Improving the SERS detection sensitivity of aromatic molecules by a PDMS-coated Au nanoparticle monolayer film
Chen Qian†
,
Qinghua Guo†,
Minmin Xu,
Yaxian Yuan* and
Jianlin Yao*
College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123, China. E-mail: yuanyaxian@suda.edu.cn; jlyao@suda.edu.cn
First published on 3rd June 2015
Abstract
Surface enhanced Raman spectroscopy (SERS) has been considered as a promising tool for detecting targets with single molecule sensitivity. However, the SERS detection on targets without a specific adsorption group has still remained a significant challenge. In this paper, we reported a facile strategy to fabricate a PDMS film-coated Au nanoparticle monolayer film (Au MLF) composite substrate for improving SERS detection of aromatic molecules in water and in the atmosphere. Toluene, benzene and nitrobenzene were used as the targets to evaluate the performance of the composite substrate. The results indicated that the PDMS film played the vital role to capture and preconcentrate these targets for improving the capability in SERS detection of these targets. The performance was critically dependent on the hydrophobicity, functional groups and the permeability of the targets. This composite substrate was more favorable for the detection of toluene and nitrobenzene than benzene. The limit of detection (LOD) for toluene and nitrobenzene was decreased by about two orders of magnitude on the PDMS-Au MLF compared to that on the naked Au MLF, and by one order of magnitude for benzene. It was estimated to be 0.5 ppm, 0.6 ppm and 78 ppm for toluene, nitrobenzene and benzene, respectively. The results demonstrated that this approach could be developed as a promising tool to detect numerous targets which were non-specifically adsorbed onto metallic nanostructures. It opened a window towards the general application of SERS for in situ monitoring of pollutants in water and in the atmosphere.
Introduction
Various volatile organic compounds (VOCs) and semi-volatile organic compounds (SVOCs), including benzene, toluene, nitrobenzene and their derivatives, are considered to be carcinogenic and mutagenic, and have significant harmful effects on human health and environmental security.1–3 Typical identification techniques mainly relied on expensive instrumentation, such as gas chromatography (GC), gas chromatography-mass spectrometry (GC-MS), and high performance liquid chromatography (HPLC). Although these techniques held an acceptable sensitivity and stability, the preparation processes involved and subsequent analytical procedures were still complex and time-consuming.4 Moreover, they were completely laboratory based, and lacked the instantaneous and in situ monitoring capability, particularly for remote identification and monitoring. Therefore, with the increasing diffusion and accumulation of the pollutants to the environment in water and in the atmosphere, the development of in situ technologies is highly desired for rapid identification.Surface enhanced Raman spectroscopy (SERS) has attracted considerable attention for chemical sensing, environmental monitoring and other relevant fields. With sensitivity up to the single molecule level and capability in the identification of molecules through the vibrational fingerprint, it is considered as a promising tool for the detection of trace molecules under ambient conditions, including in liquids, the atmosphere, tissue and so on.5–8 Moreover, the compact integration of the modern Raman spectrometer has improved the performance for portable and remote monitoring.9 Generally, the strong SERS effect was mainly contributed to by the electromagnetic and charge transfer enhancements. For the former, the generation of surface plasmon resonance (SPR) from the appropriate nanostructures induced a localized electric field to enhance the Raman signal of molecules which were located in a certain distance (nano/subnanometer scale) away from the surface. For the latter, it originated from the excitation photon driven charge transfer between the metal and the molecules which were immobilized at the metal surface. Therefore, it was essential to locate the targets near the enhancement source (SERS substrates). Generally, the probes attached with specific groups, involving thio, amino, nitrile, etc., were allowed to immobilize to the metal surface directly for providing the strong SERS signal, i.e. decreasing the limit of detection (LOD).10–12 Therefore, the generalities on targets for SERS detection still remained a significant challenge, particularly for the analytes without a specific adsorption group, such as aromatic molecules.
