A novel biobased plasticizer of epoxidized cardanol glycidyl ether: synthesis and application in soft poly(vinyl chloride) films†
Abstract
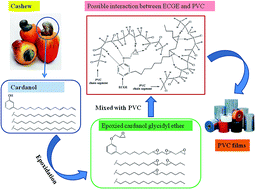
A novel plasticizer derived from cardanol, and epoxied cardanol glycidyl ether (ECGE), was synthesized and characterized by 1H-NMR and 13C-NMR. The effects of ECGE combined with the commercial plasticizer dioctyl phthalate (DOP), in soft poly(vinyl chloride) (PVC) films, were studied. The mechanical properties of PVC films showed both tensile strength and percent elongation increases with increasing ECGE content. Thermogravimetric analysis (TGA) was performed to characterize the thermal stabilities of the plasticized samples and showed the stability of films increased on increasing the content of ECGE. The properties of volatility, extraction, and exudation resistance of plasticizers were tested and analysis by means of solubility parameters as reported in the literature suggests the ECGE has similar or higher stability for these properties than DOP. FTIR analysis of the films also revealed that ECGE interacted with PVC. Due to its inherent chemical backbone and the modified epoxy groups, ECGE properly balanced the properties and improved the performance of PVC films compared with the neat DOP plasticizer.

 Please wait while we load your content...
Please wait while we load your content...