Increased active sites and their accessibility of a N-doped carbon nanotube carbocatalyst with remarkably enhanced catalytic performance in direct dehydrogenation of ethylbenzene†
Zhongkui Zhao*,
Yitao Dai,
Guifang Ge,
Xinwen Guo and
Guiru Wang
State Key Laboratory of Fine Chemicals, Department of Catalysis Chemistry and Engineering, School of Chemical Engineering, Dalian University of Technology, Dalian 116024, P. R. China. E-mail: zkzhao@dlut.edu.cn; Fax: +86-411-84986354
First published on 5th June 2015
Abstract
This work presents an efficient and low-cost one-step strategy for simultaneously N-doping and increasing surface ketonic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups and structural defects of a N-doped carbon nanotube (HN-CNT) through the explosive decomposition of hexamethylenetetramine (HTA) nitrate, a low-cost N,O-containing organic compound. The as-synthesized HN-CNT demonstrates a 1.64 and 2.19 times higher steady-state styrene rate with 98.5% selectivity towards styrene for direct dehydrogenation (DDH) than that of the parent CNT and H-CNT prepared by the similar pyrolysis procedure to that for the HN-CNT except for replacing HTA nitrate with HTA.
O groups and structural defects of a N-doped carbon nanotube (HN-CNT) through the explosive decomposition of hexamethylenetetramine (HTA) nitrate, a low-cost N,O-containing organic compound. The as-synthesized HN-CNT demonstrates a 1.64 and 2.19 times higher steady-state styrene rate with 98.5% selectivity towards styrene for direct dehydrogenation (DDH) than that of the parent CNT and H-CNT prepared by the similar pyrolysis procedure to that for the HN-CNT except for replacing HTA nitrate with HTA.
Styrene, one of the most important monomers for the synthesis of polymers and copolymers, is mostly produced by direct dehydrogenation (DDH) of ethylbenzene on alkaline promoted iron-based catalysts.1 The industrially used K–Fe catalyst suffers quick deactivation resulting from coke deposition, as well as huge energy consumption owing to the required excess of superheated steam in the feed. Therefore, to pursue an efficient alternative to iron-based catalysts is highly desirable, but remains a rigorous challenge. Nanocarbon materials have been demonstrated to be a promising, sustainable and low-cost metal-free alternative to metal-based catalysts for organic synthesis,2 hydrogen production,3 photodegradation,4 the oxygen reduction reaction,5 and as counter electrode catalysts for solar cells.6 Nowadays, carbocatalysis has already attracted great attention and become the forefront and hot topic in heterogeneous catalysis.7
The use of carbon materials as metal-free catalysts in the oxidative dehydrogenation (ODH) of ethylbenzene7a,7b,8 and light alkanes2a,2b,7a,7b,9 to produce their corresponding olefins has attracted great attention while DDH in the absence of oxygen is only scarcely reported.10 However, the ODH process presents some serious disadvantages compared to oxygen-free DDH namely the use of a mixture containing oxygen and hydrocarbon, leading to potential safety issues and low selectivity towards styrene owing to the parallel side reactions such as combustion and the possible damage to the carbocatalysts since they suffer from harsh operating conditions. DDH is considered as a promising approach for olefin production.10,11
The DDH of ethylbenzene to styrene was first reported by using nanodiamond as an efficient metal-free catalyst,11a which depicts a fascinating prospect for energy-saving styrene production via carbocatalysts in DDH. The exciting results inspired researchers to develop carbon-based catalysts for this reaction. Great advances have been made in developing the highly efficient ethylbenzene DDH carbocatalysts.12 It was previously demonstrated that the N-doping of carbon materials can efficiently enhance the DDH reaction.11b,12a Furthermore, some nanocarbon materials like few-layer graphene decorated nanodiamond, nanodiamond/CNT-SiC, N-doped mesoporous graphene/nanodiamond, nanodiamond/carbon nitride hybrid, and N-doped CNT-decorated activated carbon have demonstrated promising catalytic performances in the DDH reaction of ethylbenzene.12b–f However, further improvement in activity is essential for possible industrial application but it still remains a rigorous challenge.
It was previously established that the surface ketonic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O is the main active site for C–H activation.10–12 Recently, it was established that, besides the ketonic C
O is the main active site for C–H activation.10–12 Recently, it was established that, besides the ketonic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, the surface structural defects can also act as active sites for C–H bond activation.10a,13 The improvement in nucleophilicity of the ketonic C
O groups, the surface structural defects can also act as active sites for C–H bond activation.10a,13 The improvement in nucleophilicity of the ketonic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups by N doping benefits the promotion of DDH.11b,12a The pyrolysis of carbon materials with melamine can lead to the formation of some structural defects but the defectiveness is scarce,11b,12a,14 therefore the further improvement in defects is required. Moreover, the thick carbon nitride layers covering the N-doped carbon nanotube (M-CNT) can be formed if melamine was used as a N precursor,13a,13b which reduces the accessibility of active sites to reactants. We previously demonstrated that the addition of guanidine nitrate in the pyrolysis of melamine and the use of melamine–cyanuric acid in supramolecular assembly can eliminate the compact carbon nitride layers on a CNT, and can also simultaneously increase the structural defects and enrich the surface ketonic C
O groups by N doping benefits the promotion of DDH.11b,12a The pyrolysis of carbon materials with melamine can lead to the formation of some structural defects but the defectiveness is scarce,11b,12a,14 therefore the further improvement in defects is required. Moreover, the thick carbon nitride layers covering the N-doped carbon nanotube (M-CNT) can be formed if melamine was used as a N precursor,13a,13b which reduces the accessibility of active sites to reactants. We previously demonstrated that the addition of guanidine nitrate in the pyrolysis of melamine and the use of melamine–cyanuric acid in supramolecular assembly can eliminate the compact carbon nitride layers on a CNT, and can also simultaneously increase the structural defects and enrich the surface ketonic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups.13a,13b However, the previously reported method would consume a large amount of precious guanidine and melamine–cyanuric acid. Therefore, the more efficient and low-cost strategy for fabricating a N-doped CNT with an increased number of defects and C
O groups.13a,13b However, the previously reported method would consume a large amount of precious guanidine and melamine–cyanuric acid. Therefore, the more efficient and low-cost strategy for fabricating a N-doped CNT with an increased number of defects and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups is highly desirable, but remains a challenge. Hexamethylenetetramine was demonstrated to be a good candidate as a N precursor for fabricating novel mesoporous carbon nitride material.11b We envision that the use of low-cost hexamethylenetetramine nitrate as a N,O precursor may simultaneously increase the amount of catalytically active sites and their accessibility, which in turn further improves DDH catalysis.
O groups is highly desirable, but remains a challenge. Hexamethylenetetramine was demonstrated to be a good candidate as a N precursor for fabricating novel mesoporous carbon nitride material.11b We envision that the use of low-cost hexamethylenetetramine nitrate as a N,O precursor may simultaneously increase the amount of catalytically active sites and their accessibility, which in turn further improves DDH catalysis.
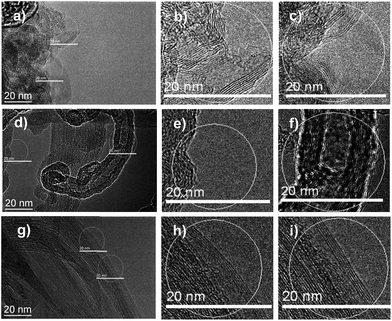
In this work, we firstly present a sophisticated approach for fabricating a novel N-doped CNT (HN-CNT) by explosive decomposition of the low-cost hexamethylenetetramine (HTA) nitrate, used as a N,O precursor; for comparison, the H-CNT was prepared by a similar pyrolysis process under the same conditions as those for the HN-CNT except for the replacement of HTA nitrate with HTA (Scheme 1). The characterization results show that the developed HN-CNT catalyst has increased structural defects, enriched surface ketonic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups and 1.3% of surface N content (by XPS analysis) in comparison with the as-synthesized H-CNT. From Fig. 1a–c, the enlarged structural defectiveness of the HN-CNT can be seen clearly. However, the perfect CNT wall for the pristine CNT can be observed (Fig. 1g–i). Fig. 1d–f shows that the carbon nitride fragments coated on the nanotube wall of the H-CNT can be formed by the pyrolysis of HTA. In comparison with the H-CNT, visible carbon nitride fragments on the developed HN-CNT can not be observed, although we use similar pyrolysis conditions. The visible structural defect and the basic disappearance of carbon nitride on the HN-CNT may be ascribed to the explosive decomposition of HTA nitrate. From our previous results, the formed carbon nitride feature on the H-CNT is different from that on a M-CNT, caused by the unique structure and chemical properties of HTA nitrate.13a Owing to the increased structural defects and the accessibility of active sites including both structural defects and surface C
O groups and 1.3% of surface N content (by XPS analysis) in comparison with the as-synthesized H-CNT. From Fig. 1a–c, the enlarged structural defectiveness of the HN-CNT can be seen clearly. However, the perfect CNT wall for the pristine CNT can be observed (Fig. 1g–i). Fig. 1d–f shows that the carbon nitride fragments coated on the nanotube wall of the H-CNT can be formed by the pyrolysis of HTA. In comparison with the H-CNT, visible carbon nitride fragments on the developed HN-CNT can not be observed, although we use similar pyrolysis conditions. The visible structural defect and the basic disappearance of carbon nitride on the HN-CNT may be ascribed to the explosive decomposition of HTA nitrate. From our previous results, the formed carbon nitride feature on the H-CNT is different from that on a M-CNT, caused by the unique structure and chemical properties of HTA nitrate.13a Owing to the increased structural defects and the accessibility of active sites including both structural defects and surface C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, the developed catalyst can exhibit outstanding DDH catalytic performance.
O, the developed catalyst can exhibit outstanding DDH catalytic performance.
 | ||
| Scheme 1 Schematic representations for the synthesis of a HN-CNT carbocatalyst by a facile one-step approach, as well as for the synthesis of a H-CNT. | ||
The textural features of the as-synthesized HN-CNT, H-CNT, and pristine CNT were investigated by N2 adsorption–desorption experiments. From Fig. 2a, the significantly decreased surface area and pore volume for the as-synthesized H-CNT in comparison with those for the pristine CNT can be observed and ascribed to the compact carbon nitride formed on the CNT wall and the possibly blocked mouth of the CNT. The formed carbon nitride fragments identified by the above HRTEM can reduce the accessibility of active sites on the H-CNT, and therefore leads to poor catalytic performance. Although no visible carbon nitride fragments on the HN-CNT can be seen HRTEM, the decrease in surface area and pore volume of the HN-CNT in comparison to the pristine CNT can be observed (Fig. 2a), showing that the carbon nitride fragments cannot be completely removed from the HN-CNT. Moreover, the micro- and meso-pore size distribution provides extra evidence for the CNT mouth being blocked by the two synthesized samples. The structural characteristics of the as-synthesized HN-CNT, H-CNT, and pristine CNT were further investigated by XRD and Raman techniques.
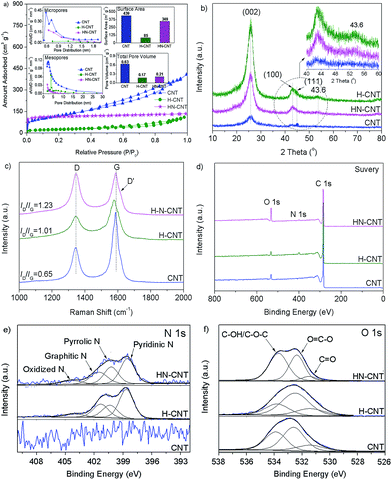 | ||
| Fig. 2 Nitrogen adsorption–desorption results (a), XRD patterns (b), Raman spectra (c), XPS survey (d), N 1s (e), and O 1s (f) of the HN-CNT, H-CNT, and pristine CNT samples. | ||
The structural characteristics of the as-synthesized N-doped CNT (HN-CNT and H-CNT) and pristine CNT were further investigated by XRD and Raman techniques. From Fig. 2b, towards the HN-CNT, H-CNT, and pristine CNT, the diffraction peaks corresponding to (002), (100) and (111) planes can be identified, indicating well-formed graphitic structures.12c–e More interestingly, the peak at 43.6° in the XRD patterns of the as-synthesized HN-CNT and H-CNT, caused by the formed carbon nitride fragments on the HN-CNT and H-CNT, can be observed; however, no peak at this angle can be resolved in the pattern of the pristine CNT. The sharper and stronger peaks towards the (002), (100) and (111) planes in the H-CNT in comparison with those in the pristine CNT imply that graphitic carbon nitride fragments are present, which is different from those in the M-CNT.15 The medium diffraction peaks towards the (002), (100) and (111) planes for the HN-CNT in comparison with those for the H-CNT and pristine CNT can be seen, suggesting the partial removal of the formed carbon nitride fragments, which is consistent with the HRTEM and BET results. From Fig. 2c, we could observe that there are two main first-order Raman modes at around 1334–1337 and 1596 cm−1, corresponding to the A1g mode in disordered carbon or structural defects and to the E2g mode of ideal graphitic carbon, respectively.16 Correlated to the HRTEM results illustrated in Fig. 1, the higher ID/IG for the HN-CNT in comparison with that for the pristine CNT is mainly caused by more structural defects and lattice edges in the as-prepared hybrid composite,17 but the higher ID/IG for the H-CNT in comparison with that for the pristine CNT suggests that graphitic carbon nitride fragments are present on the material. The visible D′ peak in the Raman spectrum of the HN-CNT is a further indicator of there being more defects than in the other two.13a The enlarged structural defectiveness identified by HRTEM and Raman experiments allows the HN-CNT to be an excellent DDH catalyst. Moreover, the developed HN-CNT in this work exhibits a higher ID/IG value (1.23) in comparison with the previously reported G-M-CNT,13a although the cost was higher and a larger amount of guanidine nitrate was used. This shows that HN is more efficient and has a lower cost for producing structural defects, which may result in the higher catalytic performance. The better behavior of HN than guanidine nitrate might be ascribed to the different molecular structures. Further investigations should be performed to reveal why HN is more efficient for increasing structural defects on a CNT in comparison with guanidine nitrate.
XPS experiments were performed to investigate the nature and coordination of the carbon, nitrogen, and oxygen in the as-synthesized N-doped CNT and the pristine CNT, since the surface chemistry of carbon materials significantly affects their catalysis in the DDH reaction.11–13 From Fig. 2d and Table S1,†in comparison with the pristine CNT, increased surface O content in the HN-CNT but decreased surface O content in the H-CNT can be seen. The former is caused by the explosive decomposition of HTA nitrate; but the latter may be due to the O escaping from the H-CNT in the pyrolysis process at high temperature or owing to the coating effect of compact carbon nitride fragments. From Fig. S1, 2e and Table S1,† N incorporation into the carbon matrix can be identified. Furthermore, the as-prepared HN-CNT has lower N content than the H-CNT, ascribed to the explosive decomposition of the O-containing nitrate motif in HTA nitrate. The main side-products for DDH are benzene and toluene resulting from the cracking of ethylbenzene, which is consistent with the results reported in the literature.11,12c–e The surface phenolic hydroxyl group and/or possible COOH may promote the cracking of ethylbenzene due to its acidity.18 The incorporated N atom into the carbon matrix can increase the electron density of carbon materials, and therefore strengthens the basic properties but weakens the acidity of the catalyst, which may result in an improvement in catalytic activity for styrene production and simultaneously compress the benzene and toluene formation.12c–e From Fig. 2d and Table S1,† HTA nitrate explosive decomposition also leads to a clear difference in the surface N and O content and their chemical state. The O 1s XPS spectra of the three samples were deconvoluted into three peaks and assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, O
O, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O and C–O–C/C–OH containing groups, respectively (Fig. 2f and Table S1†).13a,13b The content of surface ketonic C
C–O and C–O–C/C–OH containing groups, respectively (Fig. 2f and Table S1†).13a,13b The content of surface ketonic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, the main active sites for the DDH reaction on the HN-CNT, H-CNT, and pristine CNT are 0.95, 0.65, and 0.65, respectively. The higher C
O groups, the main active sites for the DDH reaction on the HN-CNT, H-CNT, and pristine CNT are 0.95, 0.65, and 0.65, respectively. The higher C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O content of the HN-CNT compared to the other two allows it to exhibit superior DDH catalytic performance.
O content of the HN-CNT compared to the other two allows it to exhibit superior DDH catalytic performance.
The DDH of ethylbenzene to styrene is an industrially important catalytic process. Herein, the catalytic performance of the HN-CNT, H-CNT, and pristine CNT catalysts was tested. Fig. 3 presents the catalytic properties and the industrially used K–Fe catalyst was also included for comparison. From the previously reported results, the catalytic activity can basically reach the steady state after the reaction runs for more than 15 h. Herein, the steady-state styrene rate and selectivity at 20 h on stream was used to evaluate the catalytic performance of the as-synthesized catalysts. The developed HN-CNT catalyst gives a steady-state styrene rate of 4.6 mmol g−1 h−1 with 98.5% of high styrene selectivity. The catalytic activity of the HN-CNT is 2.19 and 1.64 times higher than that of the as-synthesized H-CNT and the pristine CNT, respectively. Moreover, from our previously reported results,13a,13b although their is a lower amount of surface C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups on the M-CNT prepared by pyrolysis of melamine, it exhibits higher catalytic activity than the pristine CNT, ascribed to the promoting effect of N-doping. However, the as-synthesized N-doped CNT in this work (H-CNT) formed by pyrolysis of HTA shows lower catalytic activity than the pristine CNT, although there is almost the same amount of surface ketonic C
O groups on the M-CNT prepared by pyrolysis of melamine, it exhibits higher catalytic activity than the pristine CNT, ascribed to the promoting effect of N-doping. However, the as-synthesized N-doped CNT in this work (H-CNT) formed by pyrolysis of HTA shows lower catalytic activity than the pristine CNT, although there is almost the same amount of surface ketonic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups on the two samples, caused by the formed compact carbon nitride fragments on the H-CNT, whose feature is different from that on M-CNT identified by the HRTEM, N2 adsorption–desorption, XRD, and Raman characterization results. Moreover, the developed HN-CNT in this work exhibits a higher catalytic performance in comparison to the previously reported G-M-CNT,13a ascribed to producing structural defects by HN more efficiently than guanidine nitrate, identified by Raman characterization. Furthermore, the lower cost of HTA nitrate with lower usage is required to prepare the HN-CNT with higher catalytic performance in comparison to the previously reported guanidine nitrate, which shows a large advance in the modified CNT for DDH reaction.
O groups on the two samples, caused by the formed compact carbon nitride fragments on the H-CNT, whose feature is different from that on M-CNT identified by the HRTEM, N2 adsorption–desorption, XRD, and Raman characterization results. Moreover, the developed HN-CNT in this work exhibits a higher catalytic performance in comparison to the previously reported G-M-CNT,13a ascribed to producing structural defects by HN more efficiently than guanidine nitrate, identified by Raman characterization. Furthermore, the lower cost of HTA nitrate with lower usage is required to prepare the HN-CNT with higher catalytic performance in comparison to the previously reported guanidine nitrate, which shows a large advance in the modified CNT for DDH reaction.
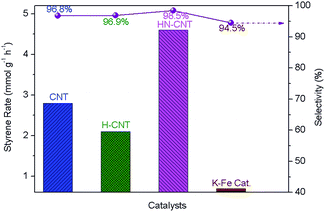 | ||
| Fig. 3 Catalytic properties of the HN-CNT, H-CNT, and pristine CNT as a function of time on stream for DDH of ethylbenzene to styrene. | ||
Correlated to the nature of the HN-CNT as shown above, the outstanding catalytic performance of the developed HN-CNT benefits from the simultaneous enlargement in structural defectiveness, the increase in surface ketonic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups as well as the improvement in nucleophilicity of surface ketonic C
O groups as well as the improvement in nucleophilicity of surface ketonic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and basic properties of materials caused by N-doping. Moreover, the removal of compact carbon nitride fragments enhances the accessibility of active sites to ethylbenzene, which also benefits the DDH reaction.13a,13b Furthermore, the blocked mouth of the HN-CNT identified by the N2 adsorption–desorption measurement results (very low pore volume and very fewer pores in HN-CNT) doesn’t reduce its catalytic activity in the DDH reaction, suggesting the DDH reaction mainly takes place on the external surface of HN-CNT. The developed HN-CNT catalyst exhibits 6.57 times the steady-state styrene rate of the K–Fe catalyst, implying a bright future for the clean, highly efficient and energy-saving industrial production of styrene under oxidant- and steam-free conditions.
O and basic properties of materials caused by N-doping. Moreover, the removal of compact carbon nitride fragments enhances the accessibility of active sites to ethylbenzene, which also benefits the DDH reaction.13a,13b Furthermore, the blocked mouth of the HN-CNT identified by the N2 adsorption–desorption measurement results (very low pore volume and very fewer pores in HN-CNT) doesn’t reduce its catalytic activity in the DDH reaction, suggesting the DDH reaction mainly takes place on the external surface of HN-CNT. The developed HN-CNT catalyst exhibits 6.57 times the steady-state styrene rate of the K–Fe catalyst, implying a bright future for the clean, highly efficient and energy-saving industrial production of styrene under oxidant- and steam-free conditions.
Conclusion
In summary, we developed a facile and low-cost approach for synthesizing a N-doped carbon nanotube catalyst with increased structural defects and enriched surface ketonic carbonyl groups through a one-step method including the explosive decomposition of hexamethylenetetramine nitrate. The as-synthesized HN-CNT carbocatalyst in this work demonstrates remarkably higher catalytic activity in the direct dehydrogenation of ethylbenzene for styrene production, which benefits from the increased catalytically active sites, the improved nucleophilicity of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and basic properties of materials caused by N-doping, as well as high accessibility of active sites to ethylbenzene owing to the removal of the carbon nitride fragments. Moreover, the combination of the reaction results and the N2 adsorption–desorption measurement results suggest that the DDH reaction mainly takes place on the external surface of HN-CNT. The developed facile and efficient one-step approach for simultaneously producing defects, enriching with surface C
O and basic properties of materials caused by N-doping, as well as high accessibility of active sites to ethylbenzene owing to the removal of the carbon nitride fragments. Moreover, the combination of the reaction results and the N2 adsorption–desorption measurement results suggest that the DDH reaction mainly takes place on the external surface of HN-CNT. The developed facile and efficient one-step approach for simultaneously producing defects, enriching with surface C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, and achieving N-doping also paves a new way for the preparation of other N-doped nanocarbon catalysts with outstanding catalytic performance in diverse transformations.
O groups, and achieving N-doping also paves a new way for the preparation of other N-doped nanocarbon catalysts with outstanding catalytic performance in diverse transformations.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (grant no. 21276041), the Joint Fund of Coal, set up by National Natural Science Foundation of China and Shenhua Co., Ltd. (grant no. U1261104), and also by the Chinese Ministry of Education via the Program for New Century Excellent Talents in University (grant no. NCET-12-0079), and Fundamental Research Funds for the Central Universities (grant no. DUT15LK41).Notes and references
- (a) O. Shekhah, W. Ranke and R. Schlogl, J. Catal., 2004, 225, 56–68 CrossRef CAS; (b) S. J. Liao, T. Chen, C. X. Miao, W. M. Yang, Z. K. Xie and Q. L. Chen, Catal. Commun., 2008, 9, 1817–1821 CrossRef CAS.
- (a) J. Zhang, X. Liu, R. Blume, A. H. Zhang, R. Schlöl and D. S. Su, Science, 2008, 322, 73–77 CrossRef CAS PubMed; (b) W. Qi, W. Liu, B. Zhang, X. Gu, X. Guo and D. S. Su, Angew. Chem., Int. Ed., 2013, 52, 14224–14228 CrossRef CAS PubMed; (c) S. P. Pitre, C. D. McTiernan, H. Ismaili and J. C. Scaiano, J. Am. Chem. Soc., 2013, 135, 13286–13289 CrossRef CAS PubMed; (d) Y. Nabae, H. Rokubuichi, M. Mikuni, Y. Kuang, T. Hayakawa and M. Kakimoto, ACS Catal., 2013, 3, 230–236 CrossRef CAS; (e) D. R. Dreyer and C. W. Bielawski, Chem. Sci., 2011, 2, 1233–1240 RSC.
- K. Schwinghammer, B. Tuffy, M. B. Mesch, E. Wirnhier, C. Martineau, F. Taulelle, W. Schnick, J. Senker and B. V. Lotsch, Angew. Chem., Int. Ed., 2013, 52, 2435–2439 CrossRef CAS PubMed.
- (a) M. Shalom, S. Inal, C. Fettkenhauer, D. Neher and M. Antonietti, J. Am. Chem. Soc., 2013, 135, 7118–7121 CrossRef CAS PubMed; (b) Y. Zheng, L. Lin, X. Ye, F. Guo and X. Wang, Angew. Chem., Int. Ed., 2014, 53, 11926–11930 CrossRef CAS PubMed.
- Y. Zheng, Y. Jiao, Y. L. Ge, M. Jaroniec and S. Z. Qiao, Angew. Chem., Int. Ed., 2013, 52, 3110–3116 CrossRef CAS PubMed.
- I. V. Lightcap and P. V. Kamat, Acc. Chem. Res., 2013, 46, 2235–2243 CrossRef CAS PubMed.
- (a) W. Qi and D. Su, ACS Catal., 2014, 4, 3212–3218 CrossRef CAS; (b) D. Chen, A. Holmen, Z. Sui and X. Zhou, Chin. J. Catal., 2014, 35, 824–841 CrossRef CAS; (c) X. K. Kong, C. L. Chen and Q. W. Chen, Chem. Soc. Rev., 2014, 43, 2841–2857 RSC; (d) M. M. Titirici, R. J. White, N. Brun, V. L. Budarin, D. S. Su, F. del Monte, J. H. Clark and M. J. MacLachlang, Chem. Soc. Rev., 2015, 44, 250–290 RSC; (e) S. Navalon, A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Chem. Rev., 2014, 114, 6179–6212 CrossRef CAS PubMed.
- (a) J. Diao, H. Liu, J. Wang, Z. Feng, T. Chen, C. Miao, W. Yang and D. S. Su, Chem. Commun., 2015, 51, 3423–3425 RSC; (b) R. Rao, M. Yang, Q. Ling, C. Li, Q. Zhang, H. Yang and A. Zhang, Catal. Sci. Technol., 2014, 4, 665–671 RSC; (c) P. Janus, R. Janus, P. Kustrowski, S. Jarczewski, A. Wach, A. M. Silvestre-Albero and F. Rodriguez-Reinoso, Catal. Today, 2014, 235, 201–209 CrossRef CAS.
- (a) C. A. Gärtner, A. C. van Veen and J. A. Lercher, ChemCatChem, 2013, 5, 3196–3217 CrossRef; (b) X. Sun, Y. Ding, B. Zhang, R. Huang, D. Chen and D. S. Su, ACS Catal., 2015, 5, 2436–2444 CrossRef CAS; (c) V. Zarubina, C. Nederlof, B. van der Linden, F. Kapteijn, H. J. Heeres, M. Makkee and I. Melián-Cabrera, J. Mol. Catal. A: Chem., 2014, 381, 179–187 CrossRef CAS; (d) Y. Marco, L. Roldán, E. Muñoz and E. García-Bordejé, ChemSusChem, 2014, 7, 2496–2504 CrossRef CAS PubMed.
- (a) R. Wang, X. Sun, B. Zhang, X. Sun and D. Su, Chem.–Eur. J., 2014, 20, 6324–6331 CrossRef CAS PubMed; (b) L. Liu, Q. F. Deng, B. Agula, X. Zhao, T. Z. Ren and Z. Y. Yuan, Chem. Commun., 2011, 47, 8334–8336 RSC; (c) L. Liu, Q. F. Deng, Y. P. Liu, T. Z. Ren and Z. Y. Yuan, Catal. Commun., 2011, 16, 81–85 CrossRef CAS; (d) C. Duong-Viet, H. Ba, Y. Liu, L. Truong-Phuoc, J. M. Nhut and C. Pham-Huu, Chin. J. Catal., 2014, 35, 906–913 CrossRef CAS.
- (a) J. Zhang, D. S. Su, R. Blume, R. Schlögl, R. Wang, X. Yang and A. Gajović, Angew. Chem., Int. Ed., 2010, 49, 8640–8644 CrossRef CAS PubMed; (b) Z. K. Zhao, Y. T. Dai, J. H. Lin and G. R. Wang, Chem. Mater., 2014, 26, 3151–3161 CrossRef CAS.
- (a) J. Wang, H. Liu, J. Diao, X. Gu, H. Wang, J. Rong, B. Zong and D. S. Su, J. Mater. Chem. A, 2015, 3, 2305–2313 RSC; (b) T. T. Thanh, H. Ba, L. Truong-Phuoc, J. M. Nhut, O. Ersen, D. Begin, I. Janowska, D. L. Nguyen, P. Grangerd and C. Pham-Huu, J. Mater. Chem. A, 2014, 2, 11349–11357 RSC; (c) Z. K. Zhao, Y. T. Dai and G. F. Ge, Catal. Sci. Technol., 2015, 5, 1548–1557 RSC; (d) Z. K. Zhao, Y. T. Dai, G. F. Ge, Q. Mao, Z. M. Rong and G. R. Wang, ChemCatChem, 2015, 7, 1070–1077 CrossRef CAS; (e) Z. K. Zhao and Y. T. Dai, J. Mater. Chem. A, 2014, 2, 13442–13451 RSC; (f) H. Liu, J. Diao, Q. Wang, S. Gu, T. Chen, C. Miao, W. Yang and D. Su, Chem. Commun., 2014, 50, 7810–7812 RSC.
- (a) Z. K. Zhao, Y. T. Dai, G. F. Ge and G. R. Wang, ChemCatChem, 2015, 7, 1135–1144 CrossRef CAS; (b) Z. K. Zhao, Y. T. Dai, G. F. Ge and G. R. Wang, Chem.–Eur. J., 2015, 21, 8004–8009 CrossRef CAS PubMed; (c) J. Zhu, A. Holmen and D. Chen, ChemCatChem, 2013, 5, 378–401 CrossRef CAS.
- Y. Xue, B. Wu, L. Jiang, Y. Guo, L. Huang, J. Chen, J. Tan, D. Geng, B. Luo, W. Hu, G. Yu and Y. Liu, J. Am. Chem. Soc., 2012, 134, 11060–11063 CrossRef CAS PubMed.
- J. Chen, X. Wang, X. Cui, G. Yang and W. Zheng, Chem. Commun., 2014, 50, 557–559 RSC.
- Y. Xia and R. Mokaya, Chem. Mater., 2005, 17, 1553–1560 CrossRef CAS.
- Z. Lin, G. Waller, Y. Liu, M. Liu and C. P. Wong, Adv. Energy Mater., 2012, 2, 884–888 CrossRef CAS.
- Y. V. Kissin, Catal. Rev., 2001, 43, 85–146 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details for catalyst preparation, characterization, and catalytic performance measurement and the extra characterization results. See DOI: 10.1039/c5ra08754f |
| This journal is © The Royal Society of Chemistry 2015 |