Since the aromatic compounds, such as benzene and toluene, contained no specific functional group and held a low affinity toward the metallic plasmonic surface, it was really difficult to detect them directly by SERS. In order to overcome this drawback, various techniques have been developed to immobilize these targets onto the substrate to increase the sensitivity, i.e. locating the targets at the zone of the electromagnetic field.13 Among them, as a general strategy, different kinds of materials were modified onto the substrates, which allowed sufficient weakly adsorbed targets to be immobilized near the plasmonic nanostructures through the interaction between the modified functional layer and the targets.13–31 For example, based on host–guest molecular recognition, the targets were selectively trapped through the host–guest interactions resulting in the preconcentration of targets. For molecules lacking affinity to the metallic surface, the particular construction of host molecules has cavities to specifically trap targets to be close to the SERS substrates.14–17 In other cases, self-assembly of monolayers of thiol and alkylsilane on SERS substrates made the metal surface become hydrophobic to allow the inclusion of the targets into the zone of electromagnetic enhancement, i.e. immobilization of the targets in the range of the long distance of electromagnetic enahncement.18–20 The noble metal modified magnetic nanoparticles provided an alternative preconcentration of targets by an external magnetic field. Moreover, the magnetic field induced the aggregation of nanocomposites to generate numerous plasmonic “hot spots”, thus improving the LOD of targets without a specific group to anchor to the metal surface.21–23 Similarly, metal–organic frameworks (MOFs) were successfully modified to plasmonic nanostructures and acted as the host to capture the weakly adsorbed targets through the unique porous structures of MOFs, particularly for gas targets in the atmosphere. A few of these reports demonstrated that the nature of MOFs made it become a very promising composite substrate for the preconcentration of weakly adsorbed molecules to be close to the metal surface.24–27 The metallized polymers such as Au/Ag nanoparticle–polydimethylsiloxane (PDMS) nanocomposites exhibited a unique immobilization capability for the SERS detection of aromatic compounds.28–31 However, it was mainly used to capture targets with functional groups, such as nitro, carboxyl, and hydroxyl, to improve the SERS sensitivity.31 To the best of our knowledge, there are no reports on the SERS detection of aromatic molecules, such as benzene, toluene, ethyl benzene and xylene by combining the PDMS film and metallic plasmonic nanostructures. Actually, PDMS incorporated with Au nanoparticles exhibited an extremely high swelling capability (larger than six times). Thus, it has already been explored to capture aromatic molecules both in water and in the atmosphere, i.e. cleaning up the environment. Moreover, the composite could be regenerated simply by heating to about 300 °C in air.32 Due to its strong adsorption capability, PDMS was also used as a sorbent for the detection of benzene, toluene, ethylbenzene and xylene in water and air by solid-phase extraction.33–35 Therefore, it is certainly worth exploring the combination of PDMS and plasmonic nanostructures to detect aromatic molecules without specific affinity groups. In this paper, spin coating was explored to optimize the PDMS layer on the Au nanoparticles monolayer film (Au MLF). The layer thickness of PDMS was then tuned to achieve the maximum SERS enhancement by changing the concentration of the PDMS solution. For such a kind of composite, the inner layer of the Au MLF generated a gigantic SERS effect, while the outer layer of PDMS played the vital role in capturing the aromatic targets. A series of aromatic molecules, including benzene, toluene and nitrobenzene, served as the model system to demonstrate the capability of the composite both for capturing the targets from water or the atmosphere and SERS monitoring. This strategy provided an alternative approach to improve the SERS detection sensitivity of weakly adsorbed targets and then extended the generality of SERS for different kinds of analytes.
Experimental section
General
SYLGARD silicone elastomer base and SYLGARD 184 silicone elastomer curing agent were bought from Dow Corning Company. Polyvinylpyrrolidone was purchased from Acros. Chloroauric acid tetrahydrate (HAuCl4·4H2O), hydroxylamine hydrochloride (NH2OH·HCl), trisodium-citrate, sulfuric acid (H2SO4, 95–98%), hydrogen peroxide (H2O2, 30%), toluene, benzene, nitrobenzene, tetrahydrofuran (THF), acetone and ethanol were of analytical grade and purchased from Sinopharm Chemical reagent corporation. All aqueous solutions were prepared with Milli-Q water (≥18.2 MΩ cm).Characterizations
Scanning electron microscopy (SEM) images were taken using FEI QUANTA 200F. Raman spectroscopy was performed using a LabRam HR800 micro-Raman system (HR800, Horiba, Jobin Yvon) with 632.8 nm laser excitation from a He–Ne laser. A 50× objective lens with a working distance of about 8 mm was attached onto the Raman microscope. The slit width and confocal pinhole were 100 μm and 400 μm, respectively. The laser power was about 5 mW on the surface.Fabrication of the SERS composite substrate
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (in wt%), and were kept shaking for 1 hour followed by 20 min degassing under vacuum. The PDMS elastomer was diluted in tetrahydrofuran (THF) solution to obtain a concentration range from 0.078% to 50%. In our case, the thickness of the PDMS layer was controllable by changing the concentration of the PDMS elastomer. 5 μL PDMS elastomer solution was dropped onto the Au MLF, and the spin coating procedure was accomplished after 600 s at a spin speed of 1000 rpm with an acceleration of 500 rpm s−1. Finally, the coated films on the Au MLFs were subsequently cured in a vacuum oven at 80 °C for about 6 h.38
1 (in wt%), and were kept shaking for 1 hour followed by 20 min degassing under vacuum. The PDMS elastomer was diluted in tetrahydrofuran (THF) solution to obtain a concentration range from 0.078% to 50%. In our case, the thickness of the PDMS layer was controllable by changing the concentration of the PDMS elastomer. 5 μL PDMS elastomer solution was dropped onto the Au MLF, and the spin coating procedure was accomplished after 600 s at a spin speed of 1000 rpm with an acceleration of 500 rpm s−1. Finally, the coated films on the Au MLFs were subsequently cured in a vacuum oven at 80 °C for about 6 h.38Results and discussion
Screening the PDMS film thickness
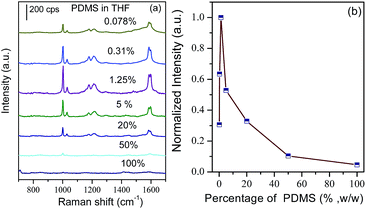
It is well known that PDMS exhibits a high swelling ability for the preconcentration of trace aromatic molecules, and the removal of benzene, toluene and oil spills from polluted water has already been explored.33–35 In our present case, in order to achieve high sensitivity of SERS detection, three issues should be taken into account: (i) the enhancement effect of the plasmonic nanostructures; (ii) the number of target molecules located at the zone of the electromagnetic field; and (iii) the propagation of the laser and the Raman signal though the PDMS film. For the first issue, the Au MLF served as the SPR source, which contributed to the strong coupling effect between adjacent nanoparticles. For the last two issues, the thickness of the PDMS film became the critical factor on the performance of the substrate. The thicker PDMS film was beneficial for inhibiting the diffusion of targets to air, i.e. immobilizing more targets around the surfaces. However, it blocked the propagation laser and Raman signal, resulting in the notable decrease of the SERS signal. Therefore, it was necessary to screen a favorable thickness of the PDMS film. The previous studies indicated that the thickness of the PDMS film was critically dependent on spin duration, spin speed, and the concentration of the PDMS base material. To some extent, a longer spin duration, faster spin speed and lower concentration of PDMS base attributed the thinner PDMS film. However, the thickness of the PDMS layer can’t be controlled by varying the spin speed and spin time to achieve a layer thickness under 5 μm.39 Thinner PDMS layers with an accurately controllable thickness have been fabricated by diluting PDMS base material in some organic solvents, such as hexane, tert butyl alcohol, n-octane, and tetrahydrofuran (THF).38–40 In our case, the THF was used to dilute uncured PDMS base materials. The SERS detection capability of the PDMS-Au MLF composite was evaluated using toluene as the target analyte. Fig. 1 presents the typical morphology of the composite. It indicates that the Au MLF was well dispersed on the Si wafer, and Au nanoparticles together with the interparticle spacing were discriminated unambiguously on the naked Au MLF. With an increase of the concentration of PDMS base material, the surface of the Au MLF was gradually covered. The interparticle spacing disappeared completely by using the pure PDMS base as a coating solution (Fig. 1d). | ||
| Fig. 1 SEM images of the naked Au MLF (a), and coated with PDMS using different concentrations of PDMS base materials, 1.25% (b), 5% (c) and 100% (d). | ||
The corresponding PDMS concentration-dependent SERS spectra of toluene are illustrated in Fig. 2 as well as the concentration intensity of the band at 1003 cm−1. It should be pointed out that the spectral features observed from the composite substrate remained unchanged, indicating the same adsorption configuration of toluene on the composite substrate. It could be observed that the SERS intensity of the band at 1003 cm−1 was dependent on the concentration of the PDMS base material. In the diluted solution, it increased with concentration, and reached a maximum at a concentration of about 1.25%. Then, it decreased remarkably as the concentration increased, and completely disappeared by using pure PDMS base material as the coating solution. It was reasonable to assume that the thickness of the PDMS exhibited a tendency to be thinner with decreasing concentrations of PDMS.39 The present experimental facts demonstrate that the thinner PDMS film resulted in difficulties for trapping the targets which contributed to the weak SERS signal, while the thicker film decreased the propagation of the laser and Raman signal to block the SERS signal dramatically. As a consequence, an appropriate thickness of the PDMS film was essential both for trapping the targets and reducing the attenuation of the laser power and Raman signal. Based on the above experimental facts, the 1.25% PDMS base material in THF was used to fabricate the coating film. Based on the measurements by spectroscopic ellipsometry and AFM, the favorable PDMS thickness of the composite substrate ranged from 129 to 139 nm.
Detection of toluene in water
Generally, the solubility of toluene was quite low in water, the saturated aqueous solution was about 7 mM.15 Fig. 3 presents a series of Raman and SERS spectra of toluene in water on different substrates. Major bands at 786 cm−1, 1004 cm−1, 1031 cm−1, 1210 cm−1 and 1604 cm−1 were observed in the normal Raman spectrum of pure liquid toluene (Fig. 3a), which were assigned to the C–C bending, ring breathing mode, C–C stretching, ring-CH3 stretching and ring-relevant modes.41,42 The bands at 1003 cm−1 and 1030 cm−1 dominated in the SERS spectrum, which were well matched to the corresponding bands of liquid toluene (Fig. 3a and e). It indicated the occurrence of a physical interaction between the Au MLF and toluene, and the toluene was trapped by the PDMS film. An unexpected change was also observed from the bands at 786 cm−1 and 1604 cm−1, i.e. a disappearance of the former and significant enhancement of the latter together with a slight red-shift in frequency. It was mainly due to the surface selection rule for the enhancement effect. The background signal was negligible on the PDMS-Au MLF without adsorption of toluene (Fig. 3d), and it should be noted that no Raman signal was observed from the toluene adsorbed on the PDMS-coated Si wafer, indicating no plasmon enhancements from the PDMS film (as shown in Fig. 3b). Therefore, the observed SERS signal originated from the toluene located at the long distance zone of electromagnetic enhancement. | ||
| Fig. 3 Normal Raman spectrum of liquid toluene (a). SERS spectra of toluene on the PDMS-coated Si wafer (b) and naked Au MLF (c). SERS spectra from PDMS-Au MLF without (d), and with (e), toluene. | ||
As a comparison, the SERS spectrum of toluene adsorbed onto a naked Au MLF substrate is presented as Fig. 3c. The similar spectral features of SERS from the Au MLF with/without PDMS indicated the same interaction between toluene and the substrate. However, the remarkable difference (about one order of magnitude) in the SERS intensity was mainly contributed to by the increase of efficient targets around the composite substrate. Normally, on the naked Au MLF surface, the polyvinylpyrrolidone (PVP), which was added in the preparation of the Au nanoparticles, played the important role in improving the chemical selectivity and weakly affected the adsorption of toluene molecules, due to its hydrogen-bonding properties.43 The PDMS film exhibited a similar function for trapping the targets. Mark and his coworkers estimated the diffusion and partition coefficient, and permeability of aromatic molecules in the PDMS membrane.44 In fact, the lower diffusion led to a stronger target–PDMS interaction, and the enrichment occurred inside the membrane. Moreover, PDMS also showed a hydrophobic interaction with the methyl and alkyl groups of the attached targets due to the van der Waals force between the methyl group of PDMS and alkyl groups of the targets.44 As a consequence, one could assume that the toluene molecules were trapped by the PDMS film efficiently, and it was believed that the PDMS film significantly improved the SERS detection sensitivity.
In order to further evaluate the performance of the PDMS-Au MLF composite, the SERS detection on toluene with different concentrations from 5 mM down to 5 μM was performed on the PDMS-Au MLF composite (as shown in Fig. 4). It can be found that, concomitantly with the decrease of the concentration of toluene solution, the SERS intensity of toluene decreased significantly both on the naked Au MLF and PDMS-Au MLF substrate. By comparison, for the same concentration, the SERS intensity of toluene adsorbed on the composite substrate was much higher than that adsorbed on the naked Au MLF. For example, for the intensity of the band at 1003 cm−1 in the 5 mM toluene solution, the SERS signal was about 9 times stronger than that from the naked Au MLF substrate. Furthermore, the SERS signal was negligible on the naked Au MLF as the concentration was less than 50 μM, while it was observed unambiguously from the composite substrate even though the concentration was reducing down to 5 μM (as shown in Fig. 4b), and it was comparable to that observed from the 0.5 mM toluene solution adsorbed on the naked Au MLF substrate. Therefore, by the spin coating of PDMS strategy, the limit of detection (LOD) of SERS was improved by two orders of magnitude to achieve 5 μM (∼0.5 ppm) for detecting toluene in water.
 | ||
| Fig. 4 SERS spectra of toluene with different concentrations using the PDMS-Au MLF and Au MLF as substrates (a); the concentration dependence of the SERS intensity of the band at 1003 cm−1 (b). | ||
Detection of toluene gas in the atmosphere
In fact, VOCs have been becoming the main pollutants in air, and thus their sensitive detection has attracted considerable attention. Based on the fact that the PDMS film exhibited a high efficiency for trapping aromatic molecules in water, it was worth exploring the performance of the PDMS-Au MLF composite in vapor detection. Due to the high vapor pressure of toluene, it was employed as a model target to evaluate the SERS capability for detecting toluene gas in the open atmosphere at room temperature. As described in the experimental section, the composition was located at nearly pure liquid toluene in the open atmosphere. With the volatilization of toluene in the open system, the target gas diffused around the liquid toluene and the PDMS-Au MLF continuously captured the target gas molecules from the atmosphere. After reaching the maximum SERS intensity, the SERS of toluene gas was subsequently recorded from the PDMS-Au MLF composition as presented in Fig. 5 together with that from the naked Au MLF as a comparison. Obviously, the SERS intensity from the former was about 5 times stronger than that from the latter, indicating the high performance of the PDMS-Au MLF for detecting toluene gas. It demonstrated that the composite could be developed as a promising substrate to detect small aromatic molecules both from aqueous and vapor phases with high sensitivity. Most importantly, this approach was also used to identify the targets by analyzing the fingerprint spectral information which was unique to each target.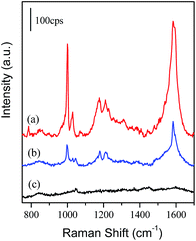 | ||
| Fig. 5 SERS spectra of toluene vapor detected using PDMS-Au MLF (a), Au MLF (b) and a blank PDMS-Au MLF in the atmosphere (c). | ||
Detection of benzene and nitrobenzene in water
As mentioned above, PDMS holds a high performance for trapping the targets due to the interaction with the PDMS film. Therefore, it was believed that the trapping capability for the weaker anchored targets should be decreased significantly, resulting in the decreasing sensitivity of SERS detection. In order to evaluate the generality of this strategy, targets with a similar structure to toluene, including benzene (about 23 mM for the saturated aqueous solution) and nitrobenzene (saturated concentration of 15.4 mM in water), were engaged as the model targets.15,45Fig. 6 presents a series of SERS spectra of benzene with different concentrations adsorbed onto the PDMS-Au MLF and naked Au MLF substrates, respectively. The band at 994 cm−1 dominated in the SERS spectrum, which was assigned to symmetric ring breathing,42 while all of the other Raman bands were comparatively weak. It should be pointed out that, for the saturated benzene solution, the SERS intensity from the composite was about 7–8 times that from the naked Au MLF substrate. The intensity decreased dramatically as the concentration was reduced from saturation to 1 mM, and it almost disappeared on the Au MLF substrate for the 1 mM solution. However, for the PDMS-Au MLF composite substrate, the strongest SERS band of benzene could be distinguished in a 1 mM solution, indicating that the LOD was lowered by about 5 times to 1 mM compared to the LOD of 5 mM for the naked Au MLF substrate. Obviously, the LOD for benzene was higher by at least two orders of magnitude than that of toluene using the same SERS substrate. It indicated that the LOD of this approach was critically dependant on the nature of the targets. Since there are van der Waals forces between the methyl groups of the polymer and the methyl group of toluene, the PDMS film offered a relatively strong hydrophobic interaction with toluene. Moreover, the diffusion coefficients illustrated the interaction between PDMS and the targets, a higher diffusion coefficient suggested a weaker interaction with the PDMS film. It was reported that the diffusion coefficient of toluene was smaller than that of benzene.34,44 Along with this, the trapping capability of PDMS was dependent on the diffusion coefficient, and it was more favorable for capturing toluene than benzene. In contrast with the diffusion coefficient, the permeability in the PDMS film exhibited the opposite effect on the trapping capability for targets, i.e. a higher permeability represented a high performance of PDMS for capturing targets. However, the permeation process depended on both the solubility and diffusion constants. Generally, permeability was higher for hydrophobic target compounds than polar ones, and it increased with hydrophobicity. Mark et al. estimated the permeability of compounds in the sequence of: aromatic > alcohols, and 3C-benzenes ≫ 2C-benzenes.44 Thus, toluene permeated to a greater extent than benzene through the PDMS layer (about 2 times as much than benzene), which reduced the LOD remarkably. As a consequence, our present results are in good agreement with the previous literature.34,44 Furthermore, as the most rigorously detected target, the LOD for benzene was about 1 mM (78 mg L−1), i.e. about 78 ppm, which was comparable with that previously reported on a functionalized Au SERS substrate and was inferior to the thiol modified SERS substrate.15
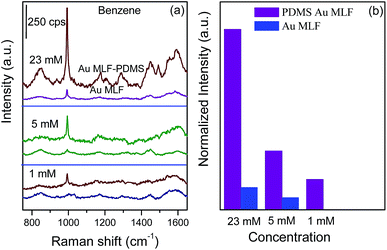 | ||
| Fig. 6 SERS spectra of benzene with different concentrations using the PDMS-Au MLF and Au MLF as substrates (a); the concentration dependence of the SERS intensity of the band at 994 cm−1 (b). | ||
For another target, the characteristic Raman peaks of nitrobenzene could be divided into two catalogues, including the nitro group relevant and the ring-relevant modes. The strongest SERS band at about 1343 cm−1 was assigned to the symmetric stretching mode of the nitro group, while the middle strong band at 1001 cm−1 corresponded to the ring breathing mode.42 These bands dominated in the SERS spectra presented in Fig. 7.
 | ||
| Fig. 7 SERS spectra of nitrobenzene with different concentrations using the PDMS-Au MLF and Au MLF as substrates (a); the concentration dependence of the SERS intensity of the band at 1343 cm−1 (b). | ||
By varying the concentration of the nitrobenzene aqueous solution, it demonstrated that the SERS signal of nitrobenzene from the naked Au MLF was relatively weaker than that from the PDMS-Au MLF composite substrate at the same concentrations. As illustrated in Fig. 7b, the LOD of SERS for nitrobenzene was about 5 μM and 0.5 mM on the PDMS-Au MLF and naked Au MLF substrates, respectively. It should be pointed out that the SERS signal of nitrobenzene with a concentration of 0.5 mM was almost negligible with a very low signal-to-noise on the naked Au MLF substrate (Fig. 7b). Therefore, the comparable LOD was attributed to toluene and nitrobenzene, respectively. Actually, the hydrophobicity and permeability of nitrobenzene lay between the benzene and toluene, the low LOD was mainly contributed to by the interaction of nitro groups with the Au surface, which increased the SERS signal remarkably. The higher permeability for toluene served as compensation against the very weak interaction with the Au surface. Nevertheless, PDMS exhibited a stronger affinity for the methyl or nitro group-substituted aromatic molecules than for benzene.
Conclusions
In summary, a facile strategy was developed to fabricate a PDMS-Au MLF composite substrate for the detection of aromatic molecules in water and in the atmosphere by SERS. The PDMS film was covered onto the Au MLF by the spin coating technique. By considering the influence on the SERS signal and the trapping capability, the concentration of the PDMS base was 1.25% to be used as the spin coating solution to reach a maximum SERS effect. The thickness of the PDMS layer was about 129 nm to 139 nm. The PDMS-Au MLF composite held a higher capability than the naked Au MLF substrate for the targets in water or in the atmospheres. Moreover, the performance was dependent on the nature of the targets, i.e. the detection performance was in the sequence of toluene ≈ nitrobenzene > benzene. The LOD reached was 0.5 ppm, 78 ppm and 0.6 ppm for toluene, benzene and nitrobenzene, respectively. Although some issues, including quantitative analysis and identification on multi-targets, should be addressed, our results demonstrate that the present approach could be developed as a promising tool to detect numerous targets which are non-specifically adsorbed onto metallic plasmonic nanostructures. It opens a window towards general application in the in situ monitoring of pollutants in environmental science.Acknowledgements
This work is supported by the National Nature Science Foundation of China (no 21033007, 21173155, 21303115 and 21473118), the National Instrumentation Program (2011YQ031240402), and the partial financial support from a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The Natural Science Foundation of Jiangsu Province (BK2012187) and the Natural Science Fundamental Research Project of Jiangsu Colleges and Universities (12KJD150011) are gratefully acknowledged.Notes and references
- M. M. Loh, J. I. Levy, J. D. Spengler, E. A. Houseman and D. H. Bennett, Environ. Health Perspect., 2007, 115, 1160–1168 CrossRef CAS PubMed.
- L. Molhave, G. Clausen, B. Berglund, J. De Ceaurriz, A. Kettrup, T. Lindvall, M. Maroni, A. C. Pickering, U. Risse, H. Rothweiler, B. Seifert and M. Younes, Indoor Air, 1997, 7, 225–240 CAS.
- S. Król, B. Zabiegała and J. Namieśnik, Anal. Bioanal. Chem., 2011, 400, 1751–1769 CrossRef PubMed.
- L. C. Sander and S. A. Wise, Anal. Chem., 1987, 59, 2309–2313 CrossRef CAS.
- B. Sharma, R. R. Frontiera, A. I. Henry, E. Ringe and R. P. Van Duyne, Mater. Today, 2012, 15, 16–25 CrossRef CAS.
- R. A. Alvarez-Puebla and L. M. Liz-Marzán, Small, 2010, 6, 604–610 CrossRef CAS PubMed.
- C. L. Haynes, A. D. McFarland and R. P. Van Duyne, Anal. Chem., 2005, 77, 338A–346A CrossRef CAS.
- A. Hakonen, M. Svedendahl, R. Ogier, Z.-J. Yang, K. Lodewijks, R. Verre, T. Shegai, P. O. Anderssonb and M. Käll, Nanoscale, 2015, 7, 9405–9410 RSC.
- A. Hakonen, P. O. Andersson, M. S. Schmidt, T. Rindzevicius and M. Kall, Anal. Chim. Acta, 2015 DOI:10.1016/j.aca.2015.04.010.
- M. Moskovits, Rev. Mod. Phys., 1985, 57, 783–826 CrossRef CAS.
- K. A. Willets and R. P. Van Duyne, Annu. Rev. Phys. Chem., 2007, 58, 267–297 CrossRef CAS PubMed.
- A. Campion and P. Kambhampati, Chem. Soc. Rev., 1998, 27, 241–250 RSC.
- D. W. Li, W. L. Zhai, Y. T. Li and Y. T. Long, Microchim. Acta, 2014, 181, 23–43 CrossRef CAS.
- W. Hill, B. Wehling, C. G. Gibbs, C. D. Gutsche and D. Klockow, Anal. Chem., 1995, 67, 3187–3192 CrossRef CAS.
- C. Marenco, C. J. M. Stirling and J. Yarwood, J. Raman Spectrosc., 2001, 32, 183–194 CrossRef CAS PubMed.
- Y. F. Xie, X. Wang, X. X. Han, X. X. Xue, W. Ji, Z. H. Qi, J. Q. Liu, B. Zhao and Y. Ozaki, Analyst, 2010, 135, 1389–1394 RSC.
- I. Lopez-Tocon, J. C. Otero, J. F. Arenas, J. V. Garcia-Ramos and S. Sanchez-Cortes, Anal. Chem., 2011, 83, 2518–2525 CrossRef CAS PubMed.
- N. S. Myoung, H. K. Yoo and I. W. Hwang, J. Nanophotonics, 2014, 8, 0830831–0830837 CrossRef PubMed.
- C. L. Jones, K. C. Bantz and C. L. Haynes, Anal. Bioanal. Chem., 2009, 394, 303–311 CrossRef CAS PubMed.
- L. G. Olson, R. H. Uibel and J. M. Harris, Appl. Spectrosc., 2004, 58, 1394–1400 CrossRef CAS PubMed.
- J. J. Du and C. Y. Jing, J. Phys. Chem. C, 2011, 115, 17829–17835 CAS.
- Q. An, P. Zhang, J. M. Li, W. F. Ma, J. Guo, J. Hu and C. C. Wang, Nanoscale, 2012, 4, 5210–5216 RSC.
- Z. Y. Bao, X. Liu, Y. Chen, Y. C. Wu, H. L. W. Chan, J. Y. Dai and D. Y. Lei, J. Hazard. Mater., 2014, 280, 706–712 CrossRef CAS PubMed.
- L. E. Kreno, N. G. Greenelth, O. K. Farha, J. T. Hupp and R. P. Van Duyne, Analyst, 2014, 139, 4073–4080 RSC.
- K. Sugikawa, Y. Furukawa and K. Sada, Chem. Mater., 2011, 23, 3132–3134 CrossRef CAS.
- D. Y. Siberio-Pérez, A. G. Wong-Foy, O. M. Yaghi and A. J. Matzger, Chem. Mater., 2007, 19, 3681–3685 CrossRef.
- Y. L. Hu, J. Liao, D. M. Wang and G. K. Li, Anal. Chem., 2014, 86, 3955–3963 CrossRef CAS PubMed.
- K. S. Giesfeldt, R. M. Connatser, M. A. De Jesús, P. Dutta and M. J. Sepaniak, J. Raman Spectrosc., 2005, 36, 1134–1142 CrossRef CAS PubMed.
- J. Olavarría-Fullerton, S. Wells, W. Ortiz-Rivera, M. J. Sepaniak and M. A. De Jesus, Appl. Spectrosc., 2011, 65, 423–428 CrossRef PubMed.
- M. A. De Jesus, K. S. Giesfeldt and M. J. Sepaniak, Appl. Spectrosc., 2004, 58, 1157–1164 CrossRef CAS PubMed.
- M. Wang, B. De Vivo, W. J. Lu and M. Muniz-Miranda, Appl. Spectrosc., 2014, 68, 784–788 CrossRef CAS PubMed.
- R. Gupta and G. U. Kulkarni, ChemSusChem, 2011, 4, 737–743 CrossRef CAS PubMed.
- M. Lamotte, P. F. De Violet, P. Garrigues and M. Hardy, Anal. Bioanal. Chem., 2002, 372, 169–173 CrossRef CAS.
- K. P. Chao, V. S. Wang, H. W. Yang and C. I. Wang, Polym. Test., 2011, 30, 501–508 CrossRef CAS PubMed.
- S. Seethapathy and T. Górecki, Anal. Chim. Acta, 2012, 750, 48–62 CrossRef CAS PubMed.
- G. Frens, Nature (London), Phys. Sci., 1973, 241, 20–22 CrossRef CAS PubMed.
- L. Scarabelli, M. Coronado-Puchau, J. J. Giner-Casares, J. Langer and L. M. Liz-Marzan, ACS Nano, 2014, 8, 5833–5842 CrossRef CAS PubMed.
- M. Bračič, T. Mohan, R. Kargl, T. Griesser, S. Hribernik, S. Köstler, K. Stana-Kleinschek and L. Fras-Zemljič, RSC Adv., 2014, 4, 11955–11961 RSC.
- J. H. Koschwanez, R. H. Carlson and D. R. Meldrum, PLoS One, 2009, 4, e4572 Search PubMed.
- T. Shahal, K. A. Melzeak, C. R. Lowe and E. Gizeli, Langmuir, 2008, 24, 11268–11275 CrossRef CAS PubMed.
- P. Gao and M. J. Weaver, J. Phys. Chem., 1985, 89, 5040–5046 CrossRef CAS.
- P. Gao and M. J. Weaver, J. Phys. Chem., 1989, 93, 6205–6211 CrossRef CAS.
- D. L. Stokes, A. Pal, V. A. Narayanan and T. vo-Dinh, Anal. Chim. Acta, 1999, 399, 265–274 CrossRef CAS.
- E. Boscaini, M. L. Alexander, P. Prazeller and T. D. Mark, Int. J. Mass Spectrom., 2004, 239, 179–186 CrossRef CAS PubMed.
- I. Arslan-Alaton and J. L. Ferry, Appl. Catal., B, 2002, 38, 283–293 CrossRef CAS.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |