can be indexed as reported cubic α-KYb3F10 (JCPDS: 74-2204) structure.21 Besides XRD pattern of the ITO substrate, there is no other impure phase. The lattice parameter of the electrodeposited α-KYb3F10 is a = 11.338 Å, a little less than the reported value (a = 11.431 Å).21 The sharp and strong peaks indicate that as electrodeposited and annealed α-KYb3F10:Er3+ thin films are well crystallized. The films show a little [111] preferred orientation. Fig. 1b shows the crystal structure of α-KYb3F10. The crystal structure of α-KYb3F10 is isotopic with γ-KYb3F10, a cubic 2 × 2 × 2 superstructure of fluorite that consists of two ionic groups [KYb3F8]2+ and [KYb3F12]2− alternating along the three crystallographic directions.21 The coordination of the Yb3+ ions in the α-KYb3F10 structure is square antiprismatic with C4v symmetry.
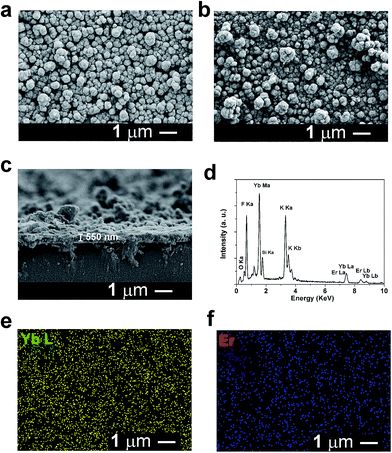
Fig. 2a and b show typical scanning electron microscopy (SEM) images of the electrodeposited and annealed α-KYb3F10:Er3+ (2 mol%) films, respectively. Both films are composed of spherical structures with size of around 400 nm in diameter and the annealing process does not change the morphology too much. Fig. 2c shows that the thickness of the annealed film obtained by cross-section SEM image is about 550 nm. Fig. 2d shows that there are K, F, Yb and Er elements existed in the annealed films in the energy dispersive X-ray spectrum (EDX). Based on the result of EDX, the content of the Er3+ ions in the films is consistent with the amount of Er3+ ions in the solution. Fig. 2e and f further show that Yb3+ and Er3+ ions are homogeneously distributed in KYb3F10 films.
The up-conversion emissions of as electrodeposited and annealed α-KYb3F10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+(2 mol%) films under excitation by a 980 nm laser were investigated. The as electrodeposited α-KYb3F10:Er3+ films show little emissions (shown in Fig. 3a using black line). Similar result was also observed in our previous works.22 Two possible paths decrease the emission intensity, one is the change of crystal structure of the host and the other one is the surface effects. The XRD patterns and width of (111) peak of electrodeposited and annealed electrodeposited films are compared and there are little differences (Fig. S1†). This indicates that the annealed process has little effect on the crystal structure and grain size of the electrodeposited films due to low annealed temperature (300 °C). Thus the change crystal structure of the annealed films is excluded on affecting the emission intensity. The surfaces effects on emission intensity are attributed to the presence of high vibrational energy ligands (e.g., –CH, –OH groups) or surface defects (e.g., vacancy, lanthanide segregation).23–25 The –OH groups are known to be efficient quenchers in Ln3+-doped phosphate glass and NaYF4:Yb, Er nanocrystals.24,26 The Fourier transform infrared (FTIR) spectra of the electrodeposited and annealed electrodeposited KYb3F10 are compared (Fig. S2†). The absorption of –OH group in annealed films decreases dramatically and –CH groups change little. Therefore, the emission might be annihilated by adsorbed water in the as electrodeposited α-KYb3F10:Er3+ films obtained from aqueous solutions.
Er3+(2 mol%) films under excitation by a 980 nm laser were investigated. The as electrodeposited α-KYb3F10:Er3+ films show little emissions (shown in Fig. 3a using black line). Similar result was also observed in our previous works.22 Two possible paths decrease the emission intensity, one is the change of crystal structure of the host and the other one is the surface effects. The XRD patterns and width of (111) peak of electrodeposited and annealed electrodeposited films are compared and there are little differences (Fig. S1†). This indicates that the annealed process has little effect on the crystal structure and grain size of the electrodeposited films due to low annealed temperature (300 °C). Thus the change crystal structure of the annealed films is excluded on affecting the emission intensity. The surfaces effects on emission intensity are attributed to the presence of high vibrational energy ligands (e.g., –CH, –OH groups) or surface defects (e.g., vacancy, lanthanide segregation).23–25 The –OH groups are known to be efficient quenchers in Ln3+-doped phosphate glass and NaYF4:Yb, Er nanocrystals.24,26 The Fourier transform infrared (FTIR) spectra of the electrodeposited and annealed electrodeposited KYb3F10 are compared (Fig. S2†). The absorption of –OH group in annealed films decreases dramatically and –CH groups change little. Therefore, the emission might be annihilated by adsorbed water in the as electrodeposited α-KYb3F10:Er3+ films obtained from aqueous solutions.
