Nanonickel catalyst reinforced with silicate for methane decomposition to produce hydrogen and nanocarbon: synthesis by co-precipitation cum modified Stöber method†
U. P. M. Ashik and
W. M. A. Wan Daud*
Department of Chemical Engineering, University of Malaya, 50603, Kuala Lumpur, Malaysia. E-mail: upmashik@gmail.com; ashri@um.edu.my; Fax: +60 379675319; Tel: +60 105023818, +919496844805
First published on 1st May 2015
Abstract
Co-precipitation cum modified Stöber method is a continuous process avoiding application of higher temperature treatment before supporting nanometal with SiO2, irrespective of pre-preparation methods. We have conducted the co-precipitation process without undertaking calcination under air in order to avoid even a partial particle agglomeration and hence maintained average particle size ∼30 nm after enforcing with SiO2. This is the first report adopting such an unceasing preparation for preparing metal/silicate nanostructures. Furthermore, n-Ni/SiO2 nanostructured catalysts were used for thermocatalytic decomposition of methane to produce hydrogen and carbon nanotubes. The catalyst was found to be very stable and the methane transformation activity proceeded for 300 min on methane stream with little deactivation in the temperature range 475–600 °C. We have also successfully extended the catalyst preparation method for Fe and Co metals and conducted preliminary catalyst examinations.
Introduction
Nanostructured materials have recently attracted intensive attention by researchers mainly because of their inbuilt characteristics. Biology, optics, electronics, magnetism, sensing, etc. are some fields, chiefly working with nanostructures. Presently, nanostructures produced by applying the Stöber method are scarcely used in catalysis.1–8 Recent studies have revealed that the enforcement of nanomaterials with inert protective support can enhance the stability of the nanomaterial as well as changing the electron charge, reactivity and functionality of the material.9–11 Furthermore, nanometal/support composites show dissimilar and advanced properties from those of the individual metal and support materials.12 Nano-Ni particles have large specific surface area and obviously have large number of active sites which leads to intrinsic surface reactivity, and such nanoparticles tend to aggregate at high temperature, which results in low catalytic stability at higher temperatures. However, shielding of nanoparticles with porous, stable and inert silicates prevents particle agglomeration and gears up the catalyst for higher temperature performance. Silicate supported materials have an advantage of exhibiting a synergetic effect of both metal and support materials. In the case of n-Ni/SiO2 materials, the Ni phase provides the activity and the porous silica support causes reaction similar to mesoporous silica.To the best of our knowledge, for the first time we apply n-Ni/SiO2 catalyst prepared with co-precipitation cum modified Stöber method for thermocatalytic decomposition of methane (TCD) for the co-production of hydrogen and nanocarbon. Establishment of clean hydrogen fuel, which does not produce any greenhouse gases (GHG) upon combustion, can profoundly impact on two major contemporary challenges – amelioration of the energy crisis and reduction of environmental pollution from GHGs. The major resources and preparation methods for hydrogen are schematized in Fig. 1. Cell technology, petroleum refining, food, electronics, metallurgical processing industries and many other fields can be fueled by hydrogen and hence its use has attracted tremendous attention by current researchers.13–16 Global statistics demonstrate that 48% of hydrogen is produced form natural gas, corresponding to 240 billion cubic meters (Bcm) per year. Of the remainder, 30% (150 Bcm per year) comes from petroleum, and 18% (90 Bcm per year) from coal. Regrettably, only 4% (20 Bcm per year) is obtained through water electrolysis without producing any GHG.17,18 There are different types of methods that have been developed for hydrogen production, such as bio-hydrogen production, reviewed elsewhere,19 steam reforming of methane (SRM), partial oxidation (POX), coal gasification, water splitting, biomass gasification and thermochemical processes.20–23 Water splitting is a clean process as it consumes only renewable solar and wind energy, but is not economical because of its very low efficiency and higher processing cost. Furthermore, gasification and reforming of biomass are extensively explored for producing hydrogen from several biomass resources such as forest residues, wood wastes, crop residues, waste water treatment, biogas, etc.24,25 However, requirement of additional separation/purification treatments are the major limitations of these technologies which reduces hydrogen selectivity.26 SRM and POX are the normally adopted methods for producing hydrogen from methane gas. Among them, SRM has been considered as the most commonly adopted technique for recent years. However, SRM needs higher processing energy and results in the production of enormous levels of COx (at least 1 mol of CO2 mol−1 of converted methane) irrespective of its comparatively higher process efficiency (50%).13 Likewise, the POX process also causes massive GHG emission. Subsequently, thermocatalytic decomposition of methane (TCD) is attractive as a novel technique for eco-friendly hydrogen production. In this moderately endothermic process, methane is thermally decomposed to solid carbon and gaseous hydrogen in a technically simple one-step process as shown in eqn (1).
| CH4 → C + 2H2: ΔH298 K = 74.52 kJ mol−1 | (1) |
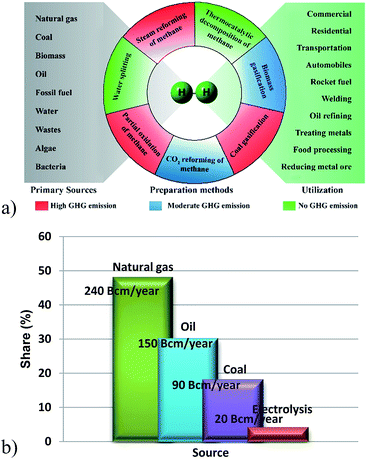 | ||
| Fig. 1 (a) Schematic representation of the sources, preparation methods and utilization of hydrogen and (b) worldwide hydrogen production by sources (reprinted with permission from Elsevier Limited).17 | ||
Moreover, the TCD process can enhance the production rate of single and multi-walled carbon nanotubes and fibers with high mechanical strength, irrespective of the arc-discharge evaporation to produce single-wall carbon nanotubes.27
In general, catalytic deactivation during the TCD process is mainly because of the substantial carbon deposition over the catalyst with time. This faster deactivation is the major challenge in TCD and studies are continuously being performed to develop catalysts with longer life as well as higher activity. It is well known that Ni-based catalysts are excellent in the TCD process.28,29 Takenaka et al.30 studied the effect of catalytic supports (MgO, Al2O3, SiO2, TiO2, ZrO2 MgO·SiO2, Al2O3·SiO2, H+-ZSM-5, etc.) for Ni for producing hydrogen and carbon nanofibers by TCD process and concluded that SiO2 is the most efficient catalyst support. Here, we have concentrated to study on SiO2 as a support to design a nanostructured catalyst with a longer life and higher activity.
In the present study, we report a new approach to prepare nanostructured n-Ni/SiO2 catalysts with a simple room-temperature processing denoted co-precipitation cum modified Stöber method; a continuous process avoiding application of higher temperature calcination before supporting metal nanoparticles with SiO2.1,31 This procedure aimed to produce fine nanoparticles avoiding n-NiO particle agglomeration which occurs if performing calcination before supporting with SiO2. Additionally, we have conducted TCD in a pilot plant to study its stability and activity at different temperatures with time on stream. We found that the as-prepared n-Ni/SiO2 catalyst exhibits high catalytic stability in comparison with traditional Ni/SiO2 catalysts. Furthermore, the co-precipitation cum modified Stöber method was extended to other metals such as iron and cobalt with the same SiO2 support, and preliminary activity inspection was conducted. Investigation of physicochemical properties of the catalyst was done by means of N2 adsorption–desorption measurement (BET), X-ray diffraction (XRD), high-resolution transmission electron microscopy (HRTEM), hydrogen temperature programmed reduction (H2-TPR), ammonia temperature programmed desorption (NH3-TPD) and thermogravimetric (TGA) analysis. In addition, the characterization of the formed nanocarbon fibers and tubes at various temperatures was made with the help of HRTEM and XRD.
Experimental section
Co-precipitation cum modified Stöber method is a combination of M–OH precipitation and SiO2 support formation over precipitated M–OH in a consecutive manner. A nanosized M–OH containing suspension was prepared by treating metal nitrate with ammonia solution at room temperature, which prevents agglomeration of metal oxides at comparatively higher temperature. The SiO2 support was fabricated through hydrolysis of a mixture of tetraethylorthosilicate (TEOS) and octadecyltrimethoxysilane (C18TMS) with aqueous ammonia.32Chemicals used
Nickel(II) nitrate hexahydrate (Ni(NO3)2·6H2O), cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O) and octadecyltrimethoxysilane (C18TMS) were purchased from Acros Organics. Iron(III) nitrate nonahydrate (Fe(NO3)3·9H2O) and tetraethyl orthosilicate (TEOS) were purchased from Aldrich and used as received. NH3 solution and ethanol bought from R&M solutions. 99.999% hydrogen, 99.995% methane and 99.99% nitrogen were purchased from Linde Malaysia Sdn. Bhd.Preparation of nano-Ni/SiO2 catalyst
200 mL of 0.1 molar Ni(NO3)2·6H2O solution was sonicated for 5 min and 6 mL of 30% NH3 solution added dropwise while sonicating. The solution precipitated the metal hydroxide under sonication for 1 h. The resulting suspension was then stirred for 1 h over a magnetic stirrer at room temperature. Then, the solution was centrifuged at 4000 rpm for 30 min and the precipitate washed twice with deionized water and once with ethanol. The product was transferred to 100 mL of ethanol and magnetically stirred for 15 h. The resulting suspension was sonicated for 10 min and 4 mL of 8 M NH3 solution added to make the suspension basic. 0.4 mL of TEOS and 0.4 mL of C18TMS were added simultaneously to the dispersion under sonication, and then the resulting mixture was sonicated for a further 60 min at room temperature. The solution was stirred for a further 5 h over a magnetic stirrer. The precipitate was separated by centrifugation and dried in an oven at 100 °C for 15 h. This was then calcined at 450 °C for 3 h to produce n-NiO/SiO2 (0.02) nanostructures. The produced nanocatalyst was treated with 30% H2 at 550 °C to reduce NiO before its activity examination and was denoted n-Ni/SiO2 (0.02). Nanostructures with higher nickel precursor concentrations such as 0.04 mol (n-Ni/SiO2 (0.04)) and 0.06 mol (n-Ni/SiO2 (0.06)) were also prepared. In order to prepare n-NiO particles, the suspension after 15 h of stirring (before adding silica precursors in the above procedure) was evaporated at 100 °C and calcined at 350 °C. The preparation method was extended to other metals such as Fe and Co with Fe(NO3)3·9H2O and Co(NO3)2·6H2O precursors, respectively.Characterization
 | (2) |
In eqn (2), the average crystallite size, peak length, line broadening full width at half-maxima after subtracting the instrumental line broadening (in radians), and the Bragg’s angle are expressed as Davg (nm), k (1.54056 Å), β, and 2θ, respectively. 0.9 is the Scherrer constant.
Catalytic activity
Results and discussion
Production of n-Ni/SiO2 nanocatalysts
Fine nanostructured Ni/SiO2 catalysts were synthesized by co-precipitation cum modified Stöber method. The Stöber method was adopted in order to safeguard nanometal active phase with SiO2 like inert materials. No surfactants were used in our method and SiO2 formation reaction was conducted in alcoholic medium avoiding water (water content may hasten hydrolysis process which results in particle agglomeration and leave free metal and SiO2 particles).34,35 Furthermore, free n-NiO particles are nearly eliminated in the final product by increasing the quantity. The overall process constituted of different stages as follows. (i) Precipitation of NiOH nanoparticles from precursor Ni(NO3)2·6H2O with NH3 solution; (ii) the produced fine nanoparticles were directly supported with SiO2 by the Stöber method.32 SiO2 protection was developed uniformly over dispersed NiOH particles with a mixture of C18TMS and TEOS. C18TMS was added to the reaction mixture so as to increase the porosity of SiO2. (iii) Porosity enhancement on SiO2 was performed by calcination under air at 450 °C and reduction at 550 °C, which removed all organic moieties and converted metal oxides to metal. It has been reported that aggregation of metal oxide nanoparticles at 450 °C is insignificant.36 The added C18TMS helps to prevent silica polymerization and produce more pores inside the silica network after calcination. Those heat treatments did not lead to metal particle agglomeration because of the silica coating. While the particle size of unprotected n-NiO were increased to large values (48.02 to 12933.53 nm) on reduction treatment, SiO2 supported structures maintained their mean size with only a minor increase from 32.19 nm to 52.78 nm (detailed BET results provided in Table 2). Different precursor quantities were experimented on in an attempt to enhance the yield without affecting its structure and properties of the catalyst. The major challenge observed in nano-compound processing is the quantity of the product employed in our method in terms of yield. However, the quality of the product in terms of activity (see Fig. S4†) and particle size distribution was found to be invariant (see HRTEM images; Fig. 5, S2 and S3†). Additionally, the method was extended to different active metals such as cobalt and iron. A series of measurements were conducted to characterize the nanostructures. Furthermore, activity and stability were studied for TCD at various temperature and methane feed flow rate in a fixed bed pilot plant.Characterization of the catalysts before TCD
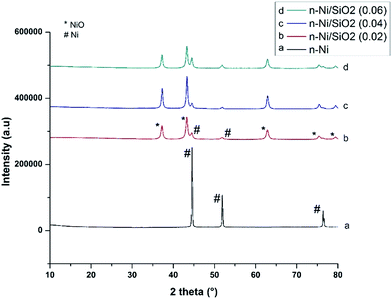 | ||
| Fig. 2 XRD patterns of (a) n-Ni, (b) n-Ni/SiO2 (0.02), (c) n-Ni/SiO2 (0.04) and (d) n-Ni/SiO2 (0.06). Peaks corresponding to NiO and Ni are indicated. | ||
| Sample | Ni(111)/nm | Ni(200)/nm | Ni(220)/nm | Avg./nm |
|---|---|---|---|---|
| n-Ni | 61.18 | 78.71 | 72.06 | 70.65 |
| n-Ni/SiO2 (0.02) | 28.54 | 43.84 | 29.26 | 33.88 |
| n-Ni/SiO2 (0.04) | 33.97 | 31.75 | 47.55 | 37.75 |
| n-Ni/SiO2 (0.06) | 31.14 | 29.11 | 29.24 | 29.83 |
| TCD-600 | 70.14 | 45.11 | 25.84 | 47.03 |
| TCD-550 | 70.15 | 25.77 | 29.45 | 41.79 |
| TCD-500 | 26.98 | 51.55 | 29.49 | 36 |
| TCD-475 | 26.97 | 72.11 | 51.58 | 50.22 |
In terms of activity, this does not influence the TCD process as methane itself acts as an excellent reducing agent, and no NiO phases were detected in XRD patterns after TCD (Fig. 10). Moreover, existing NiO phases are supposed to interact with porous silicate support resulting in accomplishment of a complex catalysis environment which likely leads to a more stable reaction course during the TCD of methane. However, the average crystallite size of the n-Ni and n-Ni/SiO2 calculated using the global Scherrer equation (Table 1) is evidently close to the mean particle size obtained from BET analysis (Table 2). The mean crystallite sizes (Table 1) clearly reveal that the protection of SiO2 over n-NiO prevents agglomeration. Hence, average crystallite size of n-NiO was 70.65 nm is reduced to around half this value when supported with SiO2. One can observe that the intensity and width of reflections of the NiO peaks in the n-Ni/SiO2 nanostructures change with precursor concentration. This can be attributed to the variation of the dispersion during silicate formation process accomplished from a mixture of TEOS and C18TMS in basic ethanol solution under sonication. Ultrasonic treatment is supposed to enhance the dispersion, while higher content of TEOS and C18TMS mixture may reduce such an effect.38 Hence, the variation in NiO dispersion at different precursor concentration shows an impact on the intensity and width of reflections of NiO.
| Catalyst | Single point SAam2 g−1 | BET SA/m2 g−1 | Micropore areab/m2 g−1 | Mesopore + external areac/m2 g−1 | Micropore volumed/cm3 g−1 | Total pore volumee/cm3 g−1 | Mesoporous volume/cm3 g−1 | BET pore size/nm | Mean particle size/nm |
|---|---|---|---|---|---|---|---|---|---|
| a Represents the values calculated at a relative pressure (P/P0) of N2 equal to 0.301.b Represents the values calculated from t-plot method.c Represents the values calculated from t-plot method.d Represents the values calculated from t-plot method.e Represents the total pore volume evaluated from nitrogen uptake at a relative pressure (P/P0) of N2 equal to 0.98. | |||||||||
| n-NiO | 62.22 | 62.46 | 5.17 | 57.28 | 0.0020 | 0.2499 | 0.2479 | 16.274 | 48.02 |
| n-NiO/SiO2 (0.02) | 91.50 | 93.18 | 5.17 | 88.01 | 0.0024 | 0.2301 | 0.2277 | 9.987 | 32.19 |
| n-NiO/SiO2 (0.04) | 90.62 | 92.53 | 6.24 | 86.28 | 0.0030 | 0.2036 | 0.2006 | 8.901 | 32.42 |
| n-NiO/SiO2 (0.06) | 102.64 | 104.6 | 6.47 | 98.21 | 0.0031 | 0.2148 | 0.2117 | 8.235 | 28.65 |
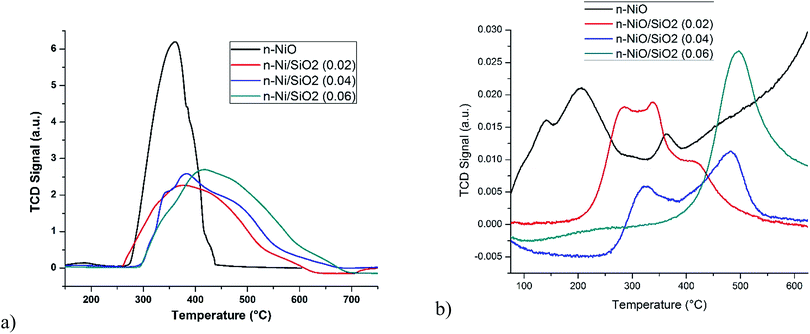
Fig. 6b shows the NH3-TPD profile for determining the number of surface Ni sites which adsorb NH3 per unit mass of catalyst. Because of the diffusional limitations, the acid sites computed with NH3-TPD is not very accurate in relation to the actual acidity strength measured with quantitative measurements.43 Hence, NH3-TPD is not commonly accepted as a reliable characterization method for computing the precise quantity of acid sites. However, NH3-TPD can provide a qualitative indication of the conjugation intensity of NH3 molecule with acid sites. As shown in Fig. 6b, the NH3-TPD curves shows that the acidity sites increase with the precursor concentrations. n-NiO reveals only a weak interaction of NH3 with acid sites with peaks from 100 to 255 °C. However, desorption chromatograms of n-NiO/SiO2 catalysts start from only above 200 °C, which indicates the occurrence of more strong acid sites after supporting n-NiO with SiO2.
 | ||
| Fig. 7 Temperature programmed methane decomposition over 1 g of n-Ni and n-Ni/SiO2 (0.02) catalyst. Temperature range 200–900 °C, flow rate 0.64 L min−1. | ||
Based on the results from temperature programmed methane decomposition, it was decided to carry out isothermal catalytic trials in the temperature range of 475–600 °C over n-Ni/SiO2 (0.02) catalyst. Fig. 8a shows the changes in hydrogen production percentage with time on stream for the TCD over n-Ni/SiO2 (0.02) catalyst at 475–600 °C. The experiments were conducted to evaluate activity stability of nanostructured catalyst materials as well as its ability to tolerate higher temperature environments. n-Ni/SiO2 (0.02) catalysts were evaluated with 99.995% methane. During the entire process, methane and hydrogen only were detected as gaseous products according to the reaction CH4 → 2H2 + C. In general, hydrogen production is high just after the contact of methane with the catalyst and decreases gradually with time. It is found from temperature programmed methane decomposition (Fig. 7) that n-Ni/SiO2 (0.02) was undergoing fast deactivation above 700 °C because of its high temperature sensitivity, supporting previous reports,44 and hence such high temperature studies were omitted from our analysis. Furthermore, according to Takenaka et al.,44 Ni-based catalysts are effective for methane decomposition in the temperature range of 400–600 °C, but deactivated immediately at temperatures above 600 °C. Thermal degradation of the n-NiO/SiO2 occurring above 600 °C, could be a reason for a rapid deactivation at higher temperature. Hence, a gradual weight loss was observed in thermogravimetric analysis results of n-NiO/SiO2 as shown in S5,† which might be attributed to thermal degradation. Significantly, n-Ni/SiO2 (0.02) catalyst maintained its activity even after 300 min with very low catalytic deactivation rate in the temperature range of 475–600 °C. Activity loss of n-Ni/SiO2 (0.02) catalyst in percentage terms is displayed in Fig. 8b. The initial catalytic activity became higher and catalytic deactivation rate was found to increase with increasing reaction temperature, clearly indicating the influence of temperature on TCD. Throughout the experimental duration of 300 min, n-Ni/SiO2 (0.02) catalyst showed activity in a wide range between 12 to 40.4% at different temperatures, and no sharp deactivation was observed at any experimented temperatures, indicating relatively stable catalytic activity of the catalysts under the experimental conditions. We found that the minimum deactivation was occurred at 500 °C. We have extended our examination up to 300 min in order to reveal the stability of nanostructured catalyst. One can see that our n-Ni/SiO2 (0.02) catalysts are significantly more active and stable than the naked counterpart as well as those prepared by conventional methods (see Table 3).
| Catalyst | Reaction parameters | Initial | Time t | t | td | ||||
|---|---|---|---|---|---|---|---|---|---|
| T | CH4 flow | Total flow | CH4 | H2 | CH4 | H2 | |||
| a F, flow rate (mL min−1), conversion (%); t, time (h); td, time of complete deactivation (h); –, not cited in the original reference.b Flow rate (N mL min−1). | |||||||||
| Ni/SiO2 (ref. 48) | 650 | 15a | – | 42 | – | 5 | – | 4 | – |
| Ni–Ca/SiO2 (ref. 49) | 580 | – | 100a | 39 | – | 12 | – | 3 | – |
| Ni–K/SiO2 (ref. 49) | 580 | – | 100a | 40 | – | 5 | – | 2.5 | 3 |
| Ni–Fe/SiO2 (ref. 48) | 650 | 15a | – | 46 | – | 27 | – | 4 | – |
| Ni/MgAl2O4 (ref. 50) | 550 | – | 80a | 34 | – | 23 | – | 3 | 4 |
| Ni–Cu/La2O3 (ref. 51) | 600 | – | 110b | 35 | – | 60 | – | 10 | – |
| n-Ni/SiO2 (0.02) (this work) | 600 | 640a | 640a | 57.2 | 40.4 | 79.5 | 19.9 | 5 | – |
| n-Ni/SiO2 (0.02) (this work) | 550 | 640a | 640a | 68.5 | 29.4 | 76.9 | 22.9 | 5 | – |
| n-Ni/SiO2 (0.02) (this work) | 500 | 640a | 640a | 74.4 | 17.2 | 85.3 | 14.6 | 5 | – |
| n-Ni/SiO2 (0.02) (this work) | 475 | 640a | 640a | 90.1 | 11.5 | 90.9 | 9.1 | 5 | – |
| n-Ni/SiO2 (0.02) (this work) | 550 | 1070a | 1070a | 72.9 | 25.6 | 84.2 | 15.7 | 2 | – |
| n-Ni/SiO2 (0.02) (this work) | 550 | 1430a | 1430a | 78 | 21 | 87.3 | 11.9 | 2 | – |
| n-Ni/SiO2 (0.04) (this work) | 550 | 640a | 640a | 69.4 | 29.3 | 79.8 | 20.1 | 5 | – |
| n-Ni/SiO2 (0.06) (this work) | 550 | 640a | 640a | 72.3 | 27.6 | 79.4 | 20 | 5 | – |
Furthermore, the isothermal methane conversion percentage as well as the activity range clearly follow the temperature range observed in the temperature programmed methane decomposition (Fig. 7). However, it is worth pointing out that in the temperature range of 475–600 °C, the methane conversions and hydrogen production percentage as well as nanocarbon yield (Fig. 11) over the n-Ni/SiO2 (0.02) catalyst are considerably superior to previously reported Ni-based catalysts in Table 3. TCD experiments were conducted over n-Ni/SiO2 (0.04) and n-Ni/SiO2 (0.06) at 550 °C and the results were compared with that of n-Ni/SiO2 (0.02) as shown in Fig. S3.† It can be seen that all the prepared catalysts behave in a similar way. The results clearly indicate that the examined nanocatalysts are more stable than that of normally supported or naked catalysts. Hence, n-Ni/SiO2 (0.02) nanostructured catalyst can be assumed as micro-capsular like reactors45–47 in which the reactant molecules have sufficient space for catalytic activity within the porous support. Furthermore, the reactant can get adsorbed within the support through highly porous silicate and accordingly results in higher catalytic activity. The very high stability of n-Ni/SiO2 catalyst can be attributed to effective prevention of aggregation of the active Ni-phase by the silica support.
The effect of methane feed flow rate on hydrogen production in percentage terms with time on stream is shown in Fig. 9. Flow rates of 0.64, 1.07 and 1.43 L min−1 were analyzed at 550 °C over 0.5 g of catalyst. It is observed from Fig. 9 that the initial hydrogen production decreased from 26.8 to 21.04% when the flow rate was increased from 0.64 to 1.43 L min−1. It can be speculated that higher methane flow rate results in the lower contact time with catalyst and hence resulted in the lower hydrogen production.15,52 Furthermore, it is found that the catalytic deactivation rate is also increases with increasing flow rate.
Characterization of produced nanocarbon
XRD patterns of the produced carbon at 475–600 °C are shown in Fig. 10. The diffraction peaks at 2θ = 26.26 and 44.45 are characteristic of graphite (JCPDS no. 98-005-3781). The peaks at 2θ = 44.5, 51.83 and 76.28° corresponds to Ni-phases showing good agreement with JCPDS no. 01-070-1849. It is found that the graphitization intensity of carbon nanofibers improved on increasing the temperature from 475 to 600 °C which is clear from the alteration of 2θ values corresponding to nanocarbon to higher values in a similar manner to those reported with Ni-supported Y zeolite.53 | ||
| Fig. 10 XRD patterns of produced nanocarbon over n-Ni/SiO2 (0.02) at different temperatures. Peaks corresponding to graphite and Ni are indicated. | ||
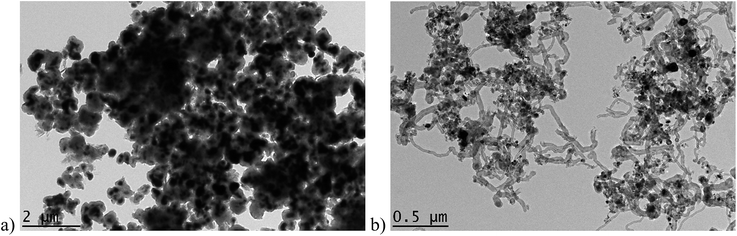
HRTEM images of n-Ni and n-Ni/SiO2 (0.02) catalyst after temperature programmed methane decomposition are shown in Fig. (11(a and b)). The unsupported n-Ni particles undergo strong sintering which results in giant agglomerate formation and the particles are covered by a carbon crust which isolates them from the reaction medium and prevents further methane decomposition over n-NiO (Fig. 11a). Hence, such catalysts are incapable to produce longer carbon nanofilaments as well, supporting our temperature programmed methane decomposition results (Fig. 7). Kim et al.54 reported the same observation that unsupported nickel powder is not suitable for production of nanofilaments in hydrocarbon media. By contrast, one can see longer nanocarbon filaments formed over n-Ni/SiO2 (0.02) catalyst after temperature programmed methane decomposition (Fig. 11b) which can be attributed to the stronger protection of n-NiO after supporting with SiO2.
Fig. 12a–c show HRTEM images of the produced nanocarbon by TCD over n-Ni/SiO2 (0.02) at different temperatures of 600, 550 and 500 °C, respectively. Accordingly, external diameter distribution of the nanocarbon at each temperature was also measured using ImageJ software considering 75 nanocarbons for diameter measurement. Large quantities of nanocarbons were deposited in the catalysts during the TCD process. The carbon yield percentage was calculated using eqn (3)55,56 and the results are shown in Fig. 13. The carbon yield of the catalysts was evaluated based on the extent of methane conversion against time on stream at a CH4 flow rate of 0.64 L min−1 for 5 h run time.
 | (3) |
A huge carbon yield of ∼5000% were obtained at 600 °C. Such observed carbon yield is outstanding compared to many other available results over Ni-based catalysts.55 The majority of produced nanocarbon is in the form of tubes and very minor quantity can be categorized as very small nanofibers. The main difference between nanotubes and nanofibers is the lack of a hollow cavity for the latter.57 Many nickel particles were located at the tip of the nanocarbons. It is apparent from the Fig. 12a–c that the carbon nanotubes are formed with thick walls and an internal cavity are posturing “fish-bone” or “bamboo” morphology. The varieties of nanocarbon found after the decomposition process can be categorized as follows; (i) nanocarbons with opening filled with pear shaped Ni particles (indicated in Fig. 12 with  symbol), (ii) fish-bone nanocarbon (
symbol), (ii) fish-bone nanocarbon ( ), (iii) carbon nanotubes with open end (
), (iii) carbon nanotubes with open end ( ), (iv) carbon nanotubes with closed end (
), (iv) carbon nanotubes with closed end ( ) and (v) carbon nanotube with Ni particle embedded in it (
) and (v) carbon nanotube with Ni particle embedded in it ( ). The diameter distribution illustrates that more than 90% of nanocarbons had an external diameter of less than 100 nm. In addition, it can be speculated that the diameter distribution shifted towards a lower diameter range on lowering the decomposition temperature (Fig. 12d–f). The fraction of carbon nanotubes with diameters above 50 nm is higher when decomposition took place at 600 °C, while it is comparatively lower at lower temperatures of 550 and 500 °C. Furthermore, previously conducted thorough studies on produced nanocarbons reveal that the outer diameter of the carbon nanotubes greatly depends on the size of Ni particles: larger Ni particles lead to carbon nanotubes with larger diameter.58 The Ni metal particle found at the tip of the carbon nanotubes are of pear or diamond shape with the sharp tail inserted into the carbon nanotube following tip-growth carbon formation mechanism,59 which is reinforcing many previous works.60–62 It is noted that the Ni particles were spherical or sphere shaped when embedded in SiO2 before the decomposition process (Fig. 5a). This structural change stipulates the possibility of the existence of Ni particles in the quasi-liquid state during the process, even at lower experimental temperature than its melting point (1452 °C) and Tamman temperature (726 °C). The occurrence of lower temperature quasi-liquid is because of formation of highly unstable, compared to Ni and graphite, Ni3C metastable compound as an intermediate product in the methane transformation process, which can be decomposed to metallic Ni and graphite at lower temperature of 400 °C. Furthermore, the higher gradient of Ni3C concentration over Ni particle during the process because of the uninterrupted graphite formation sets up a pressure at the graphitic envelope.58 Hence, mass transfer of carbon occurred by diffusion through the bulk particle as a consequence of built up pressure which tries to squeeze out the Ni particle in the quasi-liquid state. The lower temperature Ni3C to metallic Ni and graphite conversion and internal pressure build up explain the change in the shape of Ni particle after TCD process as well as the manifestation of Ni particles inside the carbon nanotubes.
). The diameter distribution illustrates that more than 90% of nanocarbons had an external diameter of less than 100 nm. In addition, it can be speculated that the diameter distribution shifted towards a lower diameter range on lowering the decomposition temperature (Fig. 12d–f). The fraction of carbon nanotubes with diameters above 50 nm is higher when decomposition took place at 600 °C, while it is comparatively lower at lower temperatures of 550 and 500 °C. Furthermore, previously conducted thorough studies on produced nanocarbons reveal that the outer diameter of the carbon nanotubes greatly depends on the size of Ni particles: larger Ni particles lead to carbon nanotubes with larger diameter.58 The Ni metal particle found at the tip of the carbon nanotubes are of pear or diamond shape with the sharp tail inserted into the carbon nanotube following tip-growth carbon formation mechanism,59 which is reinforcing many previous works.60–62 It is noted that the Ni particles were spherical or sphere shaped when embedded in SiO2 before the decomposition process (Fig. 5a). This structural change stipulates the possibility of the existence of Ni particles in the quasi-liquid state during the process, even at lower experimental temperature than its melting point (1452 °C) and Tamman temperature (726 °C). The occurrence of lower temperature quasi-liquid is because of formation of highly unstable, compared to Ni and graphite, Ni3C metastable compound as an intermediate product in the methane transformation process, which can be decomposed to metallic Ni and graphite at lower temperature of 400 °C. Furthermore, the higher gradient of Ni3C concentration over Ni particle during the process because of the uninterrupted graphite formation sets up a pressure at the graphitic envelope.58 Hence, mass transfer of carbon occurred by diffusion through the bulk particle as a consequence of built up pressure which tries to squeeze out the Ni particle in the quasi-liquid state. The lower temperature Ni3C to metallic Ni and graphite conversion and internal pressure build up explain the change in the shape of Ni particle after TCD process as well as the manifestation of Ni particles inside the carbon nanotubes.
Extension of method to Fe and Co metals
Co-precipitation cum modified Stöber method to prepare nanostructured catalyst was successfully extended to other metals such as Fe and Co with the same SiO2 support. HRTEM images of n-FeO/SiO2 (0.02) and n-CoO/SiO2 (0.02) are shown in Fig. 14. n-FeO and n-CoO were prepared from iron(III) nitrate nonahydrate (Fe(NO3)3·9H2O) and cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O), respectively. Partial agglomeration was observed in both n-FeO/SiO2 (0.02) and n-CoO/SiO2 (0.02) because of the magnetic coupling of adjacent metallic phases during silica feeding process. Our results reveal that co-precipitation cum modified Stöber method can be used as a general method for preparing silica supported metal nanostructures for high temperature requirements.Preliminary catalytic activity evaluation were conducted over n-Fe/SiO2 (0.02) and n-Co/SiO2 (0.02) catalysts. Temperature programmed methane decomposition results are shown in Fig. 15.
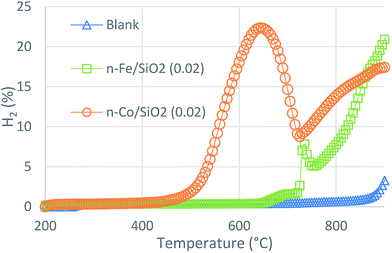 | ||
| Fig. 15 Temperature programmed methane decomposition over 1 g of n-Fe/SiO2 (0.02) and n-Co/SiO2 (0.02) catalysts. Temperature range 200–900 °C, flow rate 0.64 L min−1. | ||
Results disclose that n-Fe/SiO2 (0.02) and n-Co/SiO2 (0.02) are active for TCD but not as effective as that of n-Ni/SiO2. n-Fe/SiO2 (0.02) is active in the range of 730–760 °C, while n-Co/SiO2 (0.02) is active at 500 to 650 °C. Both of them give comparatively very less methane conversion than that of n-Ni/SiO2. Further isothermal activity studies and mechanism have yet to be conducted. It can be concluded that the activity of studied catalysts are in the following order n-Ni/SiO2 > n-Co/SiO2 > n-Fe/SiO2.
Conclusion
n-Ni/SiO2 catalysts were prepared by co-precipitation cum modified Stöber method and examined for thermocatalytic decomposition of methane. Hydrogen free from GHG was produced over n-Ni/SiO2 (0.02) catalyst without any significant deactivation at an active temperature range (475–600 °C) for examined duration of 300 min, owing to the fundamental stable nanostructure. Maximum hydrogen production of 40.4% was observed at 600 °C, while minimum deactivation after 300 min of examination was found at 500 °C. Moreover, it was observed that a higher methane flow rate results a lower methane conversion as well as a higher catalytic deactivation rate. Four different types of carbon nanotubes with inner and outer diameter in tens of nm and length in the range of hundreds of nm were observed after the decomposition process. Growth of nanocarbons was found to follow the tip-growth mechanism. Furthermore, the existence of quasi-liquid state of Ni-metal explained the encapsulation of metal particles inside the carbon nanotubes as well as the pear/diamond shape of Ni metal after decomposition. Considering the cheap nickel precursors as well as considerably simple and room-temperature catalyst production method, nanostructured n-Ni/SiO2 is a promising material for the production of GHG free H2 through the catalytic decomposition of methane. The extension of the study for nano-Fe and nano-Co active phases with SiO2 support reveals that n-Ni/SiO2 is superior to them in terms of activity and stability for thermocatalytic decomposition of methane. The activity and stability of the examined catalysts are in the following order: n-Ni/SiO2 > n-Co/SiO2 > n-Fe/SiO2. It can be predicted that these types of metal/SiO2 nanostructures with suitable metals could possibly serve as catalysts for many high-temperature reactions.Acknowledgements
The authors gratefully acknowledge financial support from the postgraduate Research Fund (UM.C/HIR/MOHE/ENG/11), University of Malaya, Malaysia.Notes and references
- Y. Li, S. Liu, L. Yao, W. Ji and C.-T. Au, Catal. Commun., 2010, 11, 368–372 CrossRef CAS PubMed.
- M. Danek, K. F. Jensen, C. B. Murray and M. G. Bawendi, Chem. Mater., 1996, 8, 173–180 CrossRef CAS.
- P. Reiss, J. Bleuse and A. Pron, Nano Lett., 2002, 2, 781–784 CrossRef CAS.
- M. L. Breen, A. D. Dinsmore, R. H. Pink, S. B. Qadri and B. R. Ratna, Langmuir, 2001, 17, 903–907 CrossRef CAS.
- V. Skumryev, S. Stoyanov, Y. Zhang, G. Hadjipanayis, D. Givord and J. Nogues, Nature, 2003, 423, 850–853 CrossRef CAS PubMed.
- H. Zeng, J. Li, Z. L. Wang, J. P. Liu and S. Sun, Nano Lett., 2004, 4, 187–190 CrossRef CAS.
- T. Tsoncheva, L. Ivanova, A. R. Lotz, J. H. Smått, M. Dimitrov, D. Paneva, I. Mitov, M. Linden, C. Minchev and M. Fröba, Catal. Commun., 2007, 8, 1573–1577 CrossRef CAS PubMed.
- Y. Deng, D. Qi, C. Deng, X. Zhang and D. Zhao, J. Am. Chem. Soc., 2008, 130, 28–29 CrossRef CAS PubMed.
- K. P. Velikov, A. Moroz and A. van Blaaderen, Appl. Phys. Lett., 2002, 80, 49–51 CrossRef CAS PubMed.
- F. Liu, Y. Qu, Y. Yue, G. Liu and Y. Liu, RSC Adv., 2015, 5, 16837–16846 RSC.
- M. A. Ebiad, D. R. Abd El-Hafiz, R. A. Elsalamony and L. S. Mohamed, RSC Adv., 2012, 2, 8145–8156 RSC.
- R. Z. Sørensen, L. J. E. Nielsen, S. Jensen, O. Hansen, T. Johannessen, U. Quaade and C. H. Christensen, Catal. Commun., 2005, 6, 229–232 CrossRef PubMed.
- T. Zhang and M. D. Amiridis, Appl. Catal., A, 1998, 167, 161–172 CrossRef CAS.
- R. Aiello, J. E. Fiscus, H.-C. zur Loye and M. D. Amiridis, Appl. Catal., A, 2000, 192, 227–234 CrossRef CAS.
- Y. Li, J. Chen, Y. Qin and L. Chang, Energy Fuels, 2000, 14, 1188–1194 CrossRef CAS.
- J. W. Gosselink, Int. J. Hydrogen Energy, 2002, 27, 1125–1129 CrossRef CAS.
- U. P. M. Ashik, W. M. A. Wan Daud and H. F. Abbas, Renewable Sustainable Energy Rev., 2015, 44, 221–256 CrossRef CAS PubMed.
- M. Balat and M. Balat, Int. J. Hydrogen Energy, 2009, 34, 3589–3603 CrossRef CAS PubMed.
- L. B. Brentner, J. Peccia and J. B. Zimmerman, Environ. Sci. Technol., 2010, 44, 2243–2254 CrossRef CAS PubMed.
- H. F. Abbas and W. M. A. W. Daud, Appl. Catal., A, 2010, 388, 232–239 CrossRef CAS PubMed.
- S. Wang, Environ. Sci. Technol., 2008, 42, 7055–7063 CrossRef CAS.
- V. M. Shinde and G. Madras, RSC Adv., 2014, 4, 4817–4826 RSC.
- R. A. Elsalamony, D. R. Abd El-Hafiz, M. A. Ebiad, A. M. Mansour and L. S. Mohamed, RSC Adv., 2013, 3, 23791–23800 RSC.
- P. Westermann, B. Jørgensen, L. Lange, B. K. Ahring and C. H. Christensen, Int. J. Hydrogen Energy, 2007, 32, 4135–4141 CrossRef CAS PubMed.
- M. N. Uddin, W. M. A. W. Daud and H. F. Abbas, RSC Adv., 2014, 4, 10467–10490 RSC.
- P. Jana, V. A. de la Peña O’Shea, J. M. Coronado and D. P. Serrano, Int. J. Hydrogen Energy, 2012, 37, 7034–7041 CrossRef CAS PubMed.
- K. Otsuka, S. Kobayashi and S. Takenaka, J. Catal., 2001, 200, 4–9 CrossRef CAS.
- T. V. Choudhary, C. Sivadinarayana, C. C. Chusuei, A. Klinghoffer and D. W. Goodman, J. Catal., 2001, 199, 9–18 CrossRef CAS.
- K. Otsuka, S. Kobayashi and S. Takenaka, Appl. Catal., A, 2000, 190, 261–268 CrossRef CAS.
- S. Takenaka, H. Ogihara, I. Yamanaka and K. Otsuka, Appl. Catal., A, 2001, 217, 101–110 CrossRef CAS.
- L. Li, S. He, Y. Song, J. Zhao, W. Ji and C.-T. Au, J. Catal., 2012, 288, 54–64 CrossRef CAS PubMed.
- W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- A. A. Al-Hassani, H. F. Abbas and W. M. A. Wan Daud, Int. J. Hydrogen Energy, 2014, 39, 7004–7014 CrossRef CAS PubMed.
- M. Stjerndahl, M. Andersson, H. E. Hall, D. M. Pajerowski, M. W. Meisel and R. S. Duran, Langmuir, 2008, 24, 3532–3536 CrossRef CAS PubMed.
- J. Zou, Y.-G. Peng and Y.-Y. Tang, RSC Adv., 2014, 4, 9693–9700 RSC.
- J. C. Park, J. U. Bang, J. Lee, C. H. Ko and H. Song, J. Mater. Chem., 2010, 20, 1239–1246 RSC.
- Y. Li, J. Chen, L. Chang and Y. Qin, J. Catal., 1998, 178, 76–83 CrossRef CAS.
- L. Yao, T. Shi, Y. Li, J. Zhao, W. Ji and C.-T. Au, Catal. Today, 2011, 164, 112–118 CrossRef CAS PubMed.
- S. D. Robertson, B. D. McNicol, J. H. De Baas, S. C. Kloet and J. W. Jenkins, J. Catal., 1975, 37, 424–431 CrossRef CAS.
- L. Li, P. Lu, Y. Yao and W. Ji, Catal. Commun., 2012, 26, 72–77 CrossRef CAS PubMed.
- X.-K. Li, W.-J. Ji, J. Zhao, S.-J. Wang and C.-T. Au, J. Catal., 2005, 236, 181–189 CrossRef CAS PubMed.
- R. Xie, D. Li, B. Hou, J. Wang, L. Jia and Y. Sun, Catal. Commun., 2011, 12, 380–383 CrossRef CAS PubMed.
- A. Romero, A. Garrido, A. Nieto-Márquez, P. Sánchez, A. D. Lucas and J. L. Valverde, Microporous Mesoporous Mater., 2008, 110, 318–329 CrossRef CAS PubMed.
- S. Takenaka, S. Kobayashi, H. Ogihara and K. Otsuka, J. Catal., 2003, 217, 79–87 CAS.
- S. H. Joo, J. Y. Park, C.-K. Tsung, Y. Yamada, P. Yang and G. A. Somorjai, Nat. Mater., 2009, 8, 126–131 CrossRef CAS PubMed.
- J. Wu, F. Hu, X. Hu, Z. Wei and P. K. Shen, Electrochim. Acta, 2008, 53, 8341–8345 CrossRef CAS PubMed.
- W.-M. Zhang, J.-S. Hu, Y.-G. Guo, S.-F. Zheng, L.-S. Zhong, W.-G. Song and L.-J. Wan, Adv. Mater., 2008, 20, 1160–1165 CrossRef CAS PubMed.
- W. Wang, H. Wang, Y. Yang and S. Jiang, Int. J. Hydrogen Energy, 2012, 37, 9058–9066 CrossRef CAS PubMed.
- B. Zapata, M. A. Valenzuela, J. Palacios and E. Torres-Garcia, Int. J. Hydrogen Energy, 2010, 35, 12091–12097 CrossRef CAS PubMed.
- G. D. B. Nuernberg, E. L. Foletto, C. E. M. Campos, H. V. Fajardo, N. L. V. Carreño and L. F. D. Probst, J. Power Sources, 2012, 208, 409–414 CrossRef CAS PubMed.
- J. L. Figueiredo, J. J. M. Órfão and A. F. Cunha, Int. J. Hydrogen Energy, 2010, 35, 9795–9800 CrossRef CAS PubMed.
- A. F. Cunha, J. J. M. Órfão and J. L. Figueiredo, Appl. Catal., A, 2008, 348, 103–112 CrossRef CAS PubMed.
- M. Nasir Uddin, W. M. A. Wan Daud and H. F. Abbas, Energy Convers. Manage., 2015, 90, 218–229 CrossRef CAS PubMed.
- M. S. Kim, N. M. Rodriguez and R. T. K. Baker, J. Catal., 1991, 131, 60–73 CrossRef CAS.
- S. K. Saraswat and K. K. Pant, Int. J. Hydrogen Energy, 2011, 36, 13352–13360 CrossRef CAS PubMed.
- S.-P. Chai, C.-M. Seah and A. R. Mohamed, J. Nat. Gas Chem., 2011, 20, 84–89 CrossRef CAS.
- P. Serp, M. Corrias and P. Kalck, Appl. Catal., A, 2003, 253, 337–358 CrossRef CAS.
- J. C. Guevara, J. A. Wang, L. F. Chen, M. A. Valenzuela, P. Salas, A. García-Ruiz, J. A. Toledo, M. A. Cortes-Jácome, C. Angeles-Chavez and O. Novaro, Int. J. Hydrogen Energy, 2010, 35, 3509–3521 CrossRef CAS PubMed.
- A. K. Sinha, D. W. Hwang and L.-P. Hwang, Chem. Phys. Lett., 2000, 332, 455–460 CrossRef CAS.
- T. Baird, J. R. Fryer and B. Grant, Carbon, 1974, 12, 591–602 CrossRef CAS.
- R. T. K. Baker, M. A. Barber, P. S. Harris, F. S. Feates and R. J. Waite, J. Catal., 1972, 26, 51–62 CrossRef CAS.
- P. A. Tesner, E. Y. Robinovich, I. S. Rafalkes and E. F. Arefieva, Carbon, 1970, 8, 435–442 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra07098h |
| This journal is © The Royal Society of Chemistry 2015 |