Synthesis of poly-functionalized imidazoles via vinyl azides annulation†
Jing Luo‡
,
Wenteng Chen‡,
Jiaan Shao,
Xingyu Liu,
Ke Shu,
Pai Tang and
Yongping Yu*
Zhejiang Province Key Laboratory of Anti-Cancer Drug Research, College of Pharmaceutical Science, Zhejiang University, Hangzhou 310058, P. R. China. E-mail: yyu@zju.edu.cn; Tel: +86-571-88208452
First published on 19th June 2015
Abstract
An efficient annulation of vinyl azides with activated imines has been developed for the synthesis of poly-functionalized imidazole derivatives. This reaction includes thermal decomposition of vinyl azides to 2H-azirines, nucleophilic attack of 2H-azirines with activated imines and subsequent cyclization to desired imidazoles.
Functionalized imidazoles are very important in natural products, pharmaceuticals, and materials science. They constitute platforms for biologically active molecules,1 chemical ligands2 and advanced materials.3 In every application of imidazoles, the substitution pattern of the imidazole ring is of importance as it impacts on both the chemical and physical properties of the resultant molecules. Therefore, various synthetic methods have been developed, such as the functionalization of already constructed imidazole rings4 and other cascade reactions involving cycloaddition reactions of activated isocyanides,5 reaction of amidines with α-haloketones6a,b or nitroallylic acetates.6c Despite these advances, the development of efficient strategies for the construction of poly-functionalized imidazoles is highly desirable.
Vinyl azides have long proved to be versatile building blocks in the synthesis of nitrogen-containing heterocycles. Our group and others have reported the utility in the construction of azaheterocycles, such as anilines,7a pyrrolo[1,2-a]pyrazine,7b 4-aminopyridines,7c pyrazoles,7d imidazoles7e or other heterocycles.7f–j
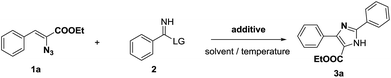
The continued interest in electrophilic reactivity of vinyl azides motivated us to investigate the reaction of vinyl azides with activated imines. We began our studies using vinyl azide (1a) with ethoxyimidate as a model reaction. The ethoxyimidates were usually prepared from nitriles through a Pinner reaction.8 Unexpectedly, the reaction failed to proceed under the Michael addition conditions previously reported (Table 1, entries 1–6).7 To our surprise, the reaction proceeded when using EtOH as solvent in the presence of Et3N (1.0 equiv.) under the 110 °C for 10 h (Table 1, entry 8). The main product was then characterized as 3a in 30% yield. It was found that the reaction could also lead to the formation of 3a in 32% yield without the addition of base (Table 1, entry 9). The low yields were due to the decomposition of vinyl azides.
| Entry | Base | Solvent | LG | T/°C | t/h | Yieldb/% |
|---|---|---|---|---|---|---|
| a Unless otherwise specified, reactions were performed in a sealed tube using 1a (0.1 mmol) and 2 (0.11 mmol) in various solvents (1.0 mL) at different temperature in the presence or absence of the additive (0.1 mmol) for a period of time.b Isolated yields are the means from two independent experiments.c No reaction. | ||||||
| 1 | Et3N | DMF | OEt | 25 | 10 | nrc |
| 2 | Et3N | DMF | OEt | 60 | 10 | Trace |
| 3 | Et3N | EtOH | OEt | 25 | 10 | nrc |
| 4 | t-BuOK | EtOH | OEt | 25 | 10 | nrc |
| 5 | Cs2CO3 | EtOH | OEt | 25 | 10 | nrc |
| 6 | K2CO3 | EtOH | OEt | 25 | 10 | nrc |
| 7 | Et3N | EtOH | OEt | 80 | 10 | Trace |
| 8 | Et3N | EtOH | OEt | 110 | 10 | 30 |
| 9 | — | EtOH | OEt | 110 | 8 | 32 |
| 10 | — | DMF | OEt | 110 | 8 | 37 |
| 11 | — | Toluene | OEt | 110 | 8 | 22 |
| 12 | — | t-BuOH | OEt | 110 | 8 | 92 |
| 13 | — | t-BuOH | Ot-Bu | 110 | 8 | 10 |
| 14 | — | t-BuOH | SCH3 | 110 | 8 | 90 |
| 15 | — | t-BuOH | OEt | 110 | 1 | 23 |
| 16 | — | t-BuOH | OEt | 110 | 4 | 48 |
| 17 | — | t-BuOH | OEt | 110 | 10 | 91 |