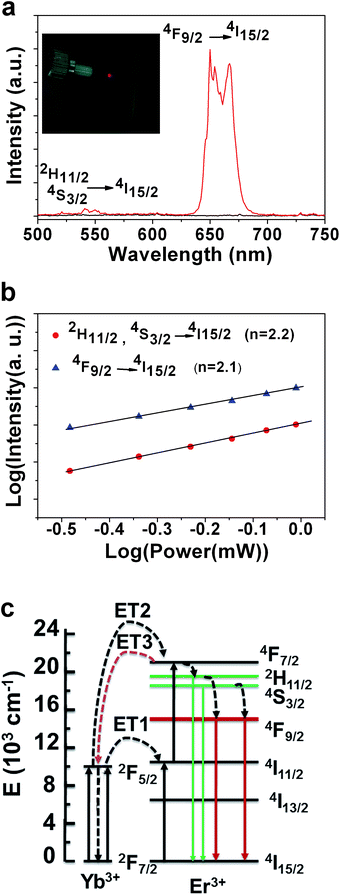
For annealed films, Fig. 3a shows that there are a weak green emission at 542 and 549 nm and a strong red emission peak at 654 nm. The ratio of red/green is about 22.5 for the annealed α-KYb3F10:Er3+(2 mol%) film. Inset image in Fig. 3a shows that the annealed film exhibits intense red emission upon excited with a 0.5 W 980 nm laser. The green emission bands at 542 and 549 nm accounts for the 2H11/2, 4S3/2 → 4I15/2 transition and the red luminescence centered at 654 nm is ascribed to the 4F9/2 → 4I15/2 transition of Er3+, respectively. The quantum efficiency of the film is measured by the method developed by Zhang and Zhao27 and is about 0.036%, which is higher than our reported value in electrodeposited NaGdF4:Yb3+/Er3+ film.22
To investigate the up conversion mechanism of α-KYb3F10:Er3+, the intensities of the up conversion emissions were recorded as a function of the power of 980 nm laser. For an unsaturated up conversion process, the emission intensity (I) is proportional to power (n) of laser power (P):
| I ∝ Pn | (5) |
Therefore, the energy level diagrams of Er3+ and Yb3+ ions as well as the up conversion mechanism are presented in Fig. 3c. Firstly, Yb3+ ion is excited to 2F2/5 state by absorbing a near-infrared photon, and then the state 4I11/2 of Er3+ is populated by energy transfer (ET1) occurred from Yb3+ ion to Er3+ ion. In the meantime, Er3+ ion itself can also absorb a 980 nm photon directly from the laser. Another energy transfer (ET2) between Yb3+ and Er3+ can populate the 4F7/2 level of the Er3+ ion. Then Er3+ ion relax non-radiatively to the 2H11/2 and 4S3/2 levels. When 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transitions of Er3+ ions occur, green light can be observed. As for red light emission, it comes from 4F9/2 → 4I15/2 transition. There are two pathways for the population of 4F9/2 level. One is the relaxation of 2F7/2 and the other is excitation from 4I13/2 by the energy transfer of Yb3+ or absorption of a 980 nm photon.29 It was reported that an ET3 process: 4F7/2(Er) + 2F7/2(Yb) → 4I11/2(Er) + 2F5/2(Yb),30 can depopulate the 4F7/2 excited level and reduce the intense of green emission. Therefore, the red emission is dominated in the electrodeposited α-KYb3F10:Er3+ films.
In summary, we have demonstrated that α-KYb3F10:Er3+ thin films shown nearly single red emissions excited by a 980 nm laser can be obtained from an aqueous solution by a facile electrodeposition method near room temperature. It is expected that the α-KYb3F10:Er3+ thin films on transparent ITO substrate will be suitable for applications in optical display devices and bioimaging.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (21373183), the National Basic Research Program of China (Grant no. 2011CB936003), and Zhejiang Provincial Natural Science Foundation of China (LY12B07001).Notes and references
- F. Auzel, Chem. Rev., 2004, 104, 139 CrossRef CAS PubMed.
- E. Downing, L. Hesselink, J. Ralston and R. Macfarlane, Science, 1996, 273, 1185 CAS.
- T. Sandrock, H. Scheife, E. Heumann and G. Huber, Opt. Lett., 1997, 22, 808 CrossRef CAS.
- B. E. Cohen, Nature, 2010, 467, 407 CrossRef CAS PubMed.
- G. Wang, Q. Peng and Y. Li, Acc. Chem. Res., 2011, 44, 322 CrossRef CAS PubMed.
- A. Shalav, B. S. Richards, T. Trupke, K. W. Krämer and H. U. Güdel, Appl. Phys. Lett., 2005, 86, 013505 CrossRef PubMed.
- M. Haase and H. Schäfer, Angew. Chem., Int. Ed., 2011, 50, 5808 CrossRef CAS PubMed.
- B. M. Van der Ende, L. Aarts and A. Meijerink, Phys. Chem. Chem. Phys., 2009, 11, 11081 RSC.
- N. Menyuk, K. Dwight and J. W. Pierce, Appl. Phys. Lett., 1972, 21, 159 CrossRef CAS PubMed.
- R. T. Wegh, H. Donker, K. D. Oskam and A. Meijerink, Science, 1999, 283, 663 CrossRef CAS.
- J. F. Suyver, J. Grimm, K. W. Krämer and H. U. Güdel, J. Lumin., 2005, 114, 53 CrossRef CAS PubMed.
- F. Wang and X. Liu, J. Am. Chem. Soc., 2008, 130, 5642 CrossRef CAS PubMed.
- D. Chen, L. Lei, R. Zhang, A. Yang, J. Xu and Y. Wang, Chem. Commun., 2012, 48, 10630 RSC.
- J. A. Switzer and G. Hodes, MRS Bull., 2010, 35, 743 CAS.
- R. M. Penner, J. Phys. Chem. C, 2014, 118, 17179 CAS.
- M. J. Siegfried and K.-S. Choi, Angew. Chem., Int. Ed., 2005, 44, 3218 CrossRef CAS PubMed.
- T. Yoshida and H. Minoura, Adv. Mater., 2000, 16, 1219 CrossRef.
- D. Lincot, Thin Solid Films, 2005, 487, 40 CrossRef CAS PubMed.
- M. Dinamani, P. V. Kamath and R. Seshadri, Chem. Mater., 2001, 13, 3981 CrossRef CAS.
- S. J. Limmer, E. A. Kulp and J. A. Switzer, Langmuir, 2006, 22, 10535 CrossRef CAS PubMed.
- M. Labeau, S. Aleonard, A. Vedrine, R. Boutonnet and J. C. Cousseins, Mater. Res. Bull., 1974, 9, 615 CrossRef CAS.
- L. Tian, P. Wang, H. Wang and R. Liu, RSC Adv., 2014, 4, 19896 RSC.
- M. C. Tan, G. A. Kumar, R. E. Riman, M. G. Brik, E. Brown and U. Hommerich, J. Appl. Phys., 2009, 106, 063118 CrossRef PubMed.
- Y. Yan, A. J. Faber and H. de Waal, J. Non-Cryst. Solids, 1995, 181, 283 CrossRef CAS.
- F. Wang, J. Wang and X. Liu, Angew. Chem., Int. Ed., 2010, 49, 7456 CrossRef CAS PubMed.
- D. Yuan, M. C. Tan, R. E. Riman and G. M. Chow, J. Phys. Chem. C, 2013, 117, 13297 CAS.
- X. M. Li, D. K. Shen, J. P. Yang, C. Yao, R. C. Che, F. Zhang and D. Y. Zhao, Chem. Mater., 2013, 25, 106 CrossRef CAS.
- M. Pollnau, D. R. Gamelin, S. R. Luthi, H. U. Gudel and M. P. Hehlen, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 3337 CrossRef CAS.
- J. Wang, R. R. Deng, M. A. MacDonald, B. L. Chen, J. K. Yuan, F. Wang, D. Z. Chi, T. S. A. Hor, P. Zhang, G. K. Liu, Y. Han and X. Liu, Nat. Mater., 2014, 13, 157 CrossRef CAS PubMed.
- H. Guo, N. Dong, M. Yin, W. Zhang, L. Lou and S. Xia, J. Phys. Chem. B, 2004, 108, 19205 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra07346d |
| This journal is © The Royal Society of Chemistry 2015 |