The impact of zinc oxide nanoparticles on nitrification and the bacterial community in activated sludge in an SBR
S. T. Wang*a,
S. P. Lib,
W. Q. Wanga and
H. Youc
aSchool of Municipal and Environmental Engineering, State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology, 73, Huanghe Rd., Nangang Dist., Harbin 150090, China. E-mail: wshutao@126.com; 734067974@qq.com
bEnvironmental Monitoring Station of Guangzhou Military Region, Guangzhou 510517, P. R. China. E-mail: lisuping1010@163.com
cSchool of Marine Science and Technology, Harbin Institute of Technology at Weihai, Weihai 264209, China. E-mail: youhong@hit.edu.cn
First published on 31st July 2015
Abstract
Zinc oxide (ZnO) nanoparticles (NPs) have been reported to induce adverse effects on organisms. The impacts of ZnO NPs on nitrification and the nitrobacteria community in activated sludge were investigated in a simulated SBR. It revealed that ZnO NPs at low concentrations (5 and 10 mg L−1) slightly inhibited nitrification. At these concentrations the activity of ammonia monooxygenase (AMO) and nitrite oxidoreductase (NOR) as well as the cell membrane integrity of Nitrosomonas europaea were almost unaffected. Concentrations of 20 mg L−1 and 50 mg L−1 ZnO NPs had significantly adverse effects on the activity of AMO and NOR and on the transformation of NH4+–N to NO2−–N and NO2−–N to NO3−–N. Analysis by denaturing gradient gel electrophoresis (DGGE) revealed that higher concentrations of ZnO NPs significantly inhibited the growth of the typical ammonia-oxidizing bacteria (AOB) that were mainly responsible for oxidation of ammonia to nitrate. Moderate concentrations of ZnO NPs could accelerate the growth of some types of denitrifying bacteria and promote the growth of some pathogenic bacteria. Moderate and high concentrations of ZnO NPs could obviously destroy the integrity of the cell membrane of Nitrosomonas europaea. These findings meaningfully assessed the adverse effects of ZnO NPs on activated sludge in wastewater treatment.
1. Introduction
The development and application of nanotechnology have raised significant concerns about the adverse effects of nanoparticles (NPs) on human health and the environment.1 NPs can be more toxic than larger particles of the same composition because of their large specific surface area and unique size-effect.2,3 Many studies have thus been conducted to predict their environmental concentrations and investigate the behavior of NPs in the environment.4–6 The increasing utilization of products containing NPs, however, was observed to result in the release of NPs into wastewater treatment plants (WWTPs).7–9 Large amounts of zinc oxide nanoparticles (ZnO NPs) have been used in semiconductors, plastic additives, pigments and cosmetics.10 A recent study confirmed that ZnO NPs were present in sewage sludge and effluents. According to the report issued by the USEPA in 2009, an examination of 84 WWTPs showed that the zinc content in WWTP biosolids was 8.55 g kg−1-SS.11 The investigations in China in 2011 (139 WWTPs in total) and in 2009 (107 WWTPs in total) showed that the average concentration of Zn in biosolids was 1.03 g kg−1-SS and that the maximum concentration was 9.14 g kg−1-SS.12–14 Concerns can therefore be raised about whether the NPs in WWTPs have negative impacts on the microbial community in activated sludge, which may eventually hamper the function of WWTPs in removing pollutants from wastewater, such as chemical oxygen demand (COD), nitrogen and phosphorous.Recently, studies have started to address this issue. Zheng et al.15 reported that the presence of 10 and 50 mg L−1 of ZnO NPs decreased the total nitrogen removal efficiencies from 81.5% to 75.6% and 70.8%, respectively, compared with the absence of ZnO NPs. Additionally, several other publications indicated that different NPs and exposure times showed different effects on biological nitrogen removal. For instance, Zheng et al.15 reported that 1 mg L−1 of SiO2 NPs caused no adverse acute and chronic effects on sludge viability and wastewater nitrogen removal, while chronic exposure to 50 mg L−1 SiO2 NPs depressed the total nitrogen (TN) removal efficiency from 79.6% to 51.6% after a 70 day exposure. Chen et al.16 found that short-term exposure to 1 and 50 mg L−1 Al2O3 NPs induced only marginal influences on wastewater nitrification and denitrification. Nevertheless, prolonged exposure to 50 mg L−1 Al2O3 NPs was observed to decrease the TN removal efficiency from 80.4% to 62.5%. Ni et al.17 found that the short-term presence of 50–200 mg L−1 of NPs decreased the TN