In order to improve the yields, we screened other solvents including t-BuOH, DMF and toluene, t-BuOH was found to be favor for this reaction with higher yield of 92% (Table 1, entry 12 vs. entries 9–11). Additionally, the inductive effect of the leaving groups was tested in this reaction. It was surprising to find that only 10% of the desired product was obtained from the t-BuO-substituted imidate, while the formation of product 3a proceeded in near quantitative yield from vinyl azide (1a) with ethyoxyimidate (Table 1, entry 12 vs. entry 13). Gratifyingly, the analogues thioimidate9 gave the desired product 3a in the yield of 90% (Table 1, entry 14). These results showed the importance in the selection of the leaving groups. After optimization of the reaction conditions, an acceptable yield of 92% was obtained in t-BuOH at 110 °C for 8 h (Table 1, entry 12). When the reaction was performed for 10 h, the yields did not show significant difference (Table 1, entry 12 vs. entry 17). But when the reaction times were shorten to 1 h or 4 h, the yield of 3a decreased to 23% and 48%, respectively.
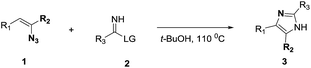
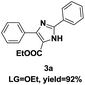
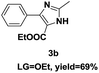
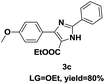
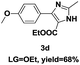
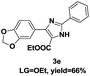
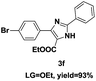
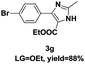
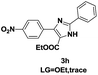
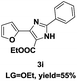
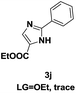
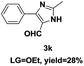
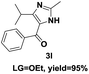
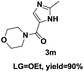
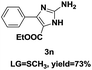
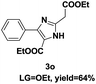
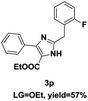
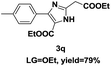
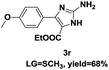
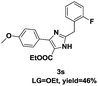
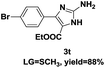
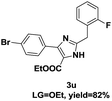
On the basis of our initial results outlined above, we explored the reaction scope. The electronic effects of the substituents adjacent to the vinyl azide 1 were examined. The reaction showed tolerance for various R1 and R2 groups of vinyl azides 1 (Table 2, 3a–3u, except 3h and 3j). For example, when vinyl azides were substituted at the aryl ring (R1) with H or Br, the desired product 3a and 3g were obtained in near quantitative yields of 92% and 93%, respectively. Vinyl azide with heterocycle substituent also smoothly reacted with imidate and provided the desired product 3i in 55% yield. However, when H was substituted at the R1 position of vinyl azide 1, the desired product 3j was obtained in trace (<5%). It may be due to the instability of aziridine formed in situ. Instead of the ester group at the R2 position, vinyl azides substituted with carbonyl group or amide group were also tested in this reaction conditions to give the corresponding products in 28%, 95% and 90% yields, respectively (3k–3m). Moreover, the chemical reactivity of leaving groups (–OEt, –SCH3) on the activated imines 2 was evaluated with various substituted vinyl azides. Imidate and thioamidium reacted with vinyl azides in a similar fashion and the reaction proceeded well, affording the imidazoles in moderate to good yields (Table 2). Additionally, aliphatic substitution as well as bearing an activated methylene on the R3 position of activated imines (2) were compatible for the tandem cyclization (Table 2, 3o–3q, 3s and 3u).
| a Reaction conditions: vinyl azides 1 (1.0 equiv.) and activated imines 2 (1.1 equiv.) in t-BuOH heated in a sealed tube (110 °C). Yields shown are those of the isolated products. | |
|---|---|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
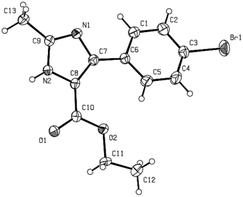
The structures of the products synthesized in this study were characterized from their 1H NMR, 13C NMR spectroscopies and HRMS analysis. The structure of compound 3g was further confirmed by X-ray analysis (Fig. 1) (ESI†).
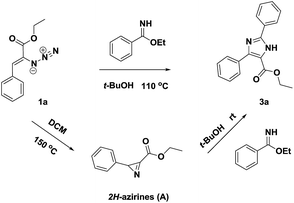
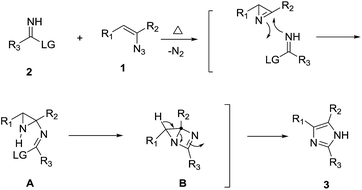
Based on the results of the study, we supposed that the reaction may proceed through 2H-azirines involving tandem cyclization. To this end, we prepared the 2H-aziridine (A)10 by thermolysis of vinyl azide 1a.11 To our expectation, the reaction of 2H-aziridine (A) with ethoxyimidate proceeded well at room temperature to give the desired product 3a (Scheme 1). This result finally supported our hypothesis.
Accordingly, we proposed a mechanism for this tandem cyclization as shown in Scheme 2. Firstly, vinyl azides 1 can be transformed to the corresponding 2H-aziridines in situ by thermal elimination of N2.10 The reaction may proceed through the addition of activated imines 2 to the imino carbon of 2H-aziridine, following by the intramolecular nucleophilic attack following with the ring opening of the strained three-membered ring to obtain the imidazole 3.
In summary, we have developed a concise approach for the synthesis of poly-functionalized imidazoles by tandem annulation of vinyl azides and activated imines, such as imidate or thioamidium. An attractive concept for the synthesis of imidazoles is the utilization of the ring strain and chemical reactivity of 2H-azirines. Aldehyde groups and halide substituents were compatible with the developed reaction conditions, thus providing convenient handles for further functionalization.
Acknowledgements
This project was supported from the National Natural Science Foundation of China (no. 81273356 and 81473074), National Science & Technology Major Projects for "Major New Drugs Innovation and Development" of China (2014ZX09304002-007), the Program for Zhejiang Leading Team of S&T Innovation Team (2011R50014), and Arthritis & Chronic Pain Research Institute, USA, to Y. Yu; National Natural Science Foundation of China (no. 81402778) and the Fundamental Research Funds for the Central Universities (no. 2015QNA7029) to W. Chen; the China Postdoctoral Science Foundation Funded Project (2015M570520) to J. Shao.Notes and references
- For the biological application of imidazoles, see: (a) G. J. Atwell, J. Fan, K. Tan and W. A. Denny, J. Med. Chem., 1998, 41, 4744 CrossRef CAS PubMed; (b) P. B. Koswatta and C. H. Lovely, Nat. Prod. Rep., 2011, 28, 511 RSC; (c) F. V. Lali, A. E. Hunt, S. J. Turner and B. M. J. Foxwell, J. Biol. Chem., 2000, 275, 7395 CrossRef CAS PubMed; (d) R. R. Wexler, W. J. Greenlee, J. D. Irvin, M. R. Goldberg, K. Prendergast, R. D. Smith and P. B. M. W. M. Timmermans, J. Med. Chem., 1996, 39, 625 CrossRef CAS PubMed; (e) S. Riduan and Y. Zhang, Chem. Soc. Rev., 2013, 42, 9055 RSC.
- For publications on imidazole coordination chemistry, see: (a) Y. Sunatsuki, Y. Motoda and N. Matsumoto, Coord. Chem. Rev., 2002, 226, 199 CrossRef CAS; (b) Y. Sunatsuki, R. Kawamoto, K. Fujita, H. Maruyama, T. Suzuki, H. Ishida, M. Kojima, S. Iijima and N. Matsumoto, Coord. Chem. Rev., 2010, 254, 1871 CrossRef CAS PubMed; (c) K. M. Lancaster, J. B. Gerken, A. C. Durrell, J. H. Palmer and H. B. Gray, Coord. Chem. Rev., 2010, 254, 1803 CrossRef CAS PubMed.
- (a) T. Iwamura and S. Nakamura, Polymer, 2013, 54, 4161 CrossRef CAS PubMed; (b) S. Yuan, Y. Deng and D. Sun, Chem.–Eur. J., 2014, 20, 1 CrossRef PubMed.
- C. Sämann, E. Coya and P. Knochel, Angew. Chem., Int. Ed., 2014, 53, 1430 CrossRef PubMed.
- (a) R. V. A. Orru and V. G. Nenajdenko, Isocyanide Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2012, p. 109 Search PubMed; (b) A. V. Lygin and A. de Meijere, Angew. Chem., Int. Ed., 2010, 49, 9094 CrossRef CAS PubMed; (c) A. V. Gulevich, A. G. Zhdanko, R. V. A. Orru and V. G. Nenajdenko, Chem. Rev., 2010, 110, 5235 CrossRef CAS PubMed; (d) U. Schöllkopf, Angew. Chem., Int. Ed. Engl., 1977, 16, 339 CrossRef PubMed.
- (a) H. Bredereck, R. Gompper and D. Hayer, Chem. Ber., 1959, 92, 338 CrossRef CAS PubMed; (b) T. J. Donohoe, M. A. Kabeshov, A. H. Rathi and I. E. D. Smith, Org. Biomol. Chem., 2012, 10, 1093 RSC; (c) T. Kumar, D. Verma, R. F. S. Menna-Barreto, W. O. Valenca, E. N. da Silva Júnior and I. N. N. Namboothiri, Org. Biomol. Chem., 2015, 13, 1996 RSC.
- (a) S. Liu, W. Chen, J. Luo and Y. Yu, Chem. Commun., 2014, 50, 8539 RSC; (b) W. Chen, M. Hu, J. Wu, H. Zou and Y. Yu, Org. Lett., 2010, 12, 3863 CrossRef CAS PubMed; (c) J. Shao, W. Yu, Z. Shao and Y. Yu, Chem. Commun., 2012, 48, 2785 RSC; (d) G. Zhang, H. Ni, W. Chen, J. Shao, H. Liu, B. Chen and Y. Yu, Org. Lett., 2013, 15, 5967 CrossRef CAS PubMed; selected references: (e) L. Xiang, Y. Niu, X. Pang, X. Yang and R. Yan, Chem. Commun., 2015, 51, 6598 RSC; (f) X. Zhu, Y.-F. Wang, F.-L. Zhang and S. Chiba, Chem.–Asian. J., 2014, 9, 2458 CrossRef CAS PubMed; (g) S. Chiba, Chimia, 2012, 66, 377 CrossRef CAS PubMed; (h) Y.-F. Wang, K. K. Toh, E. P. J. Ng and S. Chiba, J. Am. Chem. Soc., 2011, 133, 6411 CrossRef CAS PubMed; (i) E. P. J. Ng, Y.-F. Wang and S. Chiba, Synlett, 2011, 783 CAS; (j) Y.-F. Wang and S. Chiba, J. Am. Chem. Soc., 2009, 131, 12570 CrossRef CAS PubMed.
- (a) V. K. Yadav and K. G. Babu, Eur. J. Org. Chem., 2005, 452 CrossRef CAS PubMed; (b) R. Morgentin, F. Jung, M. Lamorlette, M. Maudet, M. Plé, P. Ménard, G. Pasquet and F. Renaud, Tetrahedron, 2009, 65, 757 CrossRef CAS PubMed.
- S. Caron, L. Wei, J. Douville and A. Ghosh, J. Org. Chem., 2010, 75, 945 CrossRef CAS PubMed.
- 2H-Aziridine (A), yellow oil; 1H NMR (500 MHz, CDCl3) δ 7.32 (m, 3H), 7.15–7.12 (m, 2H), 4.48 (m, 2H), 3.47 (s, 1H), 1.42 (t, J = 7.1 Hz, 3H).
- (a) S. Chiba, Y.-F. Wang, G. Lapointe and K. Narasaka, Org. Lett., 2008, 10, 313 CrossRef CAS PubMed; (b) Ǻ. S. Timén, E. Risberg and P. Somfai, Tetrahedron Lett., 2003, 44, 5339–5341 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1045551. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra07320k |
| ‡ Jing Luo and Wenteng Chen contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |