Green surfactant of marine origin exerting a cytotoxic effect on cancer cell lines
Palashpriya Das*a,
Siddik Sarkarb,
Mahitosh Mandalb and
Ramkrishna Sen*a
aDepartment of Biotechnology, Indian Institute of Technology, Kharagpur 721302, India. E-mail: write2palashpriya@gmail.com; rksen@yahoo.com; Fax: +91-3222-278707; Tel: +91-3222-283752
bSchool of Medical Science and Technology, Indian Institute of Technology, Kharagpur, India
First published on 10th June 2015
Abstract
The present work reveals the efficacy of a marine antimicrobial lipopeptide biosurfactant in blocking proliferation of breast cancer and colon cancer cell lines, without displaying any significant antioxidant activity. A novel isoform of 1382 Da played the key role, in sharp contrast to proliferation-blocking marine biosurfactant isoforms detected earlier in the range of 996–1077 Da and 1470–1509 Da. Inhibition of cancer cells was promoted by nanomolar concentrations of the test compound whereas much higher concentrations were reported for a few biosurfactants of marine origin as well as those of terrestrial origin like surfactin and rhamnolipids. Dual staining with annexin V and propidium iodide followed by FACS analysis showed an increased population of cancer cells at the sub G0/G1 phase indicative of the programmed cell death after treatment. Although in vivo studies are yet to be done, the results of the in vitro studies displaying the cytotoxicity of this non-hemolytic marine biosurfactant product advocates for its exploitation as a potential drug candidate in anticancer chemotherapy.
Introduction
The current trends in cancer research are mainly driven by the need to discover and develop anticancer agents with better efficacy and less toxicity. The use of anticancer molecules derived from microorganisms of terrestrial origin is limited by their safety and efficacy. For example, the chemotherapeutic agent doxorubicin, an anthracycline antibiotic isolated from Streptomyces sp., is toxic, inhibits the growth of the producer itself and becomes ineffective after a few cycles of chemotherapy.1 In contrast, the so far unexplored marine environment encompassing a vast area of the world's surface may serve as a rich repertoire of biomolecules of therapeutic as well as industrial importance.The first marine bioactive compounds, spongouridine and spongothymidine, were isolated from the Caribbean sponge Cryptotheca crypta in the early 1950s which were later found to possess anti-cancer and anti-viral activity.2 Marine peptides from various sea anemones are lead drug candidates as they play roles of phospholipases, K+ channel inhibitors, Na+ channel toxins etc.3 An anticancer peptide from a fish had been reported to induce apoptosis in human U937 lymphoma cells through enhancement of caspase-3 and caspase-8 activity.4 Fish protein hydrolysates have also been found to exhibit antiproliferative roles on human breast cancer cell lines.5 Squalamine, an aminosterol isolated from the liver of the dogfish shark Squalus acanthias,6 inhibited tumour growth in several animal models.7 Halobacillin is yet another compound isolated from a marine Bacillus sp. claimed to inhibit the growth of a human colon tumor cell line.8 This list is further adorned by the antimicrobial linear peptides from a marine Bacillus subtilis displaying cytotoxicity against six human cancer cell lines with GI50 values of 4.6–19.6 μg ml−1 (ref. 9) and a lipopeptide extract from a Bacillus amyloliquefaciens inducing cell death in human oral squamous cell carcinoma cell lines.10
Biosurfactants are green microbial surface-active agents. Reports on biosurfactants of marine origin are scarce, mostly stating their potentials of crude oil emulsification, enhancement of oil bioavailability and PAH remediation.11–17 A lipopeptide biosurfactant from marine Bacillus circulans was reported for the first time for its heavy metal remediation property.18 The marine B. circulans biosurfactant was non-hemolytic and possessed biofilm disruption potential along with antimicrobial action against MDR strains.19,20 These noteworthy biomedical potentials set stage for the present study of determination of its cytotoxic activity. In normal cell metabolism, oxidation fueling biological processes generates free radicals which are associated with ageing and are causes of many diseases. Although superoxide dismutase, glutathione peroxidase and catalase enzymes along with chemical compounds like ascorbic acid, carotenoids etc. protect the organisms, they often are incapable of preventing the total damage and thus one looks for natural products with antioxidant activity for reducing oxidative damage.21 Antioxidant compounds are attributed with the property of cancer prevention.22,23 Lignan, the major antioxidant component of the phenolic fraction of olive oil inhibits skin, breast and colon carcinogenesis through antioxidant mechanism.24 The extract of an edible mushroom, Agaricus brasiliensis, used for prevention and treatment of cancer is also a source of antioxidant compounds.21 Maslinic acid, a triterpenoid compound is known for both of its antioxidant and anticancer effects.25 As antioxidant activity is often co-related to anticancer activity, this work was undertaken to determine both of these action potentials of the test marine antimicrobial lipopeptide biosurfactant. The results were very encouraging not only for the antiproliferative activity being exhibited at such low concentrations, (ng ml−1), not stated earlier for other biosurfactants, but also for the fact that this non-hemolytic agent had no antioxidant activity and possessed a novel isoform of mass of 1382 Da, not reported earlier to the best of our knowledge.
Materials and methods
Isolation of the biosurfactant from the marine bacterium
The biosurfactant producer isolated from a marine water sample of Andaman and Nicobar Islands, India, was grown in glycerol mineral salts medium26 for biosurfactant production. The biosurfactant was precipitated from the production medium by acidification. It was collected, lyophilized and extracted with methanol. The methanol soluble fraction was dried at 60 °C and a stock solution (1 mg ml−1) of this partially purified biosurfactant was prepared in methanol.Ferric thiocyanate (FTC) assay
Ferric thiocyanate test was used to determine the amount of peroxide generated at the initial stage of lipid peroxidation. 1 mg ml−1 solution of each of biosurfactant and standard antioxidants like vitamin E, ascorbic acid, vitamin A or quercetin was prepared. 1 ml from all solutions was taken in separate screw capped vials. 2.88 ml of 2.5% linoleic acid in absolute ethanol and 9 ml of 40 mM phosphate buffer (pH 7.0) were added. The tube was placed at 40 °C in the dark. To 0.1 ml of this solution, 9.7 ml of 75% ethanol and 0.1 ml 30% ammonium thiocyanate were added followed by addition of 0.1 ml of 20 mM ferrous chloride in 3.5% hydrochloric acid. Precisely 3 min after that addition, the absorbance was measured at 500 nm. The control (with no biosurfactant or antioxidant) and the sample were all subjected to the same procedure. The level of lipid peroxidation inhibition was calculated from the ratio of absorbance of sample to that of control.27 Linoleic acid in presence of ethanol and ammonium thiocyanate forms peroxide. This peroxide oxidizes ferrous chloride to reddish ferric chloride, which was determined by measuring absorbance at 500 nm. However, as antioxidant activity increases, decrease in concentration of peroxide is reflected by the decreased Abs500 nm values. All tests were performed in triplicate and the results were taken as an average of three individual experiments.Lipid peroxidation assay
1 ml from a solution, containing 2.88 ml 2.5% linoleic acid in absolute ethanol, 9 ml 40 mM phosphate buffer (pH 7.0) and 1 ml antioxidants or test biosurfactant (1 mg ml−1), was taken. 2 ml of each of 20% trichloroacetic acid and 1% thiobarbituric acid (w/v) in 50 mM sodium hydroxide was then added to it. The whole mixture was placed for 10 min on a boiling water bath to develop the pink colored thiobarbituric acid reactive substances (TBARS) complex. The mixture, after cooling, was centrifuged for 15 min at 3000 rpm and the absorbance of the supernatant was read at 532 nm.28,29 The supernatant was kept at room temperature for five days and the absorbance was checked each day. As malonaldehyde formed during lipid peroxidation binds to thiobarbituric acid at high temperature to form the red colored substance absorbing at 532 nm, any agent having inhibitory effect on lipid peroxidation would show a reduced Abs532 nm. All tests were performed in triplicate and the results were taken as an average of three individual experiments.Free radical scavenging assay
1 ml biosurfactant solution in methanol (1 mg ml−1) was taken and 1 ml 2,2-diphenyl-2-picrylhydrazyl hydrate (DPPH) solution (0.2 mM in methanol) was added to it. After incubation for 30 min at room temperature; the change in color was noted by measuring the absorbance of the solution at 517 nm. The same test was repeated with standard antioxidants. Though DPPH is a stable compound, when it reacts with an antioxidant compound which can donate hydrogen, it is reduced thereby changing the color from purple to yellow. The free radical scavenging activity was determined by comparing its absorbance with that of a blank.30 More the antioxidant effect of a sample, more will be its free radical scavenging potential and lesser absorption at 517 nm will be recorded. Tests were performed in triplicate and results were taken as an average of three individual experiments. Radical scavenging activity was calculated by the following formula:Superoxide quenching assay
A mixture of phenazine methosulphate (PMS) and NADH chemically generates superoxide anions. Equal volumes of test analytes i.e. biosurfactant or antioxidants (0.5 mg ml−1, 1 mg ml−1 and 2 mg ml−1) and a mix of 20 mM Tris–HCl buffer (pH 8.0), 10 μM PMS, 50 μM NBT (nitroblue tetrazolium) and 7.5 μM NADH were taken. NBT is a yellow colored dye which interacts with superoxide to form blue formazan. Control assays in the absence of NADH ensured that the test solution underwent no reaction with NBT.29 Quenching of superoxide anion by the sample was quantified at 570 nm by noting the extent of NBT reduction. Tests were performed in triplicate and results were determined as average of three individual experiments.Purification of the biosurfactant
Biosurfactant purification was done using reverse phase high performance liquid chromatography, RP-HPLC.19 The elution was done with a 5–95% gradient of acetonitrile, with 0.1% TFA, and water for 60 min at a flow rate of 0.2 ml min−1. All isoforms were collected. The solvent of each was evaporated, the remaining was lyophilized and a stock solution of the resultant powder was made which was used for checking cytotoxic activity.Cell culture studies
Human breast cancer cell line MCF-7, human colon adenocarcinoma cell line HT-29 and normal feline cell line NIH 3T3 were cultured in Dulbecco's Modified Eagle's Medium (DMEM). The nutrient mixture (DMEM/F12) was composed of F12 (Ham) with 15 mM HEPES buffer, L-glutamine and pyridoxine hydrochloride supplemented with 1.2 g sodium bicarbonate (GIBCO™, Invitrogen, NZ), antibiotics namely 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units per litre penicillin and 10 mg l−1 streptomycin (HiMedia, India), and 10% fetal bovine serum, FBS (GIBCO). All the cells were incubated in a humidified atmosphere of 5% CO2 at 37 °C to reach confluence. The total number of cells was estimated with the help of a haemocytometer (by counting cells in the four 1 mm2 corners of the haemocytometer) after staining with trypan blue. The average number of cells per unit volume (ml) of medium was calculated as the
000 units per litre penicillin and 10 mg l−1 streptomycin (HiMedia, India), and 10% fetal bovine serum, FBS (GIBCO). All the cells were incubated in a humidified atmosphere of 5% CO2 at 37 °C to reach confluence. The total number of cells was estimated with the help of a haemocytometer (by counting cells in the four 1 mm2 corners of the haemocytometer) after staining with trypan blue. The average number of cells per unit volume (ml) of medium was calculated as the  × 104 × D, where D is the dilution factor.
× 104 × D, where D is the dilution factor.
Cell viability assay
The activity of methanol extracted biosurfactant on the cell lines was investigated using the standard MTT assay in which succinate dehydrogenase, a mitochondrial enzyme in living cells, cleaves the tetrazolium ring, converting MTT to an insoluble purple formazan. Thus the amount of formazan product produced is directly proportional to the number of viable cells.31 The cultured cells were trypsinized, collected and the viable cell suspensions (200 μl) were dispensed in quadruplicate into 96-well tissue culture plates at an optimized concentration of 104 cells per well in complete medium. After 24 h of seeding, cells were treated with different concentrations of the biosurfactant solution. The negative control consisted of cells in medium along with vehicle i.e. methanol. After 72 h, the culture medium was removed and 100 μl of MTT reagent (1 mg ml−1 in incomplete medium) was added to each well. The plates were incubated at 37 °C for 4 h followed by removal of MTT and addition of 100 μl 100% DMSO to each well. The metabolized MTT product was dissolved in DMSO and quantified by measuring the optical density at 540 nm.32 Cytotoxicity of the HPLC-purified biosurfactant isoforms on NIH 3T3, MCF-7 and HT-29 cells was also investigated using MTT assay. After 24 h of seeding the cells, they were treated with 1–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng ml−1 of the HPLC purified biosurfactant and incubated for 72 h. Cell viability was detected and measured at 540 nm.
000 ng ml−1 of the HPLC purified biosurfactant and incubated for 72 h. Cell viability was detected and measured at 540 nm.
Inhibitory concentration (IC50) determination
The concentration of any compound inhibiting cell viability by 50% is referred to as its IC50. The mean optical density value for the untreated control (methanol) was normalized as 100% OD (i.e. no growth inhibition). Cell viability of treated groups of MCF-7 and HT-29 were normalized with respect to 100% untreated control. The dose–effect curves and IC50 were analyzed as mentioned earlier32 using Prism software (GraphPad Prism 5, San Diego, CA). Three independent experiments was conducted for each set and data is presented as mean ± S.D.Detection of apoptosis induction
Cells were treated with IC50 of biosurfactant for 24 h after seeding in 60 mm tissue culture plates. After treatment, both adhered cells and the floating ones were collected and washed in phosphate-buffered saline (PBS). They were incubated in 70% ethanol and kept at −20 °C overnight for fixation. Cells were then centrifuged, washed and incubated with propidium iodide (PI) solution (40 μg ml−1 PI, 100 μg ml−1 RNase A in PBS) at 37 °C for 1 h. Apoptotic cells were determined by their hypochromic sub-diploid staining profiles. The distribution of cells in the different cell-cycle phases was analyzed from the DNA histogram using Becton-Dickinson FACS-Calibur flow cytometer and CellQuest software.32Induction of apoptosis was further confirmed by flow cytometry of cells stained with the annexin V-FITC kit (Sigma). The cells were collected after treatment with biosurfactant for 12 h and 24 h along with untreated control. They were resuspended in binding buffer at optimized concentration of 106 cells per ml. 500 μl of suspended cells were incubated with 2.5 μl of annexin V-FITC solution and 5 μl of PI for 10 min in the dark. Presence of viable (annexin V-negative and PI-negative), early apoptotic (annexin V-positive, PI-negative), and late apoptotic (annexin V-positive and PI-positive) cells was analyzed by flow cytometry using CellQuest software.
Fluorescence microscopy
Apoptosis was also studied by using fluorescence microscopy. Cells were grown on cover slips followed by treatment with biosurfactant for different time intervals. Cells were then washed with PBS and fixed in 3.7% paraformaldehyde in PBS (pH 7.2) for 15 min at room temperature. PBS wash was again conducted for 5 min followed by methanol treatment for 5 min. This was followed by washing of cells with PBS and incubation with PI (10 μg ml−1 in PBS) for 30 min in darkness. After another wash the cells were gradually dehydrated with 70%, 90%, 95% and 100% ethanol each for 10 s. The cover slips were finally washed with xylene for 10 s and then mounted with D.P.X. mountant (Merck, Darmstadt, Germany) to be observed under a fluorescence microscope.MALDI-ToF-mass spectrometry
The HPLC purified biosurfactant isoform exhibiting cytotoxicity was analyzed by MALDI-ToF mass spectrometry. Matrix used for co-crystallization was 2,5-dihydroxybenzoic acid (DHB). A 10 mg ml−1 matrix stock was prepared by dissolving 1 mg DHB in 50 μl methanol followed by diluting it with 45 μl acetonitrile and 5 μl trifluoroacetic acid. The matrix and the sample were mixed in equal volumes and the mixture was spotted on the stainless steel MALDI sample target plate.33 The plate was placed inside the sample cabinet of a Voyager DE-Pro MALDI-ToF spectrometer (Applied Biosystems, USA). Under a high vacuum condition, a pulsed nitrogen UV laser (337 nm) and accelerating voltage of 20 kV were applied for desorption and ionization of samples. Separated molecules were detected in the ion detector set in the reflector mode.Results
Antioxidant activity determination assays
Assays conducted to determine antioxidant activity indicated that the test marine biosurfactant lacked this property. FTC assay measures the total amount of peroxide generated at the initial stages of lipid peroxidation, by noting the absorbance at 500 nm. Thus, more the absorbance less is the antioxidant power. Absorbance value of the biosurfactant was nearer to the control and higher than standard antioxidants, indicating that it was devoid of antioxidant property (Fig. 1A).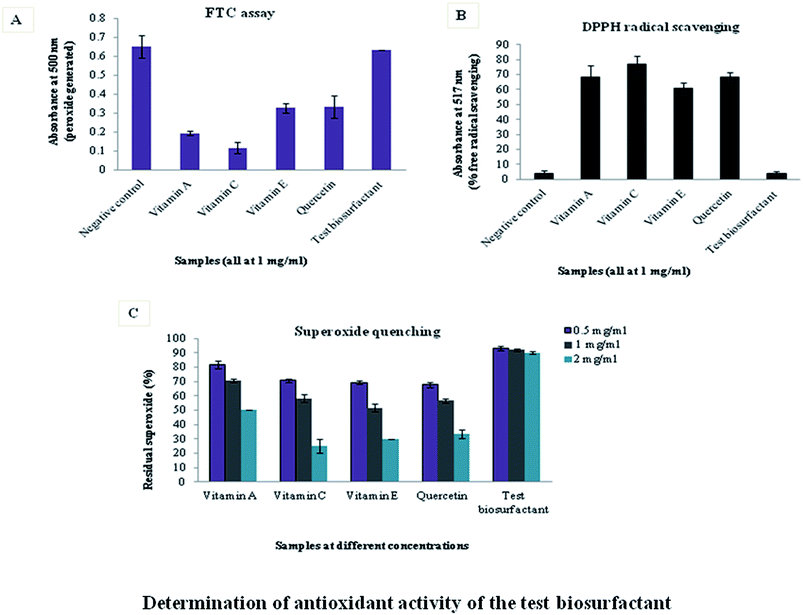 | ||
| Fig. 1 Determination of antioxidant activity of the test biosurfactant [(A): FTC assay; (B): DPPH radical scavenging assay; (C): superoxide quenching assay]. | ||
This was substantiated by the TBARS assay. Malonaldehyde formed during lipid peroxidation binds to thiobarbituric acid to form a red colored TBARS complex absorbing at 532 nm. Thus any agent like antioxidants, having inhibitory effect on lipid peroxidation, produced low amount of malonaldehyde and showed a reduced Abs532 nm but the biosurfactant failed to do so.
DPPH assay measures the free radical scavenging property of samples. DPPH is a stable free radical with an absorption maximum at 517 nm. It gets reduced in presence of an antioxidant and the color of the solution changes from purple to yellow. The degree of discoloration indicates the radical scavenging potential of a compound. If a sample has higher antioxidant activity, it will scavenge more free radicals and purple coloration will fade as was the case with standard antioxidants. However, this discoloration was not observed with the test biosurfactant thereby proving that it lacked free radical scavenging potential (Fig. 1B).
Superoxide quenching assay was quantified by coupling superoxide generation by PMS and NADH to NBT reduction. Superoxide scavenging activity was measured by noting the absorbance of blue formazan, produced by the interaction of residual superoxide (remaining after its quenching by antioxidants), with NBT. Biosurfactant-mediated superoxide quenching was not appreciable like the antioxidants. Hence, residual superoxide in presence of biosurfactant was high and NBT reduction was maximum (Fig. 1C).
Biosurfactant purification, structural determination and cytotoxicity assay
Isoforms of the test biosurfactant were separated through HPLC (Fig. 2A) and interestingly the isoform with antimicrobial activity against a battery of pathogenic microorganisms (marked with an arrow in Fig. 2A), as reported earlier,19 was the one having cytotoxic activity. | ||
| Fig. 2 Purification and MALDI-ToF spectroscopic analysis [(A): HPLC purification of the test biosurfactant and (B): MALDI-ToF analysis of the bioactive biosurfactant isoform]. | ||
The bioactive biosurfactant isoform (marked with an arrow in Fig. 2A) when subjected to MALDI-ToF mass spectrometry (Fig. 2B), separation of ionized molecules according to their respective molecular masses in the m/z range of 1326–1426 Da was revealed. The major component in the bioactive isoform was of a molecular mass of 1382 Da. Its cytotoxic effect varied with the type of cancer cell line, in a dose dependent manner.
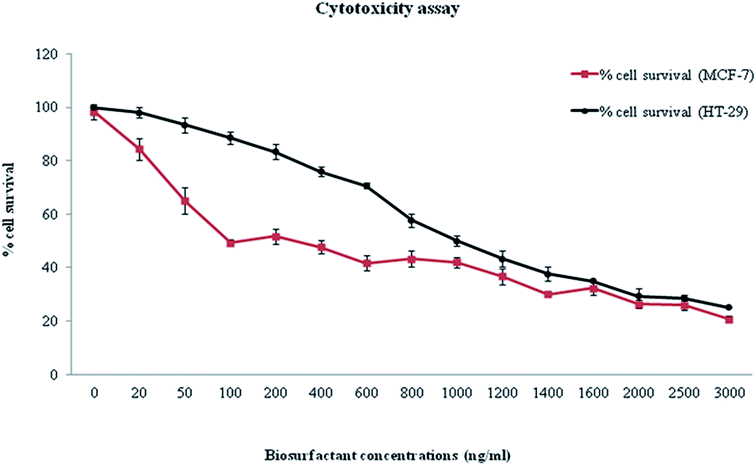
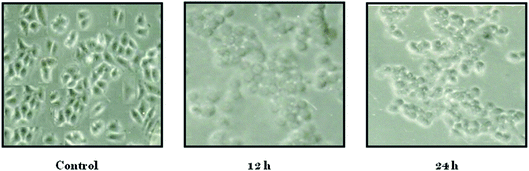
Inhibition of 50% of MCF-7 cells was achieved with ∼100 ng ml−1 of biosurfactant while same percentage of HT-29 cells was inhibited at a biosurfactant concentration of ∼1000 ng ml−1 (Fig. 3). All results were recorded as mean ± S.D. As MIC against normal cells was much higher than that for cancer cells, it indicated the efficacy of the compound in reducing cancer cell viability. Monitoring of morphology of untreated and biosurfactant-treated MCF-7 cells showed that the control cells were normal, spindle-shaped and adhered to the substratum while biosurfactant-treated cells shrunk and became round-shaped, a salient feature of apoptosis (Fig. 4).
Apoptosis detection
The dual staining technique with annexin V and PI indicated the occurrence of programmed cell death of the MCF-7 cancer cell lines due to biosurfactant treatment (Fig. 5A and B). The sub G0/G1 phase population of the cells detected by FACS was indicative of apoptosis. The percentage of apoptosis increased in a time-dependent manner due to biosurfactant action. Almost 30% of the cells underwent apoptosis within a 24 h period (Fig. 5C). Phosphatidylserine externalization is the hallmark of the occurrence of apoptosis followed by loss of membrane integrity. Thus Annexin-V binding assay was done to detect the surface exposure of phosphatidylserine. The dual parametric dot plot combining annexin V-FITC and PI fluorescence (Fig. 6) showed the extent of apoptosis by analysis of phosphatidylserine externalization and loss of membrane integrity. The most supportive proof of biosurfactant mediated apoptosis was the study of nuclear morphology of the cancer cells (Fig. 7). In apoptotic cells the nuclei are condensed or fragmented which results in the differential staining with PI. The dark field image (Fig. 7A) of the untreated cells (control) showed uniform distribution of PI within the nuclei of the cells. However, diffused staining of the nuclei (fragmented nuclei) was observed for the cells treated with the biosurfactant. This was also evidenced by the bright-field images (Fig. 7B) of the cells treated with the biosurfactant for different time periods. | ||
| Fig. 7 Fluorescence micrographs of MCF-7 cells undergoing apoptosis (arrows indicate apoptotic nuclei) [dark field image (A) and bright field image (B)]. | ||
Discussions and conclusion
Screening of marine compounds in search of novel anticancer agents led to the identification of a marine lipopeptide biosurfactant isoform, which holds promise for development of new compounds to treat human malignant cells. To the best of our knowledge, this is the first report of a novel isoform of a marine lipopeptide biosurfactant being effective on both MCF-7 breast cancer cell line and HT-29 colon cancer cell line. The isoform with cytotoxic action was of 1382 Da, in contrast to isoforms in the range of 996–1077 Da and 1470–1509 Da in a lipopeptide biosurfactant of marine origin reported to be active against colon cancer cell lines.33 The inhibition of cancer cell lines, largely attributable to programmed cell death, was brought about by much lower concentrations of the test compound (of the order of ng ml−1) than 120 μg ml−1 for HT-29 cells,33 ∼46 μg ml−1 for MCF-7,34 0.2 mg ml−1 for MDA-MB-231 and 0.1 mg ml−1 for T47D.35 A comparison of the cytotoxicity of the bioactive marine lipopeptide isoform with that of four major anticancer drugs, as they appear in the database of the Development Therapeutic Program of the National Cancer Institute on the three cell lines36 is presented in Table 1 (http://dtp.nci.nih.gov/dtpstandard/cancerscreeningdata/index.jsp) and the data advocated for the potency of the test biosurfactant. The general belief of a compound possessing both anticancer and antioxidant activity is exemplified by many plant products e.g. litchi fruit pericarp extract37 and extract of a mangrove associated bacterium.35 The lack of antioxidant property of the test biosurfactant is much in contrast to this belief. However, few studies have reported that antioxidant substances might cause DNA damage.28 Therefore the inability of the test green surfactant to block oxidative process is rather overshadowed by its marked cytotoxicity and biomedical application potentials. Although studies elucidating the detailed structure of the test marine biosurfactant as well as elaborated work on the mode of its anticancer action form the future studies to further this research, its antiproliferative role in conjugation with antimicrobial as well as non-hemolytic properties definitively advocates for its potency and paves the pathway for the development of improved antimicrobial anticancer chemotherapy.| Cytotoxic agent | MCF-7 (IC50) μM |
|---|---|
| 5-Fluorouracil | 1.75 |
| Cisplatin | 3.01 |
| Etoposide | 5.73 |
| Melphalan | 11.1 |
| Biosurfactant | ∼0.7 |
Acknowledgements
P. Das and S. Sarkar acknowledge IIT, Kharagpur for the financial assistances. R. Sen acknowledges the Department of Biotechnology (DBT), Government of India for the project grant (BT/PR-6827/AAQ/03/263/2005) in marine biotechnology.References
- S. Malla, N. P. Niraula, B. Singh, K. Liou and K. Sohng, Limitations in doxorubicin production from Streptomyces peucetius, Microbiol. Res., 2010, 165, 427–435 CrossRef CAS PubMed.
- D. Leary, M. Vierros, G. Hamon, S. Arico and C. Monagle, Marine genetic resources: a review of scientific and commercial interest, Mar. Pollut., 2009, 33(2), 183–194 CrossRef PubMed.
- R. Nuñez, A. Garateix, A. Laguna, M. D. Fernández, E. Ortiz, M. Llanio, O. Valdés, A. Rodríguez and R. Menéndez, Caribbean marine biodiversity as a source of new compounds of biomedical interest and others industrial applications, Pharmacology, 2006, 3, 111–119 Search PubMed.
- Y. G. Lee, J. Y. Kim, K. W. Lee, K. H. Kim and H. J. Lee, Peptides from anchovy sauce induce apoptosis in a human lymphoma cell (U937) through the increase of caspase-3 and -8 activities, Ann. N. Y. Acad. Sci., 2003, 1010, 399–404 CrossRef CAS PubMed.
- L. Picot, S. Bordenave, S. Didelot, I. Fruitier-Arnaudin, F. Sannier, G. Thorkelsson, J. P. Bergé, F. Guérard, A. Chabeaud and J. M. Piot, Antiproliferative activity of fish protein hydrolysates on human breast cancer cell lines, Process Biochem., 2006, 41, 1217–1222 CrossRef CAS PubMed.
- K. S. Moore, S. Wehrli, H. Roder, M. Rogers, J. N. Forrest and D. McCrimmon, Squalamine: an aminosterol antibiotic from the shark, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 1354–1358 CrossRef CAS.
- A. K. Sills, J. I. Williams, B. M. Tyler, D. S. Epstein, E. P. Sipos and J. D. Davis, Squalamine inhibits angiogenesis and solid tumor growth in vivo and perturbs embryonic vasculature, Cancer Res., 1998, 58, 2784–2792 CAS.
- J. A. Trischmann, P. R. Jensen and W. Fenical, Halobacillin: a cytotoxic cyclic acylpeptide of the iturin class produced by a marine Bacillus, Tetrahedron Lett., 1994, 35, 5571–5574 CrossRef.
- F. S. Tareq, M. A. Lee, H. S. Lee, J. S. Lee, Y. J. Lee and H. J. Shin, Gageostatins A–C, Antimicrobial linear lipopeptides from a marine Bacillus subtilis, Mar. Drugs, 2014, 12, 871–885 CrossRef CAS PubMed.
- C. H. Kuo, Y. W. Lin and R. S. Chen, Lipopeptides extract from Bacillus amyloliquefaciens induce human oral squamous cancer cell death, Asian Pac J Cancer Prev, 2015, 16, 91–96 CrossRef.
- S. S. Zinjarde and A. Pant, Emulsifier from a tropical marine yeast, Yarrowia lipolytica NCIM 3589, J. Basic Microbiol., 2002, 42, 67–73 CrossRef.
- A. Passeri, M. Schmidt, T. Haffner, V. Wray, S. Lang and F. Wagner, Marine biosurfactants IV. Production, characterization and biosynthesis of an anionic glucose lipid from the marine bacterial strain MM1, Appl. Microbiol. Biotechnol., 1992, 37, 281–286 CrossRef CAS.
- F. Peng, Z. Liu, L. Wang and Z. Shao, An oil-degrading bacterium: Rhodococcus erythropolis strain 3C-9 and its biosurfactants, J. Appl. Microbiol., 2007, 102, 1603–1611 CrossRef CAS PubMed.
- R. Thavasi, S. Jayalakshmi, T. Balasubramanian and I. M. Banat, Biosurfactant production by Corynebacterium kutscheri from waste motor lubricant oil and peanut oil cake, Lett. Appl. Microbiol., 2007, 45, 686–691 CrossRef CAS PubMed.
- P. N. Golyshin, A. P. Vitor, M. D. Santos, O. Kaiser, M. Ferrer, S. Yulia, H. Sabirova, T. N. Lünsdorf, O. V. Chernikova, M. M. Golyshina, A. P. Yakimov and N. T. Kenneth, Genome sequence completed of Alcanivorax borkumensis, a hydrocarbon degrading bacterium that plays a global role in oil removal from marine systems, J. Biotechnol., 2003, 106, 215–220 CrossRef CAS PubMed.
- S. Maneerat, T. Bamba, K. Harada, A. Kobayashi, H. Yamada and F. Kawai, A novel crude oil emulsifier excreted in the culture supernatant of a marine bacterium, Myroides sp. strain SM1, Appl. Microbiol. Biotechnol., 2006, 70, 254–259 CrossRef CAS PubMed.
- P. Das, S. Mukherjee and R. Sen, Improved bioavailability and biodegradation of a model polyaromatic hydrocarbon by a biosurfactant producing bacterium of marine origin, Chemosphere, 2008, 72, 1229–1234 CrossRef CAS PubMed.
- P. Das, S. Mukherjee and R. Sen, Biosurfactant of marine origin exhibiting heavy metal remediation properties, Bioresour. Technol., 2009, 100, 4887–4890 CrossRef CAS PubMed.
- P. Das, S. Mukherjee and R. Sen, Antimicrobial potential of a lipopeptide biosurfactant derived from a marine Bacillus circulans, J. Appl. Microbiol., 2008, 104, 1675–1684 CrossRef CAS PubMed.
- P. Das, S. Mukherjee and R. Sen, Antiadhesive action of a marine microbial surfactant, Colloids Surf., B, 2009, 71, 183–186 CrossRef CAS PubMed.
- A. A. Soares, C. G. M. de Souza, F. M. Daniel, G. P. Ferrari, S. M. G. da Costa and R. M. Peralta, Antioxidant activity and total phenolic content of Agaricus brasiliensis (Agaricus blazei Murril) in two stages of maturity, Food Chem., 2009, 112, 775–781 CrossRef CAS PubMed.
- M. M. Manson, Cancer prevention – the potential for diet to modulate molecular signalling, Trends Mol. Med., 2003, 9, 11–18 CrossRef CAS.
- Y. J. Surh, Cancer chemoprevention with dietary phytochemicals, Nat. Rev. Cancer, 2003, 3, 768–780 CrossRef CAS PubMed.
- R. W. Owen, A. Giacosa, W. E. Hull, R. Haubner, B. Spiegelhalder and H. Bartsch, The antioxidant/anticancer potential of phenolic compounds isolated from olive oil, Eur. J. Cancer, 2000, 36, 1235–1247 CrossRef CAS.
- F. J. Reyes-Zurita, E. E. Rufino-Palomares, J. A. Lupiáñez and M. Cascante, Maslinic acid, a natural triterpene from Olea europaea L., induces apoptosis in HT29 human colon-cancer cells via the mitochondrial apoptotic pathway, Cancer Lett., 2009, 273, 44–54 CrossRef CAS PubMed.
- P. Das, S. Mukherjee and R. Sen, Substrate dependent production of extracellular biosurfactant by a marine bacterium, Bioresour. Technol., 2009c, 100, 1015–1019 Search PubMed.
- J. Y. Lee, W. I. Hwang and S. T. Lim, Antioxidant and anticancer activities of organic extracts from Platycodon grandiflorum A. De Candolle roots, J. Ethnopharmacol., 2004a, 93, 409–415 Search PubMed.
- K. W. Lee, H. J. Lee and C. Y. Lee, Vitamins, Phytochemicals, Diets and Their Implementation in Cancer Chemoprevention, Crit. Rev. Food Sci. Nutr., 2004b, 44, 437–452 Search PubMed.
- S. D. Cox, C. Jayasinghe and J. L. Markham, Antioxidant activity in Australian native sarsaparilla (Smilax glyciphylla), J. Ethnopharmacol., 2005, 101, 162–168 CrossRef PubMed.
- M. S. Blois, Antioxidant determinations by the use of a stable free radical, Nature, 1958, 181, 1199–1200 CrossRef CAS PubMed.
- T. Mosmann, Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS.
- S. Sarkar, A. Mazumdar, R. Dash, D. Sarkar, P. B. Fisher and M. Mandal, ZD6474 enhances paclitaxel antiproliferative and apoptotic effects in breast carcinoma cells, J. Cell. Physiol., 2011, 226, 375–384 CrossRef CAS PubMed.
- C. Sivapathasekaran, P. Das, S. Mukherjee, J. Saravanakumar, M. Mandal and R. Sen, Marine bacterium derived lipopeptides: characterization and cytotoxic activity against cancer cell lines, Int. J. Pept. Res. Ther., 2010, 16, 215–222 CrossRef CAS.
- R. Ramasubburayan, S. Sumathi, D. M. Bercy, G. Immanuel and A. Palavesam, Antimicrobial, antioxidant and anticancer activities of mangrove associated bacterium Bacillus subtilis subsp. subtilis RG, Biocatal. Agric. Biotechnol., 2015, 4, 158–165 Search PubMed.
- C. Duarte, E. J. Gudiña, C. F. Lima and L. R. Rodrigues, Effects of biosurfactants on the viability and proliferation of human breast cancer cells, AMB Express, 2014, 4, 40 CrossRef PubMed , http://www.amb-express.com/content/4/1/40.
- A. Chodoeva, J. J. Bosc, J. Guillon, A. Decendit, M. Petraud, C. Absalon, C. Vitry, C. Jarryc and J. Roberta, 8-O-Azeloyl-14-benzoylaconine: a new alkaloid from the roots of Aconitum karacolicum and its antiproliferative activities, Bioorg. Med. Chem., 2005, 13, 6493–6501 CrossRef CAS PubMed.
- X. Wang, S. Yuan, J. Wang, P. Lin, G. Liu, Y. Lu, J. Zhang, W. Wang and Y. Wei, Anticancer activity of litchi fruit pericarp extract against human breast cancer in vitro and in vivo, Toxicol. Appl. Pharmacol., 2006, 215, 168–178 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |