Hydrogenation of (N,N-disubstituted aminomethyl)nitrobenzenes to (N,N-disubstituted aminomethyl)anilines catalyzed by palladium–nickel bimetallic nanoparticles†
Hailin
Bao
,
Dingsheng
Wang
,
Xinyan
Wang
*,
Chuanjie
Cheng
,
Yadong
Li
and
Yuefei
Hu
*
Department of Chemistry, Tsinghua University, Beijing 100084, P. R. China. E-mail: yfh@mail.tsinghua.edu.cn; wangxinyan@mail.tsinghua.edu.cn; Fax: +86-10-62771149; Tel: +86-10-62795380
First published on 20th May 2015
Abstract
Since palladium-catalysts have strong abilities for both hydrogenation of nitro-group and hydrogenolysis of benzylamine, they have a much lower chemoselectivity for the hydrogenation of (N,N-disubstituted aminomethyl)nitrobenzenes. In this article, component stable Pd–Ni bimetallic nanoparticles were prepared by simply heating RANEY®-Ni and Na2PdCl4 together in water. They demonstrated novel synergistic effects when they were used as a bimetallic catalyst, by which a highly efficient and chemoselective hydrogenation of (N,N-disubstituted aminomethyl)nitrobenzenes to (N,N-disubstituted aminomethyl)anilines was achieved.
Introduction
The catalytic hydrogenation of nitrobenzenes is the most preferred method for the synthesis of anilines due to its high efficiency and easy procedures. Pd-catalysts are often employed for this purpose in both academic and industry laboratories because they have lower costs than Pt-catalysts and higher efficiency than Ni-catalysts.1 Since Pd-catalysts also have a strong ability for the catalytic hydrogenolysis of carbon-heteroatom bonds, they have a much lower chemoselectivity for the hydrogenation of (N,N-disubstituted aminomethyl)nitrobenzenes 1.2,3In 1999, the first small-molecule, non-peptide CCR5 antagonist TAK-779 was reported to have highly potent and selective anti-HIV-1 activity.4 As shown in Fig. 1, its molecule contains a structural unit of (N,N-disubstituted aminomethyl)aniline 2. In recent years, the syntheses of structural units 2 have been gaining importance because they have been recognized as key pharmacophores and synthetic intermediates in drug discovery.5–7 The most convenient synthesis of 2 can be achieved by chemoselective reduction of 1. Unfortunately, the most reliable method for this conversion was the dissolving metal reduction5 rather than the catalytic hydrogenation.7
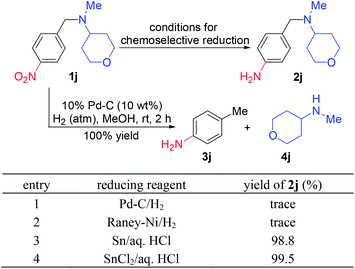
As shown in Scheme 1, a detailed study was reported by Hashimoto et al.5f for the synthesis of N-[(4-aminophenyl)-methyl]tetrahydro-N-methyl-2H-pyran-4-amine (2j) from N-[(4-nitrophenyl)methyl]tetrahydro-N-methyl-2H-pyran-4-amine (1j). Under the catalytic hydrogenation conditions, the desired product 2j was obtained in trace yield over Pd–C or RANEY®-Ni catalyst (entries 1 and 2). Finally, the combination of SnCl2/aq. HCl was employed as a reducing reagent for this conversion. The experiment in entry 1 was carefully repeated in our laboratory and the benzyl–nitrogen bond in 1j was hydrogenolyzed completely to give the corresponding 3j and 4j.
Herein, we would like to report a convenient preparation of Pd–Ni bimetallic nanoparticles. When they were used as a bimetallic catalyst, a general method was developed for highly efficient and chemoselective hydrogenation of 1 to 2.
Results and discussion
Traditionally, the chemoselectivity of Pd-catalysts are modulated by using the catalyst poisons. For example, PbO/PbAc2 or quinoline is employed for such purpose in Lindlar catalysts.8 Rosenmund reduction can be well modulated by in situ adding thioquinanthrene or thiourea into the hydrogenation processes.9 Pd–C(en) is a versatile catalyst for chemoselective hydrogenations, in which ethylenediamine is used as the poison.10 However, these reported catalytic systems are not all suitable for the chemoselective hydrogenation of 1 to 2. As shown in Scheme 2, this conversion was first achieved in our previous work,11 but the concentrated aqueous HCl was used as the poison for the Pd–C catalyst.Recently, the catalytic applications of bimetallic nanoparticles have been developing quickly.12 These bimetallic catalysts often showed novel catalytic activity and chemoselectivity based on their unique electronic effect (or ligand effect) and geometric effect (or strain effect). Many methods have been reported for the preparation of bimetallic catalysts in literature. However, our attention was attracted by several early reported references,13 in which the bimetallic catalysts were prepared by simply mixing a RANEY®-metal [M = Ni(0), Cu(0)] with a noble metal precursor [M1 = Pt(IV), Ru(III), Au(III), Pd(II)] together. Since the RANEY®-metal is a porous solid and has low redox potential, the noble metal precursor is reduced to a zero-valent metal [M1(0)] to disperse on the surface of the RANEY®-metal. In these preparations, RANEY®-metal plays three roles as a reducing reagent, a support and one component of the bimetallic catalyst. Thus, we were inspired to prepare a Pd–Ni bimetallic catalyst by the similar method. As shown in Scheme 3, when the mixture of RANEY®-Ni (99 wt%) and Na2PdCl4 (containing 1 wt% of Pd) in water was heated at 120 °C for 6 h in an autoclave, a component stable Pd–Ni bimetallic catalyst was obtained as black powders.
ICP analyses showed that Pd/Ni ratio in this bimetallic catalyst was about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
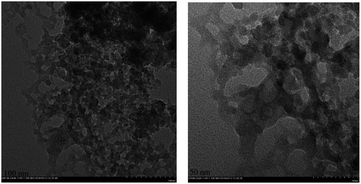
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 ± 3 (by wt%). TEM images showed that the bimetallic catalyst was nanosized particles (Fig. 2). Since it contains only 1 wt% of Pd(0), its XRD patterns had no significant differences with RANEY®-Ni (Fig. 3) and the peak of only Pd(111) was observed.
99 ± 3 (by wt%). TEM images showed that the bimetallic catalyst was nanosized particles (Fig. 2). Since it contains only 1 wt% of Pd(0), its XRD patterns had no significant differences with RANEY®-Ni (Fig. 3) and the peak of only Pd(111) was observed.
Then, the catalytic property of this Pd–Ni bimetallic catalyst was tested by using the hydrogenation of 4-(N,N-dimethyl-aminomethyl)nitrobenzene (1a) into 4-(N,N-dimethyl-aminomethyl)aniline (2a) as a model reaction. As shown in entry 1 in Table 1, 2a was obtained in 90% yield and 93% chemoselectivity over Pd–C catalyst. Low conversion of 1a was observed over RANEY®-Ni and a mixture was obtained caused by the hydrogenation intermediates (entry 2).14 As was expected, 1a was hydrogenated into 2a in quantitative yield and chemoselectivity over Pd–Ni bimetallic catalyst (entry 3). Since this experiment employed the same amounts of palladium (net weight) as that in entry 1 and nickel (net weight) as that in entry 2, the Pd–Ni bimetallic catalyst clearly demonstrated its advantages and synergistic effects. Use of 30 wt% of the Pd–Ni bimetallic catalyst gave the same excellent results (entry 4), while use of 20 wt% of the Pd–Ni bimetallic catalyst resulted in low conversion of 1a (entry 5). However, this problem can be solved easily by slightly increasing the hydrogen pressure (entries 6 and 7).
| Entry | Catalyst (wt%) | Net weight of metal (mg) | Time (min) | Yield of 2ab (%) | Selectivity (%) |
|---|---|---|---|---|---|
| a A mixture of 1a (180 mg, 1 mmol) and a catalyst in MeOH (10 mL) was stirred under H2 at room temperature and atmospheric pressure (on an atmospheric pressure hydrogenation apparatus). b Separated yield was obtained. c 85% conversion of 1a was obtained. d The hydrogenation proceeded under 80 psi. | |||||
| 1 | 5% Pd–C (10) | Pd (0.9) | 120 | 90 | 95 |
| 2 | RANEY®-Ni (50) | Ni (90) | 150 | 85c | Mixture |
| 3 | 1% Pd–Ni (50) | Pd (0.9), Ni (89.1) | 50 | 99 | 100 |
| 4 | 1% Pd–Ni (30) | Pd (0.54), Ni (53.5) | 90 | 99 | 100 |
| 5 | 1% Pd–Ni (20) | Pd (0.36), Ni (25.6) | 140 | 85 | 100 |
| 6 | 1% Pd–Ni (10) | Pd (0.18), Ni (17.8) | 180 | 55 | 100 |
| 7d | 1% Pd–Ni (5) | Pd (0.09), Ni (8.91) | 210 | 93 | 100 |
Unlike the commercial RANEY®-Ni catalyst, Pd–Ni bimetallic catalyst (in both wet and dry forms) is stable to air and no auto-ignition has occurred so far. However, its catalytic activity was influenced significantly by the reaction solvents. As shown in Table 2, all tested lower alcohols were excellent solvents for this catalytic hydrogenation (entries 1–4). But non-alcohol solvents, both polar solvents (entries 5–6) and non-polar solvents (entries 7–9), were not suitable for this purpose. This phenomenon may be partly caused by the fact that the Pd–Ni bimetallic nanoparticles could be fully dispersed in the lower alcohols, while it formed agglomerated particles in the non-alcohol solvents.
| Entry | Solvent | Timeb (min) | Yield of 2ac (%) |
|---|---|---|---|
| a A mixture of 1a (180 mg, 1 mmol) and Pd–Ni bimetallic catalyst in the tested solvent (10 mL) was stirred under H2 at room temperature and atmospheric pressure (on an atmospheric pressure hydrogenation apparatus). b The time was when the absorption of hydrogen ceased. c Separated yield was obtained. | |||
| 1 | MeOH | 90 | 99 |
| 2 | EtOH | 110 | 96 |
| 3 | i-PrOH | 210 | 94 |
| 4 | n-BuOH | 330 | 93 |
| 5 | THF | 600 | 81 |
| 6 | EtOAc | 720 | 75 |
| 7 | DCM | 480 | 47 |
| 8 | Cyclohexane | 300 | 5 |
| 9 | Toluene | 300 | 4 |
Finally, the standard procedure was assigned as shown in Scheme 4 and the reaction scope was tested. There was no difference caused by the substituted position of a nitro-group on the benzene ring (2a–2c). But, the chemoselectivity was influenced significantly by the size of the group substituted on the benzylamine, and decreased sharply by the increase of the size (2d–2h, 2n–2p). To improve the chemoselectivity of 2h, the hydrogenation of 1h at 0 °C was tested. Unfortunately, the chemoselectivity of 2h increased 9% while the yield of 2h decreased 7% because the hydrogenation of 1h automatically stopped within 16 h. We interestingly observed that the excellent chemoselectivity could be achieved as long as one group is a methyl group (2i–2j, 2n). The cyclic substituents seemed to be “small-sized groups” and gave both quantitative yields and chemoselectivity (2k–2m). For easy purification of the analytical samples, three products were converted into their amides derivatives (2o–2q). To our surprise, 2r was prepared smoothly in the presence of 40 wt% of Pd–Ni bimetallic catalysts.
Since the Pd–Ni bimetallic catalyst has magnetic property, it can be separated and recovered conveniently in work-up process with a magnetic stirring bar, which could greatly facilitate the recycling of the catalyst. As shown in Table 3, a recycling study shows that the catalytic activity of the Pd–Ni bimetallic catalyst decreased steadily in the first three recycles (entries 1–3). But, the yield and chemoselectivity of the product 2a were not influenced by prolonging the reaction time. Unfortunately, its catalytic activity dropped sharply in the fourth recycle (entry 4), presumably because of the loss of the “nonmagnetic palladium metal” during the separation and recovery of the catalyst. In fact, the proportion of palladium metal in the Pd–Ni bimetallic catalyst in the fourth recycle was 18% lower than that in the fresh catalyst.
| Recycle times | Timeb (min) | Yield of 2ac(%) |
|---|---|---|
| a A mixture of 1a (180 mg, 1 mmol) and Pd–Ni bimetallic catalyst in MeOH (10 mL) was stirred under H2 at room temperature and atmospheric pressure (on an atmospheric pressure hydrogenation apparatus). b The time was when the absorption of hydrogen ceased. c Separated yield was obtained. d Only 1a (25%) and 2a (75%) were detected in the reaction mixture by 1H NMR. | ||
| 1 | 90 | 99 |
| 2 | 210 | 97 |
| 3 | 450 | 94 |
| 4 | 900 | 75d |
Conclusions
A highly efficient and chemoselective hydrogenation of (N,N-disubstituted aminomethyl)nitrobenzenes to (N,N-disubstituted aminomethyl)anilines was developed. Its success was due to the novel catalytic activity of the Pd–Ni bimetallic nanoparticles. In literature, the reported bimetallic catalyst usually demonstrated higher catalytic activity than both componential metals by their synergistic effects. However, our Pd–Ni bimetallic catalyst demonstrated that the catalytic activity of RANEY®-Ni was enhanced and the chemoselectivity of Pd was enhanced. This hydrogenation may be widely used since the Pd–Ni bimetallic catalyst can be prepared conveniently and the products are very useful in the drug discovery.Experimental section
General information
All melting points were determined on a Yanaco melting point apparatus and were uncorrected. IR spectra were recorded as KBr pellets on a Nicolet FT-IR 5DX spectrometer. All spectra of 1H NMR and 13C NMR were recorded on a JEOL JNM-ECA 300 or 400 spectrometers in CDCl3 and TMS was used as an internal reference. HRMS were obtained on a Bruker micrOTOF-Q II spectrometer.The similar procedure was used for the chemoselective hydrogenation of 1b–1r to 2b–2r. In some cases, the flash chromatography was required for the purification of the products.
3-(N,N-Dimethyl-aminomethyl)aniline (2b)15b. Yellowish oil, 98% yield. 1H NMR (CDCl3, 400 MHz) δ 7.11–7.09 (m, 1H), 6.69–6.57 (m, 3H), 3.62 (s, 2H), 3.33 (s, 2H), 2.23 (s, 6H); 13C NMR (CDCl3, 100 MHz) δ 146.4, 140.2, 129.1, 119.4, 115.6, 113.9, 64.4, 45.4 (2C).
2-(N,N-Dimethyl-aminomethyl)aniline (2c)15c. Yellowish oil, 97% yield. 1H NMR (CDCl3, 400 MHz) δ 7.08–6.96 (m, 2H), 6.66–6.62 (m, 2H), 3.40 (s, 2H), 2.19 (s, 6H); 13C NMR (CDCl3, 100 MHz) δ 147.0, 130.3, 128.2, 123.3, 117.5, 115.4, 63.4, 44.9 (2C).
4-(N,N-Diethyl-aminomethyl)aniline (2d). Yellowish oil, 98% yield. IR (KBr) v 3449, 2969, 2801, 1620, 1616, 1281 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.10 (d, 2H, J = 8.24 Hz), 6.63 (d, 2H, J = 8.72 Hz), 3.59 (s, 2H), 3.46 (s, 2H), 2.50 (q, 4H, J = 7.36 Hz), 1.03 (t, 6H, J = 7.32 Hz); 13C NMR (CDCl3, 100 MHz) δ 145.0, 130.1 (2C), 129.5, 114.9 (2C), 56.8, 46.4 (2C), 11.6 (2C); HRMS (ESI-TOF) (m/z): calcd for C11H18N2, [M + H]+ 179.1543; found 179.1545.
4-(N,N-Dipropyl-aminomethyl)aniline (2e). Yellowish oil, 98% yield. IR (KBr) v 3349, 2798, 1621, 1516, 1459, 1274, 1173 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.10 (d, 2H, J = 11.0 Hz), 6.64 (d, 2H, J = 11.4 Hz), 3.55 (s, 2H), 3.43 (s, 2H), 2.34 (t, 4H, J = 10.08 Hz), 1.49–1.42 (m, 4H), 0.84 (t, 6H, J = 10.08 Hz); 13C NMR (CDCl3, 100 MHz) δ 144.8, 130.0 (2C), 129.8, 114.8 (2C), 57.9, 55.6 (2C), 20.1 (2C), 11.9 (2C); HRMS (ESI-TOF) (m/z): calcd for C13H22N2, [M + H]+ 207.1856; found 207.1857.
4-(N,N-Diisopyl-aminomethyl)aniline (2f). Yellowish oil, 90% yield. IR (KBr) v 3353.12, 2964, 2811, 1620, 1514, 1462, 1272 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.15 (d, 2H, J = 8.25 Hz), 6.63 (d, 2H, J = 8.58 Hz), 3.52 (s, 4H), 3.02–2.97 (m, 2H), 1.01 (S, 6H), 0.99 (S, 6H); 13C NMR (CDCl3, 75 MHz) δ 144.5, 133.0, 128.9 (2C), 114.9 (2C), 48.1, 47.3 (2C), 20.7 (4C); HRMS (ESI-TOF) (m/z): calcd for C13H22N2, [M + H]+ 207.1856; found 207.1860.
4-(N,N-Dibutyl-aminomethyl)aniline (2g). Yellowish oil, 89% yield. IR (KBr) v 3355, 2956, 2796, 1621, 1515, 1274 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.09 (d, 2H, J = 8.28 Hz), 6.63 (d, 2H, J = 8.28 Hz), 3.58 (s, 2H), 3.44 (s, 2H), 2.37 (t, 4H, J = 7.32 Hz), 1.43–1.41 (m, 4H), 1.30–1.26 (m, 4H), 0.87 (t, 6H, J = 7.32 Hz); 13C NMR (CDCl3, 75 MHz) δ 144.9, 129.9 (2C), 129.8, 114.9 (2C), 57.9, 53.2 (2C), 29.1 (2C), 20.6 (2C), 14.1 (2C); HRMS (ESI-TOF) (m/z): calcd for C15H26N2, [M + H]+ 235.2169; found 235.2165.
4-(N,N-Dicyclohexyl-aminomethyl)aniline (2h)11. Yellowish oil, 80% yield. 1H NMR (CDCl3, 400 MHz) δ 7.14 (d, 2H, J = 11.0 Hz), 6.62 (d, 2H, J = 10.6 Hz), 3.62 (s, 2H), 3.54 (s, 2H), 2.54–2.49 (m, 2H), 1.73–1.15 (m, 20H); 13C NMR (CDCl3, 75 MHz) δ 144.3, 133.5, 128.7 (2C), 114.9 (2C), 57.4 (2C), 49.3, 31.9 (4C), 26.5 (4C), 26.3 (2C).
4-(N-Methyl-N-cyclohexyl-aminomethyl)aniline (2i)5e. Yellowish oil, 99% yield. 1H NMR (CDCl3, 300 MHz) δ 7.09 (d, 2H, J = 8.25 Hz), 6.64 (d, 2H, J = 8.24 Hz), 3.57 (s, 2H), 3.44 (s, 2H), 2.44–2.39 (m, 1H), 2.16 (s, 3H), 1.85–1.16 (m, 10H); 13C NMR (CDCl3, 75 MHz) δ 144.9, 130.0, 129.8 (2C), 114.9 (2C), 62.1, 57.2, 37.4, 28.6 (2C), 26.4, 26.0 (2C).
4-[N-Methyl-N-(tetrahydropyran-4-yl)-aminomethyl]aniline (2j)5f. Yellowish oil, 98% yield. 1H NMR (CDCl3, 400 MHz) δ 7.09 (d, 2H, J = 7.76 Hz), 6.64 (d, 2H, J = 8.24 Hz), 4.00–4.03 (m, 2H), 3.49 (s, 2H), 3.39 (s, 2H), 3.38–3.35 (m, 2H), 2.65–2.63 (m, 1H), 2.20 (s, 3H), 1.75–1.68 (m, 4H); 13C NMR (CDCl3, 75 MHz) δ 145.3, 130.0 (2C), 129.0, 115.0 (2C), 67.6 (2C), 59.3, 57.2, 37.2, 29.1 (2C).
4-(Pyrrolidin-1-yl)methyl aniline (2k). Yellowish oil, 99% yield. IR (KBr) v 3372, 2929, 1623, 1582, 1382, 1288 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.11 (d, 2H, J = 8.24 Hz), 6.64 (d, 2H, J = 8.24 Hz), 3.60 (s, 2H), 3.50 (s, 2H), 2.49–2.46 (m, 4H), 1.78–1.75 (m, 4H); 13C NMR (CDCl3, 100 MHz) δ 145.2, 130.1 (2C), 129.3, 114.9 (2C), 60.1, 53.9 (2C), 23.3 (2C); HRMS (ESI-TOF) (m/z): calcd for C11H16N2, [M + H]+ 177.1386; found 177.1388.
4-(Piperidin-1-yl)methyl aniline (2l)5d. Yellowish oil, 98% yield. 1H NMR (CDCl3, 400 MHz) δ 7.09 (d, 2H, J = 8.28 Hz), 6.63 (d, 2H, J = 8.24 Hz), 3.60 (s, 2H), 3.38 (s, 2H), 2.42–2.30 (m, 4H), 1.61–1.52 (m, 4H), 1.42–1.38 (m, 2H); 13C NMR (CDCl3, 100 MHz) δ 145.2, 130.4 (2C), 128.1, 114.8 (2C), 63.3, 54.2 (2C), 25.9 (2C), 24.4.
4-(Morpholin-1-yl)methyl aniline (2m). Yellowish solid, mp 100–102 °C (lit.15d mp 100–102 °C), 97% yield. 1H NMR (CDCl3, 300 MHz) δ 7.10 (d, 2H, J = 8.24 Hz), 6.64 (d, 2H, J = 8.25 Hz), 3.70–3.67 (m, 4H), 3.62 (s, 2H), 3.37 (s, 2H), 2.42–2.39 (m, 4H); 13C NMR (CDCl3, 75 MHz) δ 145.4, 130.3 (2C), 127.5, 114.9 (2C), 67.0 (2C), 63.0, 53.5 (2C).
4-(N-Methyl-N-phenyl-aminomethyl)aniline (2n)15e. Yellowish oil, 99% yield. 1H NMR (CDCl3, 300 MHz) δ 7.21–7.19 (m, 2H), 6.75–6.58 (m, 7H), 4.38 (s, 2H), 3.55 (s, 2H), 2.93 (s, 3H); 13C NMR (CDCl3, 75 MHz) δ 149.9, 145.1, 129.0 (2C), 128.6, 127.9 (2C), 116.3, 115.2 (2C), 112.4 (2C), 56.0, 38.1.
N-[4-(N-Ethyl-N-phenyl-aminomethyl)phenyl]acetamide (2o). Yellowish oil, 88% yield. IR (KBr) v 3285, 3185, 2967, 1658, 1598, 1505, 1408, 1248 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.42–7.14 (m, 7H), 6.69–6.63 (m, 3H), 4.46 (s, 2H), 3.44 (q, 2H, J = 6.87 Hz), 2.14 (s, 3H), 1.18 (t, 3H, J = 6.87 Hz); 13C NMR (CDCl3, 75 MHz) δ 168.4, 148.3, 136.5, 135.1, 129.1 (2C), 127.0 (2C), 120.2 (2C), 116.0, 112.1 (2C), 53.4, 45.0, 24.3, 12.0; HRMS (ESI-TOF) (m/z): calcd for C17H20N2O, [M + Na]+: 291.1468; found 291.1463.
N-[4-(N-Butyl-N-phenyl-aminomethyl)phenyl]acetamide (2p). Yellowish oil, 85% yield. IR (KBr) v 3286, 2952, 2867, 1659, 1599, 1505, 1408, 1317 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.25–7.13 (m, 7H), 6.67–6.63 (m, 2H), 4.48 (s, 2H), 3.33–3.38 (m, 2H), 2.15 (s, 3H), 1.62–1.60 (m, 2H), 1.35–1.33 (m, 2H), 0.94 (t, 3H, J = 9.64 Hz); 13C NMR (CDCl3, 75 MHz) δ 168.3, 148.5, 136.4, 135.1, 129.1 (2C), 127.0 (2C), 120.2 (2C), 115.9, 112.1 (2C), 54.0, 51.0, 29.2, 24.4, 20.3, 13.9; HRMS (ESI-TOF) (m/z): calcd for C19H24N2O, [M + Na]+ 319.1781; found 319.1779.
N-[2-[N-Methyl-N-(2-chlorophenyl)-aminomethyl]phenyl]acetamide (2q). Yellowish oil, 88% yield. IR (KBr) v 3260, 2854, 2803, 1686, 1516, 1443, 1304 cm−1; 1H NMR (CDCl3, 300 MHz) δ 9.82 (s, 1H), 8.22–8.19 (m, 1H), 7.40–7.02 (m, 7H), 4.17 (s, 2H), 2.62 (s, 3H), 2.16 (s, 3H); 13C NMR (CDCl3, 75 MHz) δ 168.6, 148.4, 138.3, 130.5, 130.2, 129.5, 128.6, 127.8, 125.3, 125.2, 123.4, 121.9, 121.6, 58.8, 41.9, 24.7; HRMS (ESI-TOF) (m/z): calcd for C16H17ClN2O, [M + Na]+ 311.0922; found 311.0920.
Tris-(4-aminobenzyl)-amine (2r)15f. Yellow gum, 98% yield. 1H NMR (CDCl3, 300 MHz) δ 7.12 (d, 6H, J = 8.25 Hz), 6.66 (d, 6H, J = 8.25 Hz), 3.66 (s, 6H), 3.60 (s, 6H); 13C NMR (CDCl3, 75 MHz) δ 145.1 (3C), 130.4 (3C), 129.2 (6C), 115.0 (6C), 52.5 (3C).
Acknowledgements
This work was supported by NNSF of China (nos 21221062, 21372142 and 21472107).Notes and references
- (a) S. Nishimura, Handbook of heterogeneous catalytic hydrogenation for organic synthesis, John Wiley & Sons, Inc., New York, 2001, ch. 9 Search PubMed; (b) P. N. Rylander, Hydrogenation methods, Academic Press, London, 1985, ch. 8 Search PubMed.
- (a) S. Nishimura, Handbook of heterogeneous catalytic hydrogenation for organic synthesis, John Wiley & Sons, Inc., New York, 2001, ch. 13 Search PubMed; (b) P. N. Rylander, Hydrogenation methods, Academic Press, London, 1985, ch. 13 Search PubMed.
- In general, the order of ease of hydrogenolysis of benzylamines is tertiary > secondary > primary. N,N-Disubstituted benzylamine smoothly underwent a hydrogenolysis over Pd–C catalyst, while benzylamine and N-monosubstituted benzylamine usually stayed intact. Therefore, only N,N-disubstituted benzylamines were suitable to be used as protective groups, see: T. W. Greene and P. G. M. Wuts, Protective Groups in Organic Synthesis, John Wiley & Sons, Inc., New York, 3rd edn, 1999 Search PubMed.
- M. Baba, O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro and M. Fujino, Proc. Natl. Acad. Sci. U. S. A, 1999, 96, 5698–5703 CrossRef CAS.
- Selected references for dissolving metal reductions: (a) X. Liang, H. Pei, L. Ma, Y. Ran, J. Chen, G. Wang and L. Chen, Molecules, 2014, 19, 6163–6183 CrossRef PubMed; (b) H.-J. Kim, M. I. El-Gamal, Y. S. Lee and C.-H. Oh, Bull. Korean Chem. Soc., 2013, 34, 2480–2486 CrossRef CAS; (c) H. Konno, S. Aimoto, S. O. Smith, K. Nosaka and K. Akaji, Bioorg. Med. Chem., 2009, 17, 5769–5774 CrossRef CAS PubMed; (d) H.-R. Tsou, M. Otteng, T. Tran, M. B. Floyd Jr, M. Reich, G. Birnberg, K. Kutterer, S. Ayral-Kaloustian, M. Ravi, R. Nilakantan, M. Grillo, J. P. McGinnis and S. K. Rabindran, J. Med. Chem., 2008, 51, 3507–3525 CrossRef CAS PubMed; (e) B. Lagu, C. Gerchak, M. Pan, C. Hou, M. Singer, R. Malaviya, M. Matheis, G. Olini, D. Cavender and M. Wachter, Bioorg. Med. Chem. Lett., 2007, 17, 4382–4386 CrossRef CAS PubMed; (f) H. Hashimoto, T. Ikemoto, T. Itoh, H. Maruyama, T. Hanaoka, M. Wakimasu, H. Mitsudera and K. Tomimatsu, Org. Process Res. Dev., 2002, 6, 70–73 CrossRef CAS; (g) G. Ronsisvalle, O. Prezzavento, A. Marrazzo, F. Vittorio, M. Massimino, G. Murari and S. Spampinato, J. Med. Chem., 2002, 45, 2662–2665 CrossRef CAS PubMed; (h) M. Mitsuya, K. Kobayashi, K. Kawakami, A. Satoh, Y. Ogino, T. Kakikawa, N. Ohtake, T. Kimura, H. Hirose, A. Sato, T. Numazawa, T. Hasegawa, K. Noguchi and T. Mase, J. Med. Chem., 2000, 43, 5017–5029 CrossRef CAS PubMed.
- Selected references for catalytic transfer hydrogenation: (a) J. Ma, D. Chen, K. Lu, L. Wang, X. Han, Y. Zhao and P. Gong, Eur. J. Med. Chem., 2014, 86, 257–269 CrossRef CAS PubMed; (b) Y. Lu, T. Ran, G. Lin, Q. Jin, J. Jin, H. Li, H. Guo, T. Lu and Y. Wang, Chem. Pharm. Bull., 2014, 62, 238–246 CrossRef CAS; (c) M. Koufaki, C. Kiziridi, P. Papazafiri, A. Vassilopoulos, A. Varro, Z. Nagy, A. Farkas and A. Makriyannis, Bioorg. Med. Chem., 2006, 14, 6666–6678 CrossRef CAS PubMed.
- Selected references for catalytic hydrogenation: (a) B. J. Davie, C. Valant, J. M. White, P. M. Sexton, B. Capuano, A. Christopoulos and P. J. Scammells, J. Med. Chem., 2014, 57, 5405–5418 CrossRef CAS PubMed; (b) C. K. Arnatt, S. A. Zaidi, Z. Zhang, G. Li, A. C. Richardson, J. L. Ware and Y. Zhang, Eur. J. Med. Chem., 2013, 69, 647–658 CrossRef CAS PubMed; (c) D. Shetty, J. M. Jeong, Y. J. Kim, J. Y. Lee, L. Hoigebazar, Y.-S. Lee, D. S. Lee and J.-K. Chung, Bioorg. Med. Chem., 2012, 20, 5941–5947 CrossRef CAS PubMed; (d) J. Dietrich, C. Hulme and L. H. Hurley, Bioorg. Med. Chem., 2010, 18, 5738–5748 CrossRef CAS PubMed; (e) J. T. Kuethe, A. Wong, C. Qu, J. Smitrovich, I. W. Davies and D. L. Hughes, J. Org. Chem., 2005, 70, 2555–2567 CrossRef CAS PubMed; (f) J. Cao, J. R. Lever, T. Kopajtic, J. L. Katz, A. T. Pham, M. L. Holmes, J. B. Justice and A. H. Newman, J. Med. Chem., 2004, 47, 6128–6136 CrossRef CAS PubMed; (g) F. Stephen and S. J. A. Pope, J. Am. Chem. Soc., 2003, 125, 10526–10527 CrossRef PubMed.
- Selected references, see: (a) G. Vile, N. Almora-Barrios, S. Mitchell, N. Lopez and J. Perez-Ramirez, Chem.–Eur. J., 2014, 20, 5926–5937 CrossRef CAS PubMed; (b) J. G. Ulan, E. Kuo and W. F. Maier, J. Org. Chem., 1987, 52, 3126–3132 CrossRef CAS.
- Selected references, see: (a) H. Britton, D. Catterick, A. N. Dwyer, A. H. Gordon, S. G. Leach, C. McCormick, C. E. Mountain, A. Simpson, D. R. Stevens, M. W. J. Urquhart, C. E. Wade, J. Warren, N. F. Wooster and A. Zilliox, Org. Process Res. Dev., 2012, 16, 1607–1617 CrossRef CAS; (b) E. Mosettig and R. Mozingo, Org. React., 1948, 4, 362–377 CAS , a review.
- (a) K. Hattori, H. Sajiki and K. Hirota, Tetrahedron, 2001, 57, 4817–4824 CrossRef CAS; (b) K. Hattori, H. Sajiki and K. Hirota, Tetrahedron, 2001, 57, 2109–2114 CrossRef CAS; (c) K. Hattori, H. Sajiki and K. Hirota, Tetrahedron, 2000, 56, 8433–8441 CrossRef CAS; (d) K. Hattori, H. Sajiki and K. Hirota, Tetrahedron Lett., 2000, 41, 5711–5714 CrossRef CAS; (e) H. Sajiki, K. Hattori and K. Hirota, Chem.–Eur. J., 2000, 6, 2200–2204 CrossRef CAS; (f) H. Sajiki, K. Hattori and K. Hirota, J. Org. Chem., 1998, 63, 7990–7992 CrossRef CAS.
- C. Cheng, X. Wang, L. Xing, B. Liu, R. Zhu and Y. Hu, Adv. Synth. Catal., 2007, 349, 1775–1780 CrossRef CAS PubMed.
- (a) S. Cai, D. Wang, Z. Niu and Y. Li, Chin. J. Catal., 2013, 34, 1964–1974 CrossRef CAS; (b) W. Yu, M. D. Porosoff and J. G. Chen, Chem. Rev., 2012, 112, 5780–5817 CrossRef CAS PubMed; (c) X. Liu, D. Wang and Y. Li, Nano Today, 2012, 7, 448–466 CrossRef CAS PubMed; (d) B. Coqa and F. Figueras, J. Mol. Catal. A: Chem., 2001, 173, 117–134 CrossRef.
- (a) C. Montassier, J. C. Menezo, J. Naja, P. Granger, J. Barbier, P. Sarrazin and B. J. Didillon, J. Mol. Catal., 1994, 91, 119–128 CrossRef CAS; (b) C. Montassier, J. C. Menezo, J. Naja, J. Barbier, J. M. Dominguez, P. Sarrazin and B. J. Didillon, J. Mol. Catal., 1994, 91, 107–117 CrossRef CAS; (c) C. Montassier, J. C. Menezo, J. Moukolo, J. Naja, L. C. Hoang and J. Barbier, J. Mol. Catal., 1991, 70, 65–84 CrossRef CAS; (d) S. Nishimura, Bull. Chem. Soc. Jpn., 1959, 32, 61–64 CrossRef CAS; (e) E. Lieber and G. B. L. Smith, J. Am. Chem. Soc., 1936, 58, 1417–1419 CrossRef CAS.
- (a) L. Xu, X. Li, Y. Zhu and Y. Xiang, New J. Chem., 2009, 33, 2051–2054 RSC; (b) J. Wisniak and M. Klein, Ind. Eng. Chem. Prod. Res. Dev., 1984, 23, 44–50 CrossRef CAS; (c) H. D. Burge, D. J. Collins and B. H. Davis, Ind. Eng. Chem. Prod. Res. Dev., 1980, 19, 389–391 CrossRef CAS; (d) H. C. Yao and P. H. Emmett, J. Am. Chem. Soc., 1962, 84, 1086–1091 CrossRef CAS.
- (a) G. J. Roth, A. Heckel, F. Colbatzky, S. Handschuh, J. Kley, T. Lehmann-Lintz, R. Lotz, U. Tontsch-Grunt, R. Walter and F. Hilberg, J. Med. Chem., 2009, 52, 4466–4480 CrossRef CAS PubMed; (b) E.-S. Ibrahim, A. M. Montgomerie, A. H. Sneddon, G. R. Proctor and B. Green, Eur. J. Med. Chem., 1988, 23, 183–188 CrossRef CAS; (c) M. V. Barybin, P. L. Diaconescu and C. C. Cummins, Inorg. Chem., 2001, 40, 2892–2897 CrossRef CAS PubMed; (d) R. L. Dorow, P. M. Herrinton, R. A. Hohler, M. T. Maloney, M. A. Mauragis, W. E. McGhee, J. A. Moeslein, J. W. Strohbach and M. F. Veley, Org. Process Res. Dev., 2006, 10, 493–499 CrossRef CAS; (e) F. Picard, E. Baston, W. Reichert and R. W. Hartmann, Bioorg. Med. Chem., 2000, 8, 1479–1487 CrossRef CAS; (f) M. Alajarín, A. Pastor, R.-Á. Orenes and J. W. Steed, J. Org. Chem., 2002, 67, 7091–7095 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra for products 2a–2r. See DOI: 10.1039/c5ra07208e |
| This journal is © The Royal Society of Chemistry 2015 |