Heteroatom-doped porous carbon derived from “all-in-one” precursor sewage sludge for electrochemical energy storage†
Abstract
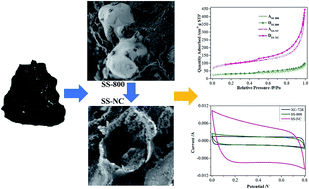
With the imposition of more stringent regulations governing the disposal and use of sewage sludge, the need to develop more cost-effective and environmentally benign re-uses of sewage sludge is of particular concern. Pyrolysis converting sewage sludge to porous carbons is an emerging technology for the disposal of huge amounts of sewage sludge. In this study, a unique heteroatom-doped porous carbon was prepared via the direct pyrolysis of sewage sludge with SiO2, transition metals and organic matter, the special components of sewage sludge, acting as an in-built template, the graphitizing catalysts, and the ideal precursor and nature dopant, respectively. The as-prepared N, O-doped porous carbon had a high specific surface area, numerous heteroatoms, good electrical transport properties and a meso-/macroporous composite. It exhibited favorable charge storage capacity and good stability over 10 000 cycles. The supercapacitor performance results from its hierarchical porous structure and heteroatom doping effects, which combine electrical double-layer capacitors and Faradaic contributions. Our protocol demonstrates a new approach for the potentially eco-friendly benign re-use of sewage sludge and provides a proven technique for synthesizing electrode materials as promising candidates for electrochemical energy storage.

 Please wait while we load your content...
Please wait while we load your content...