removal efficiency resulting from the acute toxicity of a shock load of magnetic NPs, while long-term exposure to 50 mg L−1 magnetic NPs was observed to significantly improve the TN removal efficiency. Zheng et al.7 found that concentrations of 1 and 50 mg L−1 TiO2 NPs had no acute effects on nitrogen removal from wastewater after a short-term exposure (1 day), while 50 mg L−1 TiO2 NPs (higher than its environmentally relevant concentration) was observed to significantly decrease the TN removal efficiency from 80.3% to 24.4% after long-term exposure (70 days). Li et al.18 found that 2–50 mg L−1 of TiO2 NPs did not adversely affect nitrogen removal, but when the activated sludge was exposed to 100–200 mg L−1 of TiO2 NPs, the effluent TN removal efficiencies were 36.5% and 20.3%, respectively, which were markedly lower than the values observed in the control test (80%). Most of the above studies demonstrated that NPs hampered the function of the WWTP in removing nitrogen from wastewater. However, it is still not well known how ZnO NPs affect nitrification in activated sludge and which step of nitrification is more sensitive to ZnO NPs.
As is well known, the biological removal of nitrogen in wastewater is achieved by complex microbial populations that are responsible for nitrification and denitrification.7,19,20 Therefore, the diversity of the microbial populations and a stable bacterial community structure both play important roles in achieving a high efficiency of biological nitrogen removal. Previous publications noted that silver NPs could cause a 50% inhibition of the respiration of nitrifying bacteria at a concentration of 0.14 mg L−1,21 whereas Cu NPs showed no inhibitory effect on the respiration of ammonia-oxidizing bacteria at the level of 10 mg L−1.22 Chen et al.16 indicated that, compared with the control, 50 mg L−1 Al2O3 NPs decreased the abundance of denitrifying bacteria in activated sludge according to quantitative polymerase chain reaction (PCR) assays. Ni et al.17 reported that a short-term exposure to 50 mg L−1 magnetic NPs led to the abatement of nitrifying bacteria according to fluorescence in situ hybridization (FISH) assays. According to Zheng et al.,7 denaturing gradient gel electrophoresis (DGGE) profiles showed that 50 mg L−1 TiO2 NPs clearly reduced the diversity of the microbial community in activated sludge, and FISH analysis indicated that the abundance of nitrifying bacteria, especially ammonia-oxidizing bacteria, was significantly decreased after long-term exposure to 50 mg L−1 TiO2 NPs. Similarly, according to Li et al.,18 the DGGE profiles showed that 200 mg L−1 of TiO2 NPs significantly reduced the microbial diversity in the activated sludge. Sheng et al.23 found that the microbial susceptibility to Ag NPs was different for each microorganism. For instance, Thiotrichales is more sensitive to Ag NPs than other biofilm bacteria. These results indicated that different types of NPs showed different influences on bacteria in WWTPs and that the microbial susceptibility to NPs is different. However, to date, how the presence of ZnO NPs affect the nitrifying bacterial community in activated sludge is still not well known.
The objectives of this study are to (a) evaluate how ZnO NPs affect nitrification in an aerobic activated sludge system; (b) determine the toxicity of ZnO NPs to the typical bacteria in activated sludge, which leads to the deterioration of biological nitrogen removal; and (c) explore the effects of ZnO NPs on the typical bacterial diversity in activated sludge.
2. Experimental
2.1 Materials and methods
The activated sludge was cultured in the SBR with a working volume of 4 L, which was operated to achieve biological nitrogen removal. The SBR operated at 25–28 °C with three cycles each day. Each cycle (8 h) consisted of 5.6 h of aeration, followed by 1 h for settling, 15 min for decanting and 1 h for idling. The influent pH was adjusted to 7.5 by adding NaOH, NaHCO3 and HCl. Air was provided intermittently by using an on/off controller with an online DO detector to maintain DO at an appropriate level. Sludge was wasted to keep the solids retention time (SRT) at approximately 22 days to maintain the ratio of mixed liquor volatile suspended solid (MLVSS) to mixed liquor suspended solids (MLSS), namely, MLVSS/MLSS at 0.75. The reactor was constantly mixed with a stirrer except during the settling, decanting, and idle periods. After approximately 3 months, the stable removal efficiencies of nitrogen (>90.0%) were achieved. The configuration of the reactor setup is shown in Fig. 1.
2.2. Analytical methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min at 6–10 °C, and then, the precipitate was resuspended to 30 mL and repeated twice. The pretreated activated sludge was dissolved in 15 mL of TE buffer solution. Bulk genomic DNA was extracted using sodium dodecyl sulfate (SDS) hexadecyltrimethyl ammonium bromide (CTAB), and the products were examined by agarose (1% w/v) gel electrophoresis in Tris/borate/EDTA buffer (TBE).
000g for 10 min at 6–10 °C, and then, the precipitate was resuspended to 30 mL and repeated twice. The pretreated activated sludge was dissolved in 15 mL of TE buffer solution. Bulk genomic DNA was extracted using sodium dodecyl sulfate (SDS) hexadecyltrimethyl ammonium bromide (CTAB), and the products were examined by agarose (1% w/v) gel electrophoresis in Tris/borate/EDTA buffer (TBE).The 16S rDNA variable region of the extracted DNA was amplified with primers 27F, with a GC-clamp (5′-AGAGTTTGATCMTGGCTCAG-3′), and 1492R (5′-GWATTACCGCGGCKG CTG-3′). PCR amplification was carried out in a total volume of 50 μL containing 2 μL of template DNA, 5 μL of Ex Taq reaction buffer, 0.25 μL of Ex Taq polymerase, 4 μL of dNTPs, 1 μL of forward primers and 1 μL of reverse primers (Takara, Japan) using a PE 2700 thermocycler (Biometra T-Gradient). The amplification program consisted of an initial denaturation step at 94 °C for 5 min, denaturation at 94 °C for 45 s, annealing at 55 °C for 45 s and extension at 72 °C for 60 s, followed by a final extension at 72 °C for 10 min. The PCR products were electrophoresed on 6% polyacrylamide gel in 1× TAE buffer with gradients ranging from 30% to 60% denaturant (100% denaturant: 7 M urea and 40% (v/v) deionized formamide) at a constant voltage of 60 V at 60 °C for 12 h.
The gel was stained with EB for 15 min and viewed with a BioRad Gel Documentation system (BioRad). The prominent bands were then excised from the gel, and after cleanup treatment, the recovered DNA was reamplified (initial denaturation at 94 °C for 3 min, denaturation at 94 °C for 30 s, annealing at 50 °C for 30 s and extension at 72 °C for 90 s, followed by a final extension at 72 °C for 6 min), purified, and cloned into the pMD19-T Simple vector (TaKaRa, Japan). The sequences from this study were submitted to the GenBank database. The closest matching sequences were searched using the BLAST program.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g and 4 °C for 10 min, and the crude extracts in the supernatant were obtained for the enzyme activity measurement. All of the key enzymes activities were based on the protein content as determined by the BCA method. Assays for AMO and NOR activity were performed in stoppered serum vials (10 mL) containing 100 μL of crude extract and 1.9 mL of 0.01 M phosphate buffer (containing 2 mM (NH4)2SO4, pH 7.4) or 0.01 M phosphate buffer (containing 1 mM NaNO2, pH 7.4). The vials were shaken in a water bath at 30 °C for 30 min and were then centrifuged immediately. This was followed by measuring the increase of nitrite in the AMO activity assay or the decrease of nitrite in the NOR activity assay in the supernatant. The specific AMO and NOR activities were presented as the production of μmol of nitrite per min per mg protein and the reduction of μmol of nitrite per min per mg protein, respectively.7
000g and 4 °C for 10 min, and the crude extracts in the supernatant were obtained for the enzyme activity measurement. All of the key enzymes activities were based on the protein content as determined by the BCA method. Assays for AMO and NOR activity were performed in stoppered serum vials (10 mL) containing 100 μL of crude extract and 1.9 mL of 0.01 M phosphate buffer (containing 2 mM (NH4)2SO4, pH 7.4) or 0.01 M phosphate buffer (containing 1 mM NaNO2, pH 7.4). The vials were shaken in a water bath at 30 °C for 30 min and were then centrifuged immediately. This was followed by measuring the increase of nitrite in the AMO activity assay or the decrease of nitrite in the NOR activity assay in the supernatant. The specific AMO and NOR activities were presented as the production of μmol of nitrite per min per mg protein and the reduction of μmol of nitrite per min per mg protein, respectively.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 5 min. Then, the supernatant was seeded on a 96-well plate, followed by the addition of 50 μL of substrate mix (Tiangen). After incubation at room temperature for 30 min in the dark, 50 μL of stop solution (Tiangen) was added to each well and the absorbance was recorded at 490 nm using a microplate reader (BioTek).
000g for 5 min. Then, the supernatant was seeded on a 96-well plate, followed by the addition of 50 μL of substrate mix (Tiangen). After incubation at room temperature for 30 min in the dark, 50 μL of stop solution (Tiangen) was added to each well and the absorbance was recorded at 490 nm using a microplate reader (BioTek).2.3 Statistical analysis
All of the tests were performed in triplicate, and the results were expressed as the mean ± standard deviation. Analysis of variance (ANOVA) was used to examine the significance of the results, and p < 0.05 was considered to be statistically significant.3. Results and discussion
3.1 Effects of ZnO NPs on biological nitrification
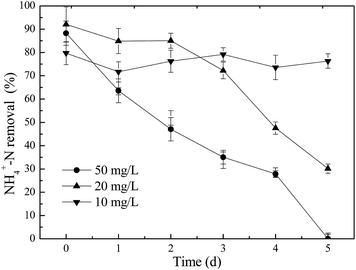
As is well known, biological nitrogen removal depends on the successful oxidation of ammonia and nitrate denitrification to N2. Fig. 2 presents the NH4+–N removal efficiency of the SBR when exposed to ZnO NPs. It can be observed that NH4+–N removal was only decreased from 79.8% to 76.3% when it was exposed to 10 mg L−1 ZnO NPs, which was not much different from the control, suggesting that 10 mg L−1 of ZnO NPs showed no measurable effect on nitrogen removal. However, the presence of 20 and 50 mg L−1 of ZnO NPs decreased NH4+–N removal efficiency from 82.4% to 20% and from 88.3% to 0%, respectively, after 5 days of exposure, which were remarkably lower values than those of the control. This indicated that a shock load of 20 and 50 mg L−1 ZnO NPs had caused significant toxicity to activated sludge and finally deteriorated the nitrogen removal performance of activated sludge. It suggests that the nitrogen removal was inhibited by higher concentrations of ZnO NPs. Similarly, Zheng et al.7 found that a low concentration of ZnO NPs showed no measurable effect on nitrogen removal. In contrast, when the concentration of ZnO NPs was 50 mg L−1, TN removal was significantly affected.To probe the effects of ZnO NPs on nitrification, the transformations of NH4+–N to NO2−–N and of NO2−–N to NO3−–N were investigated during one cycle when activated sludge was exposed to different concentrations of ZnO NPs.
As seen from Fig. 3, the concentrations of NH4+–N, NO2−–N and NO3−–N were relatively stable in the presence of 10 mg L−1 ZnO NPs during one cycle, and the variations of NH4+–N and NO2−–N were not significantly different from their respective controls. Nevertheless, in the presence of 20 mg L−1 ZnO NPs, the level of NO3−–N significantly differed from that of the control, suggesting that the presence of 20 mg L−1 ZnO NPs had an adverse effect on the transformation of NO2−–N to NO3−–N.
 | ||
| Fig. 3 Effects of ZnO NPs on the transformation of nitrogen to different forms during one cycle of the SBR. | ||
At the concentrations of 20 and 50 mg L−1 ZnO NPs (Fig. 3), the NH4+–N removal was inhibited. Moreover, little NH4+–N was oxidized into NO2−–N which appears to immediately be oxidized to nitrate, suggesting that ZnO NPs affected ammonia oxidizing bacteria not nitrite oxidizing bacteria. On the basis of Fig. 3, when the concentration of ZnO NPs reached 50 mg L−1, the variations of NH4+–N were not significantly different from 20 mg L−1 ZnO NPs, but the levels of NO2−–N and NO3−–N significantly differed from the respective control, indicating that the transformation of NH4+–N to NO2−–N were significantly inhibited.
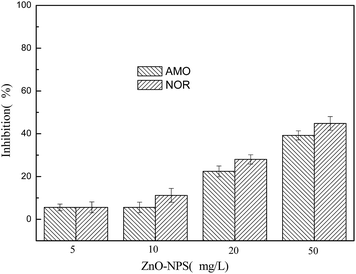
Further investigation showed that ZnO NPs influenced the activities of enzymes relevant to nitrogen removal. AMO and NOR are two key enzymes in nitrification. As seen in Fig. 4, 5 and 10 mg L−1 of ZnO NPs showed less inhibition of AMO and NOR activities. However, when the activated sludge was exposed to 20 and 50 mg L−1 of ZnO NPs for 5 days, compared to the control, the inhibition rate of AMO activity was 22.42% and 39.24%, respectively, and the inhibition rate of NOR activity was 28.03% and 44.84%, respectively. These results indicated that 20 and 50 mg L−1 of ZnO NPs significantly inhibited these two enzymes, which led to the inhibition of the normal function of nitrifying bacteria. The inhibition was almost in a dosing manner. These observations were also consistent with the changes in the transformations of NH4+–N to NO2−–N and of NO2−–N to NO3−–N during one cycle of SBR and the lower NH4+–N removal efficiencies at ZnO NPs concentrations of 20 and 50 mg L−1, as shown in Fig. 3.
 | ||
| Fig. 4 Effects of ZnO NPs on the activities of AMO and NOR during one cycle of the SBR. Exposure time: 5 days. | ||
3.2 Effects of ZnO NPs on the microbial community structure in activated sludge
Although the antimicrobial capabilities of NPs are widely reported, their impacts on ecological microbial communities are not well understood. Nitrification is carried out by a group of bacteria that are capable of using nitrate in place of oxygen as an electron acceptor for respiration. In previous studies,27 NPs were revealed to be toxic to both Gram-negative and Gram-positive bacteria, suggesting that higher concentrations of ZnO NPs in activated sludge might also decrease the abundance of denitrifying bacteria. For this reason, the effects of ZnO NPs on changes in the bacterial diversity of activated sludge were investigated. It can be seen from Fig. 5 that the activated sludge in the control showed high bacterial diversity. According to the detailed information on the bands in the DGGE profiles (Table 1), 50 mg L−1 of ZnO NPs significantly inhibited the growth of typical ammonia-oxidizing bacteria (AOB) (band b3 and b12, related to Nitrosococcus sp. and band 4, related to Nitrosomonas sp.). These microorganisms are mainly responsible for the oxidation of ammonia to nitrate. Moreover, the activity of microorganisms, such as Thioflavicoccus mobilis (band 10), which is mainly responsible for vulcanization, was also inhibited. Some bacteria associated with denitrification (band b8, related to Nitratiruptor sp. and band b14, related to Pseudomonas sp.) would be promoted by 20 mg L−1 of ZnO NPs, but inhibited by 50 mg L−1 of ZnO NPs. The activity of other pathogenic bacteria, such as Xanthomonas hortorum pv. (band b7) and Stenotrophomonas maltophilia (band b9), were promoted by ZnO NPs, two types of which could result in death and the rapid aging of activated sludge. Moreover, ZnO NPs had no significant influence on the activity of other bacteria, such as Thioalkalivibrio sulfidophilus (band b13).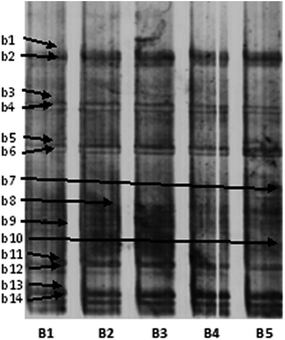 | ||
| Fig. 5 DGGE profiles of the bacterial communities of activated sludge in SBRs. (B1) Without addition of ZnO NPs (control); (B2–B5) with addition of 5, 10, 20 and 50 mg L−1 ZnO NPs, respectively. Detailed information on the bands (b1–b14) is presented in Table 1. | ||
| Band ID | Closely related sequences from GenBank | Accession no. | Identity (%) |
|---|---|---|---|
| b1 | Pseudoxanthomonas suwonensis | NC_014924.1 | 99 |
| b2 | Bifidobacterium animalis subsp. | NC_011835.1 | 100 |
| b3 | Nitrosococcus oceani | NC_007484.1 | 86 |
| b4 | Nitrosomonas sp. | NC_015222.1 | 88 |
| b5 | Verrucomicrobium spinosum | NZ_ABIZ01000001.1 | 93 |
| b6 | Arcobacter nitrofigilis | NC_014166.1 | 83 |
| b7 | Xanthomonas hortorum pv. | NZ_CM002307.1 | 96 |
| b8 | Nitratiruptor sp. | NC_009662.1 | 84 |
| b9 | Stenotrophomonas maltophilia | NC_010943.1 | 96 |
| b10 | Thioflavicoccus mobilis | NC_019940.1 | 91 |
| b11 | Pseudomonas denitrificans | NC_020829.1 | 94 |
| b12 | Nitrosococcus watsonii | NC_014315.1 | 88 |
| b13 | Thioalkalivibrio sulfidophilus | NC_011901.1 | 91 |
| b14 | Pseudomonas sp. | NC_019670.1 | 95 |
Overall, the results indicated that ZnO NPs would inhibit the growth of bacteria associated with nitrification and sulfofication. Moderate concentrations of ZnO NPs (20 mg L−1) could accelerate the growth of some types of denitrifying bacteria. ZnO NPs could promote the growth of some pathogenic bacteria, which caused activated sludge to rapidly age and die. Moreover, ZnO NPs had no obvious influence on the activity of bacteria, such as Thioalkalivibrio sulfidophilus.
3.3 Effects of ZnO NPs on Nitrosomonas europaea (ATCC 19718)
 | ||
| Fig. 6 Effects of ZnO NPs on NO2−–N production and the production difference. (A): NO2−–N concentration rate over 180 min; (B): NO2−–N production rate for the first 60 min exposure. | ||
It can be observed that concentrations of 20 and 50 mg L−1 ZnO NPs had a significant inhibitory effect on the rate of nitrite formation within 60 min. For the 5 and 10 mg L−1 ZnO NPs exposure, the NO2−–N production rate was similar to the control, suggesting that ZnO NPs did not show a significant inhibitory effect on Nitrosomonas europaea at low exposure concentrations within 60 min. At high concentrations (20 and 50 mg L−1), ZnO NPs might inhibit Nitrosomonas europaea by destroying the AMO activity damaging the integrity of the cell membrane. Similarly, Yuan et al.25 investigated the impact of Ag NPs (7 ± 3 and 40 ± 14 nm) with different coatings on Nitrosomonas europaea, and found that Ag-NPs caused damage to the cell wall and cell membrane of Nitrosomonas europaea and caused the nucleoids to disintegrate and condense, leading to the inhibition of some important protein functions.
 | ||
| Fig. 7 Effects of ZnO NPs on the integrity of the cell membrane of Nitrosomonas europaea. Exposure time: 4 h. | ||
To observe the effects of ZnO NPs on the cell membrane integrity of Nitrosomonas europaea, TEM was used to characterize the surface morphology of the cells after Nitrosomonas europaea was exposed to different concentrations of ZnO NPs (Fig. 8).
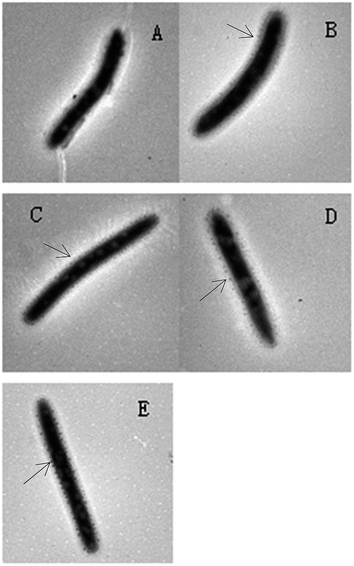 | ||
| Fig. 8 TEM images of Nitrosomonas europaea exposed to different concentrations of ZnO NPs (A) control, (B) 5 mg L−1, (C) 10 mg L−1, (D) 20 mg L−1 and (E) 50 mg L−1 of ZnO NPs. | ||
As seen from Fig. 8, Nitrosomonas europaea appeared as a rod shape, which was consistent with the description in Berger's Manual of Determinative Bacteriology (version 8).26 Compared with the control (Fig. 8(a)), adsorbed ZnO NPs can be observed around the Nitrosomonas europaea cells in all of the treatments (Fig. 8(b)–(e)). From Fig. 8(b) and (c), it can be found that only a small amount of ZnO NPs was adsorbed on the Nitrosomonas europaea surface. A lesser change in the morphology of cells as well as less damage in the cell membrane was observed. Moreover, it can clearly be seen that more and more ZnO NPs were adsorbed on the Nitrosomonas europaea surface as the concentrations of ZnO NPs increased to 20 and 50 mg L−1 (Fig. 8(d) and (e)). As is known, the adsorption of ZnO NPs might hinder the transport of organics. Additionally, damage to the cell membrane can lead to the leakage of intracellular substances, which affects the normal physiological functions of the cell. These observed results were consistent with the results above (from Fig. 2 to 7). Similarly, Yuan et al.25 also found that Ag NPs caused damage to the cell wall and cell membrane of Nitrosomonas europaea and caused the nucleoids to disintegrate and condense and thus inhibited some protein functions. In short, the impact of ZnO NPs on Nitrosomonas europaea included the adsorption of ZnO NPs onto the cell surface, damage to the cell membrane and loss of cytoplasm (intracellular plasmids and inclusions).
4. Conclusions
By adding different concentrations of ZnO NPs to SBR, we evaluated how ZnO NPs affect nitrification in activated sludge. We investigated the toxicity of ZnO NPs to typical nitrobacteria in activated sludge and explored the effect of ZnO NPs on the bacterial diversity in activated sludge. It is concluded that low concentrations of ZnO NPs slightly inhibited nitrification and that the activities of ammonia monooxygenase (AMO) and nitrite oxidoreductase (NOR), as well as the integrity of the cell membrane of Nitrosomonas europaea, were almost unaffected. Moderate and high concentrations of ZnO NPs had an adverse effect on the activities AMO and NOR and the transformation of both NH4+–N to NO2−–N. Denaturing gradient gel electrophoresis (DGGE) analysis revealed that high concentrations of ZnO NPs have a significant inhibition on the growth of many typical ammonia-oxidizing bacteria (AOB), which are mainly responsible for the oxidation of ammonia to nitrate. Moderate concentrations of ZnO NPs could promote the growth of some pathogenic bacteria, which caused activated sludge to rapidly age and die. A high concentration of ZnO NPs obviously destroyed the integrity of the cell membrane of Nitrosomonas europaea. These findings meaningfully assessed the adverse effects of ZnO NPs on activated sludge in wastewater treatment.Acknowledgements
The Project was sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, China. This work was also supported by the Key Laboratory of Urban Water Resource and Environment of Harbin institute of technology, China (ES201312). The authors acknowledge the support of Yufeng Liu, a technician from The Academy of Quality Supervision and Inspection in Heilongjiang Province.References
- G. Liu, D. Wang and J. Wang, Effect of ZnO particles on activated sludge: role of particle dissolution, Sci. Total Environ., 2011, 409(14), 2852–2857 CrossRef CAS PubMed.
- J. R. Morones, J. L. Elechiguerra and A. Camacho, The bactericidal effect of silver nanoparticles, Nanotechnology, 2005, 16(10), 2346 CrossRef CAS PubMed.
- A. Nel, T. Xia and L. Mädler, Toxic potential of materials at the nanolevel, Science, 2006, 311, 622–627 CrossRef CAS PubMed.
- H. Ma, P. L. Williams and S. A. Diamond, Ecotoxicity of manufactured ZnO nanoparticles-a review, Environ. Pollut., 2013, 172, 76–85 CrossRef CAS PubMed.
- A. García, L. Delgado and J. A. Torà, Effect of cerium dioxide, titanium dioxide, silver, and gold nanoparticles on the activity of microbial communities intended in wastewater treatment, J. Hazard. Mater., 2012, 199, 64–72 CrossRef PubMed.
- H. Mu, Y. Chen and N. Xiao, Effects of metal oxide nanoparticles (TiO2, Al2O3, SiO2 and ZnO) on waste activated sludge anaerobic digestion, Bioresour. Technol., 2011, 102(22), 10305–10311 CrossRef CAS PubMed.
- X. Zheng, R. Wu and Y. Chen, Effects of ZnO nanoparticles on wastewater biological nitrogen and phosphorus removal, Environ. Sci. Technol., 2011, 45(7), 2826–2832 CrossRef CAS PubMed.
- F. Gottschalk, T. Sonderer and R. W. Scholz, Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions, Environ. Sci. Technol., 2009, 43(24), 9216–9222 CrossRef CAS PubMed.
- M. Kiser, P. Westerhoff and T. Benn, Titanium nanomaterial removal and release from wastewater treatment plants, Environ. Sci. Technol., 2009, 43(17), 6757–6763 CrossRef CAS.
- B. Wu, Y. Wang and Y.-H. Lee, Comparative eco-toxicities of nano-ZnO particles under aquatic and aerosol exposure modes, Environ. Sci. Technol., 2010, 44(4), 1484–1489 CrossRef CAS PubMed.
- USEPA, Targeted national sewage sludge survey sampling and analysis technical report, US Environmental Protection Agency, Washington, DC, 2009, http://www.epa.gov/waterscience/biosolids/tnsss-tech Search PubMed.
- X. Mei, Z. Wang and X. Zheng, Soluble microbial products in membrane bioreactors in the presence of ZnO nanoparticles, J. Membr. Sci., 2014, 451, 169–176 CrossRef CAS PubMed.
- X. Ma, H. Weng and J. Zhang, Regional characteristics and trend of heavy metals and nutrients of sewage sludge in China, China Environ. Sci., 2011, 31(8), 1306–1313 CAS.
- J. Yang, G. H. Guo and T. B. Chen, Concentrations and variation of heavy metals in municipal sludge of China, China Water Wastewater, 2009, 25(13), 122–124 CAS.
- X. Zheng, Y. Su and Y. Chen, Acute and chronic responses of activated sludge viability and performance to silica nanoparticles, Environ. Sci. Technol., 2012, 46(13), 7182–7188 CrossRef CAS PubMed.
- Y. Chen, Y. Su and X. Zheng, Alumina nanoparticles-induced effects on wastewater nitrogen and phosphorus removal after short-term and long-term exposure, Water Res., 2012, 46(14), 4379–4386 CrossRef CAS PubMed.
- S. Q. Ni, J. Ni and N. Yang, Effect of magnetic nanoparticles on the performance of activated sludge treatment system, Bioresour. Technol., 2013, 143, 555–561 CrossRef CAS PubMed.
- D. Li, F. Cui and Z. Zhao, The impact of titanium dioxide nanoparticles on biological nitrogen removal from wastewater and bacterial community shifts in activated sludge, Biodegradation, 2013, 25(2), 167–177 CrossRef PubMed.
- T. Mino, M. van Loosdrecht and J. Heijnen, Microbiology and biochemistry of the enhanced biological phosphate removal process, Water Res., 1998, 32(11), 3193–3207 CrossRef CAS.
- R. J. Zeng, R. Lemaire and Z. Yuan, Simultaneous nitrification, denitrification, and phosphorus removal in a lab-scale sequencing batch reactor, Biotechnol. Bioeng., 2003, 84(2), 170–178 CrossRef CAS PubMed.
- O. Choi and Z. Hu, Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria, Environ. Sci. Technol., 2008, 42(12), 4583–4588 CrossRef CAS.
- R. Ganesh, J. Smeraldi and T. Hosseini, Evaluation of nanocopper removal and toxicity in municipal wastewaters, Environ. Sci. Technol., 2010, 44(20), 7808–7813 CrossRef CAS PubMed.
- Z. Sheng and Y. Liu, Effects of silver nanoparticles on wastewater biofilms, Water Res., 2011, 45(18), 6039–6050 CrossRef CAS PubMed.
- APHA, Standard Methods for the Examination of Water and Wastewater, American Public Health Association, Washington, DC, USA, 20th edn, 1998 Search PubMed.
- L. K. Adams, D. Y. Lyon and P. J. J. Alvarez, Comparative ecotoxicity of nanoscale TiO2, SiO2, and ZnO water suspensions, Water Res., 2006, 40, 3527–3532 CrossRef CAS PubMed; Z. H. Yuan, J. W. Li and L. Cui, Interaction of silver nanoparticles with pure nitrifying bacteria, Chemosphere, 2013, 90, 1404–1411 CrossRef PubMed.
- D. H. Bergey, G. K. John, R. S. Noel and H. A. Peter, Bergey's Manual of Determinative Bacteriology (Version 8), Lippincott Williams & Wilkins, 2014 Search PubMed.
- Y. Xiao and M. R. Wiesner, Transport and Retention of Selected Engineered Nanoparticles by Porous Media in the Presence of a Biofilm, Environ. Sci. Technol., 2013, 47(5), 2246–2253 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |