New efficient and recyclable catalysts for the synthesis of di- and tri-glycerol carbonates
M. Arestaab,
A. Dibenedetto*bc and
L. di Bitontob
aDepartment of Chemical and Biomolecular Engineering, NUS, Engineering Drive 4, Singapore 117585
bCIRCC, Via Celso Ulpiani n. 27, 70126 Bari, Italy
cUniversity of Bari, Department of Chemistry, Via Orabona 4, 70126 Bari, Italy. E-mail: angela.dibenedetto@uniba.it
First published on 14th July 2015
Abstract
Multifunctional monomers based on glycerol carbonate are employed in the chemical industry for the production of polyurethanes and polycarbonates. To avoid the use of toxic phosgene as carboxylating reagent, eco-friendly routes have been developed using alternative agents. In this paper, a series of binary and ternary oxides have been tested as catalysts in the synthesis of diglycerolether dicarbonate (DGDC) and diglycerol tricarbonate (DGTC) using dimethyl carbonate (DMC) and urea as carboxylating agents. The recovery and reuse of the catalysts are discussed. In the best reaction conditions, using mixed oxides La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca = 1
Ca = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as catalysts, the yields of DGDC and DGTC were >90 (pure isolated compounds) and 19.9%, respectively.
1 as catalysts, the yields of DGDC and DGTC were >90 (pure isolated compounds) and 19.9%, respectively.
1. Introduction
Over the last decade, glycerol production has considerably grown following the development of bio-oil industry for the production of biofuels and bio-lubricants.1–7 In oleo-chemistry processes glycerol represents 10% of biodiesel, so that the growing demand of biodiesel is generating in some areas a significant excess of glycerol.8–10 In addition, glycerol can be produced by enzymatic fermentation or catalytic hydrogenolysis of cellulose.11–13 There is an industrial interest to find innovative uses of glycerol: both molecular compounds and polymers are an attracting perspective (Fig. 1).Acrolein, obtained from the dehydration of glycerol,14–19 is used in situ for the synthesis of acrylic acid.20,21 The catalytic hydrogenolysis of glycerol is a route to increase the profitability of biodiesel production plants.22–27 Products such us propylene glycols are used for the synthesis of polyester resins, liquid detergents, drugs and cosmetics.28–32 Glycerol can also be converted into branched oxygen-containing components by catalytic etherification with alcohols or alkenes.33–37 It can be dehydrated to glycidol38,39 and used for the production of 1,3-propanediol via biotechnology.40,41 The production of 3-hydroxypropanoic acid (3-HPA)42,43 is also of interest as the latter can be used as monomer for polymers. Diglycerol ether is used for the production of polyesters44,45 and cosmetics.46 Glycerol carbonate is an interesting compound for the chemical industry. It is used as a component of polyurethane foams, gas separation membranes, surfactants, paints, detergents and as a non-volatile reactive solvent for several types of materials.47–52 Due to its low toxicity, vapor pressure and flammability, good biodegradability and moisturizing ability, glycerol carbonate also possesses the right characteristics of a wetting agent for cosmetic clays or of a carrier for drugs53,54 Glycerol carbonate is usually prepared by reacting glycerol with toxic phosgene:55,56 innovative processes such as the direct carboxylation of glycerol with carbon dioxide,47,57–59 the glycerolysis of urea60–62 or the trans-esterification reaction with linear or cyclic organic carbonates63–66 represent ecofriendly alternatives.
In recent years, multifunctional monomers based on glycerol carbonate have reached a particular importance in the chemical industry for the production of polyurethanes and polycarbonates (Fig. 2).67–71 Only a few methods are reported in the literature for the synthesis of such molecules. The trans-esterification of diglycerol ether for the synthesis of DGDC is a two-step process affording first 4-[(2,3 di-hydroxy-propoxy)methyl]-1,3 dioxolan-2-one, followed by the conversion of the latter into DGDC by further trans-esterification (Scheme 1).
 | ||
| Scheme 1 Schematic presentation of the products obtained from the trans-esterification of diglycerol ether with DMC for the synthesis of DGDC. | ||
Mignani et al.72 have used lanthanum oxide as catalyst in the synthesis of diglycerol dicarbonate (DGDC) from diglycerol ether and DMC. The reaction was carried out at 393 K for 48 h and the final product was crystallized from methanol with an isolated yield of 90% with respect to diglycerol ether, but neither DGDC was fully characterized nor the catalyst was recovered and reused.
Weckhuysen et al.73,74 have studied hydrotalcites Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al in the same trans-esterification process. Using hydrotalcite Mg
Al in the same trans-esterification process. Using hydrotalcite Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al = 5 molar ratio a yield of 86% of DGDC with a selectivity of 89% were obtained after 6 h of reaction (T = 403 K). The catalyst was easily recovered and reused, but the production of the cyclic carbonate decreased after the first cycle of reaction. The quantification of the products obtained from the trans-esterification reaction was made using NMR, and DGDC was not isolated. Diglycerol tricarbonate (DGTC) is reported to be obtained from the trans-esterification reaction of glycerol with DMC using potassium carbonate.71 A yield of 18% was reported after 48 h of reaction (T = 343 K), but the catalyst was not recoverable at the end of the reaction cycle.
Al = 5 molar ratio a yield of 86% of DGDC with a selectivity of 89% were obtained after 6 h of reaction (T = 403 K). The catalyst was easily recovered and reused, but the production of the cyclic carbonate decreased after the first cycle of reaction. The quantification of the products obtained from the trans-esterification reaction was made using NMR, and DGDC was not isolated. Diglycerol tricarbonate (DGTC) is reported to be obtained from the trans-esterification reaction of glycerol with DMC using potassium carbonate.71 A yield of 18% was reported after 48 h of reaction (T = 343 K), but the catalyst was not recoverable at the end of the reaction cycle.
In this paper, a series of binary and ternary oxides based on lanthanum have been synthesized and used as catalysts in eco-friendly routes for the synthesis of DGDC and DGTC. They were recovered and recycled. The various oxides are characterized by a different electronic configuration of the metal and acid/basic properties that can be tuned with the reaction they have to catalyze. In our study, we have set a relationship between catalyst composition and their properties such as: acid/basic sites, activity, recoverability and selectivity. Both dimethyl carbonate and urea have been used as reagents for building the carbonate moiety.
2. Experimental section
2.1 Materials and methods
All solvent and starting reagents were RP Aldrich products. Single metal oxides were previously calcined for 3 h at 823 K, to remove traces of moisture present. FTIR spectra were recorded with a Shimadzu Prestige 21 instrument. Mixed oxides were synthesized in the solid state by using High Energy Milling (HEM) technique with a Pulverisette 7 apparatus. Acid/basic sites were determined using a Micromeritics Chemisorb 2750 equipment. The analyses of the acid and basic sites were carried out using NH3 and CO2, respectively, as probe-gas with 100 mg of catalyst. The samples were pre-treated under N2 flow (30 mL min−1) at 823 K. The Pulse Chemisorb was performed using He as carrier gas (30 mL min−1). The TPD were performed under He flow at 30 mL min−1. GC-MS analyses were carried out with a Shimadzu 17 A gas chromatograph (capillary column: 30 m; MDN-5s, Ø 0.25 mm, 0.25 μm film) coupled to a Shimadzu QP5050 A mass spectrometer. Quantitative determinations on the reaction solution were performed using a Hewlett Packard 6850 GC-FID (capillary column: 30 m; Carbowax; Ø 0.25 mm, 0.25 μm film). The 1H NMR and 13C NMR spectra were recorded using a BRUKER 600 MHz. The synthesis and isolation of methyl-glyceryl-carbonate (MGC) was made as reported in literature.712.2 Catalysts preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca and La
Ca and La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn was carried out using a solid state method.65,75 Depending on the expected composition of mixed oxides (Table 1) weighted amounts of lanthanum, calcium and zinc oxide were mixed in High Energy Milling-HEM apparatus (spheres and basket made of agata), for 1 h at 700 rpm. Then the resulting solid was calcined at 823 K for 3 h. To check the stability of the catalyst, the CaO–La2O3 mixed oxide was analysed after 10 runs and the Ca content was found to be 10.3% with respect to the theoretical value 10.5% in the starting material. The BET and basic/acid ratio were also practically unchanged with respect to the original catalyst.
Zn was carried out using a solid state method.65,75 Depending on the expected composition of mixed oxides (Table 1) weighted amounts of lanthanum, calcium and zinc oxide were mixed in High Energy Milling-HEM apparatus (spheres and basket made of agata), for 1 h at 700 rpm. Then the resulting solid was calcined at 823 K for 3 h. To check the stability of the catalyst, the CaO–La2O3 mixed oxide was analysed after 10 runs and the Ca content was found to be 10.3% with respect to the theoretical value 10.5% in the starting material. The BET and basic/acid ratio were also practically unchanged with respect to the original catalyst.
| Catalysts | Reagents (g) | ||||
|---|---|---|---|---|---|
| La2O3 | CaO | ZnO | |||
| 1 | La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca Ca |
0.1 | 0.34 | 1.16 | — |
| 2 | 0.5 | 0.89 | 0.61 | — | |
| 3 | 1 | 1.12 | 0.38 | — | |
| 4 | La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn Zn |
0.1 | 0.25 | — | 1.25 |
| 5 | 0.5 | 0.75 | — | 0.75 | |
| 6 | 1 | 1.01 | — | 0.51 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Al = 5. Hydrotalcite Mg
Al = 5. Hydrotalcite Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al = 5 was synthesized using a co-precipitation method.74 8.55 g of Na2CO3·10H2O and 2.80 g of NaOH were dissolved into 60 mL of deionized water. A solution containing 12.82 g of Mg(NO3)2·6H2O and 3.75 g of Al(NO3)3·9H2O (molar ratio = 5
Al = 5 was synthesized using a co-precipitation method.74 8.55 g of Na2CO3·10H2O and 2.80 g of NaOH were dissolved into 60 mL of deionized water. A solution containing 12.82 g of Mg(NO3)2·6H2O and 3.75 g of Al(NO3)3·9H2O (molar ratio = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) into 100 mL of deionized water was then added dropwise. The mixture was stirred for 22 h at 373 K. Then, the white precipitate was filtered and washed (3 × 100 mL) with deionized water.
1) into 100 mL of deionized water was then added dropwise. The mixture was stirred for 22 h at 373 K. Then, the white precipitate was filtered and washed (3 × 100 mL) with deionized water.2.3 Diglycerolether dicarbonate
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diglycerol ether = 20
diglycerol ether = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The system was heated under stirring at 355 K for 48 h using an oil bath. At the end of the reaction, the catalyst was recovered by centrifugation, washed with methanol and calcined for 3 h at 773 K. The products obtained (DGDC and 4-[(2,3 dihydroxypropoxy)methyl]-1,3 dioxolan-2-one), were dissolved into DMSO and analyzed by GC and GC-MS using diphenyl ether as standard.
1). The system was heated under stirring at 355 K for 48 h using an oil bath. At the end of the reaction, the catalyst was recovered by centrifugation, washed with methanol and calcined for 3 h at 773 K. The products obtained (DGDC and 4-[(2,3 dihydroxypropoxy)methyl]-1,3 dioxolan-2-one), were dissolved into DMSO and analyzed by GC and GC-MS using diphenyl ether as standard.
 | (1.1) |
2.3.1.1 Isolation and characterization of diglycerolether dicarbonate. The crude product obtained as reported in 2.3.1 was evaporated in vacuum to eliminate excess DMC and the formed methanol that were condensed and recovered. The resulting liquid was kept at 278 K for 48 h to afford a white solid (>90%) that was separated from the residual liquid by filtration, washed with methanol (3 × 2 mL) and dried in vacuo.
Elemental analysis: calculated for C8H10O7: % C calc. 44.0, found: 43.3; % H calc. 4.58, found: 4.55.
Diglycerolether dicarbonate. 1H NMR (600 MHz, DMSO-d6) δ = 4.93 (m, 2H) OCH2C
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH2O, 4.52 (t, 2H) and 4.24 (q, 2H) OC
CH2O, 4.52 (t, 2H) and 4.24 (q, 2H) OC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CHCH2O, 3.72 (m, 4H) OCH2CHC
2CHCH2O, 3.72 (m, 4H) OCH2CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2O ppm.13C NMR (600 MHz, DMSO-d6) δ = 66.21 O
2O ppm.13C NMR (600 MHz, DMSO-d6) δ = 66.21 O![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CHCH2O, 70.65 and 70.68 OCH2CH
H2CHCH2O, 70.65 and 70.68 OCH2CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2O, 76.11 and 76.21 OCH2
H2O, 76.11 and 76.21 OCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH2O, 155.10 and 155.12 (
HCH2O, 155.10 and 155.12 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (O)OO) ppm.
(O)OO) ppm.
FTIR (KBr): 3000–2900 cm−1 (s, C–H stretch), 1790 cm−1 (s, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch), 1100 cm−1 (s, C–O–C stretch), 1401 (s, CH2 bend), 1176 cm−1 (s, CH bend).
O stretch), 1100 cm−1 (s, C–O–C stretch), 1401 (s, CH2 bend), 1176 cm−1 (s, CH bend).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diglycerol ether = 2
diglycerol ether = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 0.6 g of catalyst (weight ratio catalyst
1) and 0.6 g of catalyst (weight ratio catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diglycerol ether = 20%) were placed into the reactor connected to a vacuum system (20 Pa) to remove ammonia during the reaction (eqn (1.2)).54,75 The mixture was heated under stirring at 423 K for 15 h. At the end of the reaction, the catalyst was recovered by centrifugation, washed with methanol and calcined for 3 h at 773 K. The products, DGDC and 4-[(2,3-di-hydroxy-propoxy)methyl]-1,3-dioxolan-2-one (DHPMC), obtained, were dissolved into DMSO and analyzed by GC and GC-MS using diphenyl ether as standard. The pure solid (90% yield) was isolated following the procedure described in 2.3.1.1.
diglycerol ether = 20%) were placed into the reactor connected to a vacuum system (20 Pa) to remove ammonia during the reaction (eqn (1.2)).54,75 The mixture was heated under stirring at 423 K for 15 h. At the end of the reaction, the catalyst was recovered by centrifugation, washed with methanol and calcined for 3 h at 773 K. The products, DGDC and 4-[(2,3-di-hydroxy-propoxy)methyl]-1,3-dioxolan-2-one (DHPMC), obtained, were dissolved into DMSO and analyzed by GC and GC-MS using diphenyl ether as standard. The pure solid (90% yield) was isolated following the procedure described in 2.3.1.1.2.4 Diglycerol tricarbonate
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC = 5
DMC = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 0.1 g of catalyst (weight ratio catalyst
1) and 0.1 g of catalyst (weight ratio catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC = 20%), were placed into a glass tube under atmospheric pressure of nitrogen. The reaction was carried out at 413 K for 24 h (eqn (1.3) and (1.4)). At the end of the reaction, the catalyst was recovered by centrifugation, washed with methanol and calcined for 3 h at 773 K and eventually reused. The obtained mixture (containing methyl-glyceryl-carbonate MGC and diglycerol tricarbonate DGTC), were dissolved into DMSO and analyzed by GC and GC-MS using benzophenone as standard. The yields of MGC and DGTC were 42.7 and 15.4%, respectively, with a selectivity of 73.5 and 26.5%.
DMC = 20%), were placed into a glass tube under atmospheric pressure of nitrogen. The reaction was carried out at 413 K for 24 h (eqn (1.3) and (1.4)). At the end of the reaction, the catalyst was recovered by centrifugation, washed with methanol and calcined for 3 h at 773 K and eventually reused. The obtained mixture (containing methyl-glyceryl-carbonate MGC and diglycerol tricarbonate DGTC), were dissolved into DMSO and analyzed by GC and GC-MS using benzophenone as standard. The yields of MGC and DGTC were 42.7 and 15.4%, respectively, with a selectivity of 73.5 and 26.5%.
Diglycerol tricarbonate. 1H NMR (600 MHz, DMSO-d6) δ = 5.14 (m, 2H) OCH2C
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH2O, 4.56 (t, 2H) and 4.36 (q, 2H) OC
CH2O, 4.56 (t, 2H) and 4.36 (q, 2H) OC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CHCH2O, 4.26 (m, 4H) OCH2CHC
2CHCH2O, 4.26 (m, 4H) OCH2CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2O ppm. 13C NMR (600 MHz, DMSO-d6) δ = 65.89 O
2O ppm. 13C NMR (600 MHz, DMSO-d6) δ = 65.89 O![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CHCH2O, 67.27 and 67.32 OCH2CH
H2CHCH2O, 67.27 and 67.32 OCH2CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2O, 74.53 and 74.56 OCH2
H2O, 74.53 and 74.56 OCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH2O, 155.49 and 155.51 (
HCH2O, 155.49 and 155.51 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (O)OO) ppm.
(O)OO) ppm.
Methyl-glyceryl-carbonate. 1H NMR (600 MHz, DMSO-d6) δ = 5.01 (m, 2H) OCH2C
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH2O, 4.40 (t, 2H) and 4.30 (q, 2H) OC
CH2O, 4.40 (t, 2H) and 4.30 (q, 2H) OC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CHCH2O, 4.10 (m, 4H) OCH2CHC
2CHCH2O, 4.10 (m, 4H) OCH2CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2O ppm, 3.72 (s, OCH3). 13C NMR (600 MHz, DMSO-d6) δ = 65.43 O
2O ppm, 3.72 (s, OCH3). 13C NMR (600 MHz, DMSO-d6) δ = 65.43 O![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CHCH2O, 67.01 and 66.98 OCH2CH
H2CHCH2O, 67.01 and 66.98 OCH2CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2O, 74.19 and 74.23 OCH2
H2O, 74.19 and 74.23 OCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH2O, 154.90 and 154.94 (
HCH2O, 154.90 and 154.94 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (O)OO) ppm.
(O)OO) ppm.
 | (1.2) |
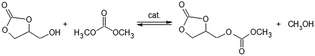 | (1.3) |
 | (1.4) |
 | (1.5) |
 | (1.6) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol carbonate = 2
glycerol carbonate = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 0.05 g of catalyst (weight ratio catalyst
1) and 0.05 g of catalyst (weight ratio catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MGC = 20%) were placed into a glass tube under atmospheric pressure of nitrogen. The reaction was carried out at 413 K for 24 h (eqn (1.5)). At the end of the reaction, the catalyst was recovered by centrifugation, washed with methanol and calcined for 3 h at 773 K. The product obtained (DGTC), was dissolved into DMSO and analyzed by GC and GC-MS using benzophenone as standard. The yield of DGTC was 19.9% with a selectivity of 23%.
MGC = 20%) were placed into a glass tube under atmospheric pressure of nitrogen. The reaction was carried out at 413 K for 24 h (eqn (1.5)). At the end of the reaction, the catalyst was recovered by centrifugation, washed with methanol and calcined for 3 h at 773 K. The product obtained (DGTC), was dissolved into DMSO and analyzed by GC and GC-MS using benzophenone as standard. The yield of DGTC was 19.9% with a selectivity of 23%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MGC = 20%) were placed into a glass tube under atmospheric pressure of nitrogen. The reaction was carried out at 413 K for 24 h (eqn (1.6)). At the end of the reaction, the catalyst was recovered by centrifugation, washed with methanol and calcined for 3 h at 773 K. The products obtained (DGTC and DMC) were dissolved into DMSO and analyzed using benzophenone as standard. The yield of DGTC was 21.2% with a selectivity of 100% towards disproportionation.
MGC = 20%) were placed into a glass tube under atmospheric pressure of nitrogen. The reaction was carried out at 413 K for 24 h (eqn (1.6)). At the end of the reaction, the catalyst was recovered by centrifugation, washed with methanol and calcined for 3 h at 773 K. The products obtained (DGTC and DMC) were dissolved into DMSO and analyzed using benzophenone as standard. The yield of DGTC was 21.2% with a selectivity of 100% towards disproportionation.3. Results and discussion
3.1 Diglycerol dicarbonate
| Catal. | % conv. diglycerol ether | % DHPMC | % DGDC | % w/w recovery of the catalysts* | |||
|---|---|---|---|---|---|---|---|
| Yield | Selectivity | Yield | Selectivity | ||||
a Reaction conditions: molar ratio DMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diglycerol ether = 20 diglycerol ether = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, weight ratio catalyst 1, weight ratio catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diglycerol ether = 20%, T = 355 K, t = 48 h. *At the end of the reaction, the catalyst was recovered and calcined for 3 h at 773 K. diglycerol ether = 20%, T = 355 K, t = 48 h. *At the end of the reaction, the catalyst was recovered and calcined for 3 h at 773 K. |
|||||||
| 1 | CaO | 87.5 | 14.2 | 16.2 | 73.3 | 83.8 | 64.2 |
| 2 | La2O3 | 70.4 | 32.0 | 45.4 | 38.4 | 54.6 | 98.5 |
| 3 | MgO | 69.6 | 33.2 | 47.7 | 36.4 | 52.3 | 82.3 |
| 4 | PdO | 40.4 | 37.8 | 93.6 | 2.6 | 6.4 | 96.4 |
| 5 | SnO | 35.9 | 33.5 | 93.3 | 2.4 | 6.7 | 98.1 |
| 6 | Fe2O3 | 35.6 | 35.6 | 100 | 0 | 0 | 97.8 |
| 7 | CeO2 | 26.4 | 26.4 | 100 | 0 | 0 | 98.4 |
| 8 | TiO2 | 25.2 | 25.2 | 100 | 0 | 0 | 98.9 |
| 9 | ZrO2 | 24.3 | 24.3 | 100 | 0 | 0 | 98.6 |
| No catal. | 12.4 | 12.4 | 100 | 0 | 0 | — | |
Calcium oxide shows the best activity in the production of DGDC with a conversion of 87.5% with a yield of 73.3% and a selectivity of 83.8% towards DGDC (Table 2, entry 1). However, the recovery of the catalyst results to be difficult due to the dissolution of calcium oxide (recovery of the catalyst = 64.2% w/w). Lanthanum oxide has a lower catalytic activity in the synthesis of DGDC (yield = 38.4%, selectivity = 54.6%, entry 2) but the catalyst loss is much less at the end of the reaction (recovery of the catalyst = 98.5% w/w). Magnesium oxide is only partly recovered (yield = 36.4%, selectivity = 52.3%, recovery of the catalyst = 82.3, entry 3).
Other metal oxides (entries 4–9, Table 2) are easily and almost quantitatively recoverable, but they show a good activity only in the first of the two steps of the trans-esterification with a 100% selectivity towards the production of 4-[(2,3-di-hydroxy-propoxy)methyl]-1,3-dioxolan-2-one. The analysis of the kinetics shows that an equilibrium position is reached after 60 h, with a yield of 75% using CaO (see Fig. 3). In absence of the catalyst, the reaction does not take place in the same experimental conditions, indicating that is not a thermal reaction. If methanol is eliminated by distillation, the conversion yield can be increased to 85% within 42 h, showing that there is an equilibrium shift to right.
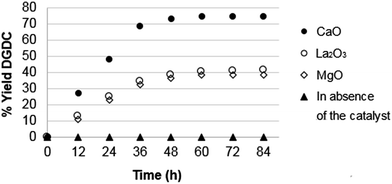 | ||
Fig. 3 Kinetic studies of the trans-esterification reaction of diglycerol ether to DGDC using single metal oxides. Reaction conditions: molar ratio DMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diglycerol ether = 20 diglycerol ether = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, T = 355 K, t = 84 h. 1, T = 355 K, t = 84 h. | ||
Fig. 4 shows that there is a correlation between the acid/basic properties of the metal oxides and their catalytic activity. Increasing the amount of the strong basic sites, an increase of the production of DGDC is observed. These data suggest that this reaction is mainly base-catalyzed. Noteworthy, most of the sites of the catalysts used (>95%) are strong sites and the catalyst properties practically depend on the strong basic sites.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca was studied in the trans-esterification of diglycerol ether with DMC for DGDC synthesis (Table 3). Entries 2–4 show that increasing the molar ratio La
Ca was studied in the trans-esterification of diglycerol ether with DMC for DGDC synthesis (Table 3). Entries 2–4 show that increasing the molar ratio La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca from 0.1 to 1, a slight decrease of the catalytic activity in the production of DGDC is observed, but the recoverability of the catalyst is noticeably increased. Fig. 5a shows the TPD profiles of CO2 release from mixed oxides La
Ca from 0.1 to 1, a slight decrease of the catalytic activity in the production of DGDC is observed, but the recoverability of the catalyst is noticeably increased. Fig. 5a shows the TPD profiles of CO2 release from mixed oxides La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca. It is evident that increasing the molar ratio La
Ca. It is evident that increasing the molar ratio La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca a decrease of strong basic sites (Fig. 5a and b) is observed, that is correlated to the lower production of DGDC (Fig. 5c). The strong basic sites due to Ca present on the surface of mixed oxides La
Ca a decrease of strong basic sites (Fig. 5a and b) is observed, that is correlated to the lower production of DGDC (Fig. 5c). The strong basic sites due to Ca present on the surface of mixed oxides La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca, play a key role in DGDC production.
Ca, play a key role in DGDC production.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca in the trans-esterification of diglycerol ether with DMC for the synthesis of DGDCa
Ca in the trans-esterification of diglycerol ether with DMC for the synthesis of DGDCa
| Catalysts | BET specific surface area (m2 g−1) | % conv. diglycerol ether | % DHPMC | % DGDC | % w/w recovery of the catalysts* | ||||
|---|---|---|---|---|---|---|---|---|---|
| Yield | Selectivity | Yield | Selectivity | ||||||
a Reaction conditions: molar ratio DMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diglycerol ether = 20 diglycerol ether = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, weight ratio catalyst 1, weight ratio catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diglycerol ether = 20%, T = 355 K, t = 48 h. *At the end of the reaction, the catalyst was recovered and calcined for 3 h at 773 K. diglycerol ether = 20%, T = 355 K, t = 48 h. *At the end of the reaction, the catalyst was recovered and calcined for 3 h at 773 K. |
|||||||||
| 1 | CaO | 0.29 ± 0.02 | 87.5 | 14.2 | 16.2 | 73.3 | 83.8 | 64.2 | |
| 2 | La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca Ca |
0.1 | 0.35 ± 0.03 | 85.4 | 15.0 | 17.6 | 70.4 | 82.4 | 75.5 |
| 3 | 0.5 | 0.51 ± 0.02 | 84.3 | 16.2 | 19.2 | 68.1 | 80.8 | 86.3 | |
| 4 | 1 | 0.72 ± 0.04 | 82.2 | 15.5 | 18.8 | 66.7 | 81.1 | 98.1 | |
| 5 | La2O3 | 0.91 ± 0.02 | 70.4 | 32.0 | 45.4 | 38.4 | 54.6 | 98.5 | |
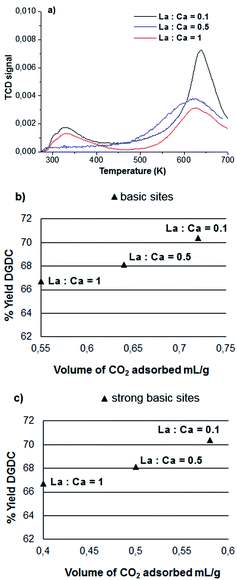 | ||
Fig. 5 TPD profiles for CO2 release from mixed oxides La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca and the correlation of the total number of basic sites and strong basic sites to the formation of DGDC. Ca and the correlation of the total number of basic sites and strong basic sites to the formation of DGDC. | ||
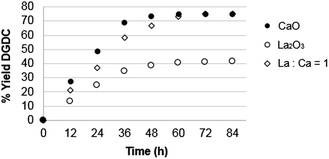
However, the presence of the hetero-metal causes an increase of the stability of the calcium oxide. La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca = 1 is easily recovered and reused with a good constancy of the catalytic activity, maintaining the same selectivity as CaO (Fig. 6). Interestingly, after 10 cycles the activity of the catalyst is the same as after 5 cycles (Fig. 6) and the elemental analyses of the La
Ca = 1 is easily recovered and reused with a good constancy of the catalytic activity, maintaining the same selectivity as CaO (Fig. 6). Interestingly, after 10 cycles the activity of the catalyst is the same as after 5 cycles (Fig. 6) and the elemental analyses of the La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca catalyst show that its composition and properties are practically unchanged with respect to the starting material (see the Experimental section). In conclusion, Ca is the most active catalytic site while La acts as a stabilizer of the structure and avoids solubilisation of Ca, preserving, thus, the catalytic activity and assuring a higher surface area (Table 3, column 3), recoverability and reusability. The catalysts after use do not show any structural change as macroscopically demonstrated by their constant activity in several cycles (Fig. 6).
Ca catalyst show that its composition and properties are practically unchanged with respect to the starting material (see the Experimental section). In conclusion, Ca is the most active catalytic site while La acts as a stabilizer of the structure and avoids solubilisation of Ca, preserving, thus, the catalytic activity and assuring a higher surface area (Table 3, column 3), recoverability and reusability. The catalysts after use do not show any structural change as macroscopically demonstrated by their constant activity in several cycles (Fig. 6).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Ca = 1 as catalyst in the trans-esterification of diglycerol ether with DMC. In Fig. 7 is reported the kinetic study of the mixed oxide La
Ca = 1 as catalyst in the trans-esterification of diglycerol ether with DMC. In Fig. 7 is reported the kinetic study of the mixed oxide La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca = 1 in the trans-esterification reaction of diglycerol ether, compared with the pure oxides. Using lanthanum oxide as catalyst in the trans-esterification reaction a conversion of 40.4% in DGDC is obtained after 60 h of reaction. Increasing the amount of calcium present, an increment of the catalytic activity in the production of the target product is observed with the same catalytic activity revealed for CaO (yield = 75%, Fig. 7). In addition, the catalyst is easily recovered and reused (Fig. 6). For the mixed oxide La
Ca = 1 in the trans-esterification reaction of diglycerol ether, compared with the pure oxides. Using lanthanum oxide as catalyst in the trans-esterification reaction a conversion of 40.4% in DGDC is obtained after 60 h of reaction. Increasing the amount of calcium present, an increment of the catalytic activity in the production of the target product is observed with the same catalytic activity revealed for CaO (yield = 75%, Fig. 7). In addition, the catalyst is easily recovered and reused (Fig. 6). For the mixed oxide La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca = 1 and CaO, the effect of the different weight ratio (catalyst
Ca = 1 and CaO, the effect of the different weight ratio (catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diglycerol ether) was also investigated (Fig. 8).
diglycerol ether) was also investigated (Fig. 8).
Reducing the amount of the catalyst from 20 to 5% w/w (catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diglycerol ether), a decrease in the production of diglycerol dicarbonate is observed for both catalysts. Conversely, a further increase of the catalyst/substrate ratio does not improve too much the conversion yield. At 30% w/w catalyst/substrate ratio the increase is only of 1.5% points from 73.5 to 75%.
diglycerol ether), a decrease in the production of diglycerol dicarbonate is observed for both catalysts. Conversely, a further increase of the catalyst/substrate ratio does not improve too much the conversion yield. At 30% w/w catalyst/substrate ratio the increase is only of 1.5% points from 73.5 to 75%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Ca = 1 with La2O3 and hydrotalcite Mg
Ca = 1 with La2O3 and hydrotalcite Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Al = 5. Once identified the mixed oxide La
Al = 5. Once identified the mixed oxide La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca = 1 as the best Ca/La catalyst in the trans-esterification reaction of diglycerol ether with DMC, a comparison was made with La2O3 and hydrotalcite Mg
Ca = 1 as the best Ca/La catalyst in the trans-esterification reaction of diglycerol ether with DMC, a comparison was made with La2O3 and hydrotalcite Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al (Table 4). These systems are reported in the literature as heterogeneous catalysts for the production of DGDC starting from diglycerol ether and DMC.72–74
Al (Table 4). These systems are reported in the literature as heterogeneous catalysts for the production of DGDC starting from diglycerol ether and DMC.72–74
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca = 1 with La2O3 and hydrotalcite Mg
Ca = 1 with La2O3 and hydrotalcite Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al = 5 in the same reaction conditionsa
Al = 5 in the same reaction conditionsa
| Catalysts | % conv. diglycerol ether | %DHPMC | % DGDC | |||
|---|---|---|---|---|---|---|
| Yield | Selectivity | Yield | Selectivity | |||
a Reaction conditions: molar ratio DMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diglycerol ether = 10 diglycerol ether = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, weight ratio catalyst 1, weight ratio catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diglycerol ether = 30%, temperature = 403 K, time = 6 h. diglycerol ether = 30%, temperature = 403 K, time = 6 h. |
||||||
| 1 | La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca = 1 Ca = 1 |
86.9 | 37.5 | 43.1 | 49.4 | 56.9 |
| 2 | Hydrotalcite Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al = 5 Al = 5 |
80.5 | 48.1 | 59.8 | 32.4 | 40.2 |
| 3 | La2O3 | 77.4 | 44.8 | 57.9 | 32.6 | 42.1 |
| No catalyst | 12.4 | 12.4 | 100 | 0 | 0 | |
Mixed oxide La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca = 1 reveals a higher catalytic activity in the synthesis of DGDC with respect to the other catalysts, in the same experimental conditions. Moreover, the characteristics of the La
Ca = 1 reveals a higher catalytic activity in the synthesis of DGDC with respect to the other catalysts, in the same experimental conditions. Moreover, the characteristics of the La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca heterogeneous catalyst make it possible to set a continuous reactor in which the reagents are flown with the recovery of the product.
Ca heterogeneous catalyst make it possible to set a continuous reactor in which the reagents are flown with the recovery of the product.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Zn in the alcoholysis of urea with diglycerol ether. Another approach studied for the production of DGDC was the alcoholysis of urea with diglycerol ether (eqn (1.2)). The alcoholysis of urea offers not only a simple and sustainable route to synthesis of cyclic carbonates, but also a positive economic impact with respect to the use of DMC that is obtained from ethylene carbonate or propylene carbonate, with the production of large amount of glycol as a by-product.84–87 Zinc oxide is one of the catalysts mostly used in the synthesis of organic carbonates, however the recovery of the catalyst at the end of the reaction cycle, results very difficult due to the formation of soluble compounds Zn(NCO)2(NH3)2.88–92 To increase the stability of the catalyst maintaining the same catalytic activity, we have studied the effect of lanthanum in the mixed oxides La
Zn in the alcoholysis of urea with diglycerol ether. Another approach studied for the production of DGDC was the alcoholysis of urea with diglycerol ether (eqn (1.2)). The alcoholysis of urea offers not only a simple and sustainable route to synthesis of cyclic carbonates, but also a positive economic impact with respect to the use of DMC that is obtained from ethylene carbonate or propylene carbonate, with the production of large amount of glycol as a by-product.84–87 Zinc oxide is one of the catalysts mostly used in the synthesis of organic carbonates, however the recovery of the catalyst at the end of the reaction cycle, results very difficult due to the formation of soluble compounds Zn(NCO)2(NH3)2.88–92 To increase the stability of the catalyst maintaining the same catalytic activity, we have studied the effect of lanthanum in the mixed oxides La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn (Table 5).
Zn (Table 5).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn in the alcoholysis of urea with diglycerol ether for the synthesis of DGDCa
Zn in the alcoholysis of urea with diglycerol ether for the synthesis of DGDCa
| Catalysts | % conv. diglycerol ether | % DHPMC | % DGDC | % w/w recovery of the catalysts* | ||||
|---|---|---|---|---|---|---|---|---|
| Yield | Selectivity | Yield | Selectivity | |||||
a Reaction conditions: molar ratio urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diglycerol ether = 2 diglycerol ether = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, weight ratio catalyst 1, weight ratio catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diglycerol ether = 20%, T = 423 K, t = 15 h, under vacuum 20 Pa. *At the end of the reaction, the catalyst was recovered and calcined for 3 h at 773 K. diglycerol ether = 20%, T = 423 K, t = 15 h, under vacuum 20 Pa. *At the end of the reaction, the catalyst was recovered and calcined for 3 h at 773 K. |
||||||||
| 1 | ZnO | 84.6 | 51.5 | 60.9 | 33.1 | 39.1 | 60.2 | |
| 2 | La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn Zn |
0.1 | 82.1 | 52.7 | 64.2 | 29.4 | 35.8 | 83.0 |
| 3 | 0.5 | 80.4 | 56.1 | 69.8 | 24.3 | 30.2 | 99.0 | |
| 4 | 1 | 78.2 | 58.1 | 74.3 | 20.1 | 25.7 | 95.4 | |
| 5 | La2O3 | 77.4 | 59.0 | 76.2 | 18.4 | 23.8 | 96.6 | |
| No catalyst | 53.4 | 53.4 | 100 | 0 | 0 | — | ||
Zinc oxide shows the best activity in the production of DGDC (yield = 33.1%), but also in this case, the recovery of the catalyst results very difficult due to the dissolution of the metal oxide in the reaction medium (catalyst recovery = 60.2% w/w as Zn(NH3)2(NCO)2). Mixed oxide La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn = 1 shows a good activity in the production of DGDC (yield = 20.1%, selectivity = 25.7%) and the catalyst is easily recovered and reused. The best catalyst results to be La
Zn = 1 shows a good activity in the production of DGDC (yield = 20.1%, selectivity = 25.7%) and the catalyst is easily recovered and reused. The best catalyst results to be La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn = 0.1 molar ratio has a conversion yield and selectivity lower than ZnO (yield = 29.4%, selectivity = 35.8%) with the advantage of a good recoverability (catalyst recovery = 83.0% w/w in its original form).
Zn = 0.1 molar ratio has a conversion yield and selectivity lower than ZnO (yield = 29.4%, selectivity = 35.8%) with the advantage of a good recoverability (catalyst recovery = 83.0% w/w in its original form).
The catalyst La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn = 0.5 shows the highest recoverability (99%) with a good conversion (80.4%) and a yield of 24.3%.
Zn = 0.5 shows the highest recoverability (99%) with a good conversion (80.4%) and a yield of 24.3%.
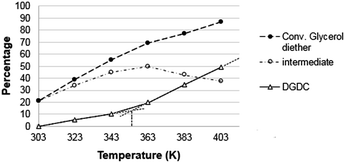
The presence of the hetero-metal increases the stability of the ZnO catalyst that maintains the same catalytic activity for several cycles of reaction.75 The reaction is not an equilibrium: in fact, the conversion yield can be improved by prolonging the reaction time (Fig. 10).
Fig. 10 shows that increasing the reaction time, the conversion of glycerol diether increases together with the formation of DGDC, while the intermediate decreases. The reaction goes to completion within 72 h, clearly showing that it is not an equilibrium reaction.
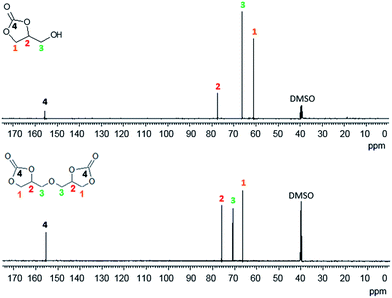
From the analysis of NMR spectra, it is possible to observe the absence of the hydroxylic group of glycerol carbonate. The bidimensional NMR spectrum definitively confirms the structure of the DGDC (Fig. 13), as clearly shown by the H–C correlation.
This represents the first full characterization of such compound as the literature gives only a partial information about its characterization in solution.72–74
3.2 Diglycerol tricarbonate
| Catalysts | % conv DMC | % MGC | % DGTC | % w/w recovery of the catalysts | |||
|---|---|---|---|---|---|---|---|
| Yield | Selectivity | Yield | Selectivity | ||||
a Reaction conditions: molar ratio glycerol carbonate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC = 5 DMC = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, weight ratio catalyst 1, weight ratio catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC = 20%, T = 413 K, time = 24 h. *At the end of the reaction the catalyst, was recovered and calcined for 3 h at 773 K. DMC = 20%, T = 413 K, time = 24 h. *At the end of the reaction the catalyst, was recovered and calcined for 3 h at 773 K. |
|||||||
| 1 | CaO | 58.1 | 42.7 | 73.5 | 15.4 | 26.5 | 67.2 |
| 2 | La2O3 | 50.5 | 40.7 | 80.6 | 9.8 | 19.4 | 97.3 |
| 3 | MgO | 45.3 | 37.9 | 83.7 | 7.4 | 16.3 | 85.4 |
| 4 | SnO | 30.2 | 28.9 | 95.7 | 1.3 | 4.3 | 96.2 |
| 5 | TiO2 | 20.4 | 20.4 | 100 | 0 | 0 | 97.1 |
| 6 | ZrO2 | 18.4 | 18.4 | 100 | 0 | 0 | 97.4 |
| 7 | Nb2O5 | 16.8 | 16.8 | 100 | 0 | 0 | 96.9 |
| 8 | No catalyst | 14.2 | 14.2 | 100 | 0 | 0 | — |
Calcium oxide shows the best catalytic activity with a yield of 15.4%. However, also in this case, the recovery of the catalyst was very difficult due to the leaching of the catalyst in solution (catalyst recovery = 67.2% w/w). The study of the basic/acid properties of the metal oxides has highlighted a correlation between the basicity and the catalytic activity of the catalysts (Fig. 14). Similarly, to the case of DGDC (Fig. 4), the data suggest that this process also is mainly base-catalyzed.
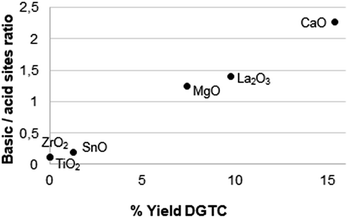 | ||
| Fig. 14 Correlation between the basic/acid sites ratio and the catalytic activity of single metal oxides in the production of DGTC. | ||
In an attempt to increase the reaction yield, the trans-esterification reaction of MGC with glycerol carbonate was also investigated (eqn (1.5), Table 7). The reaction proceeds to a conversion of MGC to 73.5%, but the yield of DGTC is only 16.9% with a selectivity of 23%. Moreover, the GC-MS analysis reveals the production of DMC during the reaction. The presence of DMC indicates that the synthesis of DGTC occurs with a different mechanism of reaction that may not imply the reaction of glycerol with MGC. In order to explain what happens in such reaction, a study of the activity of the metal oxides was carried out in presence of only MGC as reagent.
| Catalysts | % conv. glycerol carbonate | % DGTC | % w/w recovery of the catalysts* | ||
|---|---|---|---|---|---|
| Yield | Selectivity | ||||
a Reaction conditions: molar ratio MGC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol carbonate = 2 glycerol carbonate = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, weight ratio catalyst: MGC = 20%, T = 413 K, t = 24 h. *At the end of the reaction, the catalyst was recovered and calcined for 3 h at 773 K. 1, weight ratio catalyst: MGC = 20%, T = 413 K, t = 24 h. *At the end of the reaction, the catalyst was recovered and calcined for 3 h at 773 K. |
|||||
| 1 | CaO | 73.5 | 16.9 | 23.0 | 64.3 |
| 2 | La2O3 | 68.1 | 11.7 | 17.2 | 97.1 |
| 3 | MgO | 60.4 | 10.1 | 16.7 | 82.3 |
| No catalyst | 34.1 | 1.3 | 3.8 | — | |
 | (1.7) |
| Catalysts | % conv. MGC | % yield DMC | % yield DGTC | % w/w recovery of the catalysts* | |
|---|---|---|---|---|---|
a Reaction conditions: weight ratio catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MGC = 20%, T = 413 K, t = 24 h. *At the end of the reaction, the catalyst was recovered and calcined for 3 h at 773 K. MGC = 20%, T = 413 K, t = 24 h. *At the end of the reaction, the catalyst was recovered and calcined for 3 h at 773 K. |
|||||
| 1 | CaO | 42.5 | 21.3 | 21.2 | 67.3 |
| 2 | La2O3 | 33.2 | 16.6 | 16.6 | 97.4 |
| 3 | MgO | 25.4 | 12.6 | 12.8 | 83.2 |
| No catalyst | 5.6 | 2.8 | 2.8 | — | |
From the data analysis, it is possible to observe how the key step for the synthesis of DGTC is represent by the disproportionation of MGC. In fact, MGC alone reacts using CaO as catalyst with a yield of 21.2% in DGDC. DGTC and DMC are formed in equimolar amounts, as revealed by GC-MS. The conversion yield is not very high but the selectivity is 100% towards DGTC (eqn (1.8)).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Ca = 1 with K2CO3. To increase the stability of calcium oxide, the mixed oxide La
Ca = 1 with K2CO3. To increase the stability of calcium oxide, the mixed oxide La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca = 1 was tested in the disproportionation of MGC and compared with K2CO3 (Table 9) reported in literature to act as catalyst for the synthesis of DGTC starting from glycerol and DMC.71 Mixed oxide La
Ca = 1 was tested in the disproportionation of MGC and compared with K2CO3 (Table 9) reported in literature to act as catalyst for the synthesis of DGTC starting from glycerol and DMC.71 Mixed oxide La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca = 1 shows a catalytic activity better than K2CO3 in the same experimental conditions.
Ca = 1 shows a catalytic activity better than K2CO3 in the same experimental conditions.
 | (1.8) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca = 1 with K2CO3a
Ca = 1 with K2CO3a
| Catalysts | % conv. MGC | % yield DGTC | % w/w recovery of the catalysts* | |
|---|---|---|---|---|
a Reaction conditions: weight ratio catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MGC = 20%, T = 413 K, t = 24 h. *At the end of the reaction, the catalyst was recovered and calcined for 3 h at 773 K. MGC = 20%, T = 413 K, t = 24 h. *At the end of the reaction, the catalyst was recovered and calcined for 3 h at 773 K. |
||||
| 1 | La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca = 1 Ca = 1 |
37.4 | 18.7 | 96.9 |
| 2 | K2CO3 | 13.4 | 6.7 | — |
An aspect to consider is that our heterogeneous catalyst is easily recovered at the end of the reaction cycle and reused without loss of the catalytic acitivity (Fig. 15).
4. Conclusions
In this work, bi-metallic oxides based on La, Ca, Zn were synthesized and used as catalysts, in eco-friendly routes for the synthesis of DGDC and DGTC. The trans-esterification reaction of diglycerol ether with DMC, resulted to be the best method for the synthesis of DGDC.Different catalysts were tested, characterized by different acid/basic sites ratio. Mixed oxide La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca molar ratio 1 shows a good catalytic activity in the production of DGDC (yield = 66.7%) with a high selectivity (81.1%).
Ca molar ratio 1 shows a good catalytic activity in the production of DGDC (yield = 66.7%) with a high selectivity (81.1%).
Respect to calcium oxide alone, or lanthanum oxide or else hydrotalcite Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al = 5, the La
Al = 5, the La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca catalyst shows the highest yield, is more easily recoverable and reusable, with a very moderate loss of the catalytic activity. For the DGTC synthesis, we have shown that the disproportionation of MGC is the key step (CaO as catalyst, yield 21.1% and selectivity of 100%), more than the reaction of DMC with two molecules of glycerol carbonate. The mixed oxide La
Ca catalyst shows the highest yield, is more easily recoverable and reusable, with a very moderate loss of the catalytic activity. For the DGTC synthesis, we have shown that the disproportionation of MGC is the key step (CaO as catalyst, yield 21.1% and selectivity of 100%), more than the reaction of DMC with two molecules of glycerol carbonate. The mixed oxide La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca = 1 afforded much better yield (19.9) and selectivity (100%) than K2CO3 (6.7%) described in the literature. Mixed oxides were also easily recovered and reused.
Ca = 1 afforded much better yield (19.9) and selectivity (100%) than K2CO3 (6.7%) described in the literature. Mixed oxides were also easily recovered and reused.
Acknowledgements
The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 241718 EuroBioRef (DGDC) and PON 01966-MIUR (glycerol production by water-free transesterification of lipids and DGTC). IC2R srl is acknowledged for scientific support. Solvay Chemicals International is gratefully thanked for a loan of diglycerol ether.References
- M. Martín and I. E. Grossmann, Ind. Eng. Chem. Res., 2014, 53, 7730–7745 CrossRef.
- F. Bauer and C. Hulteberg, Biofuels, Bioprod. Biorefin., 2013, 7, 43–51 CrossRef CAS.
- Z. Y. Zakaria, N. Amin and J. Linnekoski, Biomass Bioenergy, 2013, 50, 370–375 CrossRef.
- J. F. Izquierdo, M. Montiel, I. Palés, P. R. Outón, M. Galán, L. Jutglar, M. Villarrubia, M. Izquierdo, M. P. Hermo and X. Ariza, Renewable Sustainable Energy Rev., 2012, 16, 6711–6724 CrossRef.
- M. Ayoub, M. S. Khayoon and A. Z. Abdullah, Bioresour. Technol., 2012, 112, 308–312 CrossRef CAS PubMed.
- N. Rahmat, A. Z. Abdullah and A. R. Mohamed, Renewable Sustainable Energy Rev., 2010, 14, 987–1000 CrossRef CAS.
- M. Pagliaro, R. Ciriminna, H. Kimura, M. Rossi and C. Della Pina, Angew. Chem., Int. Ed., 2007, 46, 4434–4440 CrossRef CAS PubMed.
- J. Janaun and N. Ellis, Renewable Sustainable Energy Rev., 2010, 14, 1312–1320 CrossRef CAS.
- C. W. Chiu, M. A. Dasari, W. R. Sutterlin and G. Suppes, Ind. Eng. Chem. Res., 2006, 45, 791–795 CrossRef CAS.
- D. T. Johnson and K. A. Taconi, Environ. Prog., 2007, 26, 338–348 CrossRef CAS.
- H. Wang, L. Zhu, S. Peng, F. Peng, H. Yu and J. Yang, Renewable Energy, 2012, 37, 192–196 CrossRef CAS.
- A. M. Ruppert, K. Weinberg and R. Palkovits, Angew. Chem., 2012, 12, 2564–2601 CrossRef PubMed.
- P. Gallezot, Catal. Today, 2007, 121, 76–91 CrossRef CAS.
- J. L. Dubois, US 8686195 B2, 2014.
- A. Talebian-Kiakalaieh, N. A. S. Amin and H. Hezaveh, Renewable Sustainable Energy Rev., 2014, 40, 28–59 CrossRef CAS.
- R. Znaiguia, L. Brandhorst, N. Christin, V. B. Baca, P. Rey, J. M. M. Millet and S. Loridant, Microporous Mesoporous Mater., 2014, 196, 97–103 CrossRef CAS.
- R. Len and R. Luque, Sustainable Chem. Processes, 2014, 2, 1–10 CrossRef.
- G. S. Foo, D. Wei, D. S. Sholl and C. Sievers, ACS Catal., 2014, 4(9), 3180–3192 CrossRef CAS.
- S. H. Chai, B. Yan, L. Z. Tao, Y. Liang and B. Q. Xu, Catal. Today, 2014, 234, 215–222 CrossRef CAS.
- X. Li, C. Qin, K. Jiao, S. Feng, Y. Zhuang, J. Ma, X. Zhu and J. Shao, US 8623780 B2, 2014.
- B. Sarkar, C. Pendem, L. N. S. Konathala, R. Tiwari, T. Sasaki and R. Bal, Chem. Commun., 2014, 50, 9707–9710 RSC.
- X. Li, C. Zhang, H. Cheng, L. He, W. Lin, Y. Yu and F. Zhao, J. Mol. Catal. A: Chem., 2014, 395, 1–6 CrossRef CAS.
- S. Jin, Z. Xiao, C. Li, C. T. Williams and C. Liang, J. Energy Chem., 2014, 9, 185–192 CrossRef.
- J. Feng, B. Xu, D. Liu, W. Xiong and J. B. Wang, in Adv. Mater. Chem., ed. B. Li and H. M. Zhang, 2013, vol. 791, pp. 16–19 Search PubMed.
- R. V. Sharma, P. Kumar and A. K. Dalai, Appl. Catal., A, 2014, 477, 147–156 CrossRef CAS.
- L. Niu, R. Wei, H. Yang, X. Li, F. Jiang and G. Xiao, Chin. J. Catal., 2013, 34, 2230–2235 CrossRef CAS.
- S. Zhu, Y. Zhu, S. Hao, H. Zheng, T. Mo and Y. Li, Green Chem., 2012, 14, 2607–2616 RSC.
- J. S. Yadav, M. Sridhar Reddy, P. Rao and A. R. Prasad, Tetrahedron Lett., 2006, 47, 4397–4401 CrossRef CAS.
- S. Fiori, A. Mariani, S. Bidali and G. Malucelli, Green Chem., 2004, 4, 1–12 Search PubMed.
- L. A. Thompson and J. A. Ellman, Chem. Rev., 1996, 96, 555–600 CrossRef CAS PubMed.
- C. Giordano, S. Cavicchioli, S. Levi and M. Villa, Tetrahedron Lett., 1988, 29, 5561–5564 CrossRef CAS.
- R. D. Bush, D. S. Connor, S. W. Heinzman and L. N. Mackey, US4663071 A, 1987.
- K. Y. Nandiwale, S. E. Patil and V. V. Bokade, Energy Technol., 2014, 5, 446–452 CrossRef.
- C. Cannilla, G. Bonura, L. Frusteri and F. Frusteri, Eur. J. Chem., 2014, 12, 1248–1254 CAS.
- P. G. Urbaneja, C. G. Sancho, R. M. Tost, J. M. Robles, J. S. González, A. J. López and P. M. Torres, Appl. Catal., A, 2014, 470, 1248–1254 Search PubMed.
- P. A. Celdeira, M. Gonçalves, C. A. Figueiredo, S. Dal Bosco, D. Mandelli and W. A. Carvalho, Appl. Catal., A, 2014, 478, 98–106 CrossRef CAS.
- Z. Gholami, A. Z. Abdullah and Z. T. Lee, Chem. Eng. Sci., 2013, 1, 79–86 CrossRef CAS.
- M. K. Munshi, S. M. Gade, V. H. Rane and A. A. Kelkar, RSC Adv., 2014, 4, 32127–32133 RSC.
- S. M. Gade, M. K. Munshi, B. M. Chherawalla, V. H. Rane and A. A. Kelkar, Catal. Commun., 2012, 27, 184–188 CrossRef CAS.
- F. Rieckenberg, I. Ardao, R. Rujananon and A. Zeng, Eng. Life Sci., 2014, 14, 380–386 CrossRef CAS.
- T. S. Kang, D. R. Korber and T. Tanaka, J. Ind. Microbiol. Biotechnol., 2014, 41(41), 629–635 CrossRef CAS PubMed.
- C. Della Pina, E. Falletta and M. Rossi, Green Chem., 2011, 13, 1624–1632 RSC.
- M. J. Burk and R. E. Osterhout, US 20100021978 A1, 2010.
- C. A. G. Quispe, C. J. R. Coronado and J. A. Carvalho Jr, Renewable Sustainable Energy Rev., 2013, 27, 475–493 CrossRef CAS.
- J. Graça and H. Pereira, Biomacromolecules, 2000, 1, 519–522 CrossRef.
- Z. Gholami, A. Z. Abdullah and K. Lee, Renewable Sustainable Energy Rev., 2014, 39, 327–341 CrossRef CAS.
- A. Dibenedetto and D. Ballivet-Tkatchenko, in Carbon dioxide as Chemical Feedstock, ed. M. Aresta, Wiley VCH, 2010, XIV, 1–14, ch. 7 Search PubMed.
- Z. Zhang, D. W. Rackemann, O. S. Doherty and I. M. O'Hara, Biotechnol. Biofuels, 2013, 6, 153–156 CrossRef CAS PubMed.
- A. Behr, P. Bahke, B. Klinger and M. Becker, J. Mol. Catal. A: Chem., 2011, 267, 149–156 CrossRef.
- M. O. Sonnati, S. Amigoni, P. T. de Givenchy, T. Darmanin, O. Choulet and F. Guittard, Green Chem., 2013, 15, 283–306 RSC.
- L. Ubaghs, N. Fricke, H. Keul and H. Höcker, Macromol. Rapid Commun., 2004, 25, 517–521 CrossRef CAS.
- J. H. Clements, Ind. Eng. Chem. Res., 2003, 42, 663–674 CrossRef CAS.
- A. Dibenedetto, F. Nocito, A. Angelini, I. Papai, M. Aresta and R. Mancuso, ChemSusChem, 2013, 6, 345–352 CrossRef CAS PubMed.
- A. Dibenedetto, A. Angelini, M. Aresta, J. Ethiraj, C. Fragale and F. Nocito, Tetrahedron, 2011, 67, 1308–1313 CrossRef CAS.
- H. Babad and A. G. Zeiler, Chem. Rev., 1973, 73, 75–91 CrossRef CAS.
- P. Strege and J. Renga, US 4344881, 1982.
- A. Dibenedetto, A. Angelini and P. Stufano, J. Chem. Technol. Biotechnol., 2014, 89, 334–353 CrossRef CAS.
- M. Tamura, M. Honda, Y. Nakagawa and K. Tomishige, J. Chem. Technol. Biotechnol., 2014, 89, 19–33 CrossRef CAS.
- M. Aresta, A. Dibenedetto, F. Nocito and C. Pastore, J. Mol. Catal. A: Chem., 2006, 257, 149–153 CrossRef CAS.
- M. Aresta, A. Dibenedetto, J. L. Dubois, C. Ferragina and F. Nocito, US 20110245513 A1, 2011.
- M. Aresta, A. Dibenedetto, F. Nocito and C. Ferragina, J. Catal., 2009, 268, 106–114 CrossRef CAS.
- M. J. Climent, A. Corma, P. De Frutos, S. Iborra, M. Noy, A. Velty and P. Concepción, J. Catal., 2010, 269, 140–149 CrossRef CAS.
- Y. T. Algoufi and B. H. Hameed, Fuel Process. Technol., 2014, 126, 5–11 CrossRef CAS.
- Y. Yia, Y. Shena, J. Sun, B. Wang, F. Xua and R. Sun, Chin. J. Catal., 2014, 35, 757–762 CrossRef.
- A. Dibenedetto, A. Angelini, A. Colucci, L. di Bitonto, C. Pastore, B. M. Aresta, C. Giannini and R. Comparelli, International Journal of Renewable Energy and Biofuels, in press Search PubMed.
- M. Malyaadri, K. Jagadeeswaraiah, P. S. Sai Prasad and N. Lingaiah, Appl. Catal., A, 2011, 401, 153–157 CrossRef CAS.
- C. Farcet and B. Lion, US 8710152 B2, 2014.
- H. S. Bevinakatti, A. G. Waite and J. Frank, US 8722814 B2, 2014.
- H. S. Bevinakatti, A. G. Waite and J. Frank, US 6620904, 2014.
- M. Völkel, R. V. Benten and S. Jain, US 8691906 B2, 2014.
- G. Rokicki, P. Rakoczy, P. Parzuchowski and M. Sobiecki, Green Chem., 2005, 7, 529–539 RSC.
- G. Mignani, J. Debray, E. Da Silva, M. Lemarie and Y. Raoul, FR 2993269 A1, 2014.
- J. A. Stewart, E. Reubsaet, B. M. Weckhuysen and P. C. A. Bruijnincx, 11th European Congress on Catalysis – EuropaCat-XI, Lyon, France, September 1st-6th, 2013 Search PubMed.
- J. A. Stewart, B. M. Weckhuysen, P. C. A. Bruijnincx, Catal. Today, DOI:10.1016/j.cattod.2014.06.035.
- M. Aresta, A. Dibenedetto, L. di Bitonto and J. L. Dubois, EP13192912.7, 2013.
- M. Aresta, A. Dibenedetto, C. Pastore, A. Angelini, B. M. Aresta and I. Papai, J. Catal., 2010, 269, 44–52 CrossRef CAS.
- A. Dibenedetto, M. Aresta, A. Angelini, J. Ethiraj and B. M. Aresta, Chem.–Eur. J., 2012, 18, 10324–10334 CrossRef CAS PubMed.
- A. Dibenedetto, A. Angelini, L. di Bitonto, E. de Giglio, S. Cometa and M. Aresta, ChemSusChem, 2014, 7, 1155–1161 CrossRef CAS PubMed.
- M. Aresta, A. Dibenedetto and A. Angelini, Chem. Rev., 2014, 114, 1709–1742 CrossRef CAS PubMed.
- A. Dibenedetto, M. Aresta, A. Angelini, J. Ethiraj and B. M. Aresta, Chem.–Eur. J., 2012, 18, 10324–10334 CrossRef CAS PubMed.
- M. Aresta, A. Dibenedetto, C. Pastore, C. Cuocci, B. M. Aresta, S. Cometa and E. De Giglio, Catal. Today, 2008, 137, 125–131 CrossRef CAS.
- A. Dibenedetto, M. Aresta, C. Fragale, M. Distaso, C. Pastore, A. M. Venezia, C. J. Liu and M. Zhang, Catal. Today, 2008, 137, 44–51 CrossRef CAS.
- M. Aresta, A. Dibenedetto, L. di Bitonto and A. Angelini, MI2013A001136, 2013.
- Z. Ga, S. F. Wang and C. G. Xia, Chin. Chem. Lett., 2009, 20, 131–134 CrossRef.
- X. Zhao, Y. Zhang and Y. Wang, Ind. Eng. Chem. Res., 2004, 15, 4038–4042 CrossRef.
- Q. Li, N. Zhao, W. Wei and Y. Sun, Stud. Surf. Sci. Catal., 2004, 153, 573–576 CrossRef CAS.
- M. Doya, T. Ohkawa, Y. Kanbara and A. Okmota, US Pat., 5349077, 1994.
- A. Dibenedetto, A. Angelini, S. Fasciano, I. Papai, D. Curulla-Ferré and M. Aresta, J. CO2 Util., 2014, 8, 27–33 CrossRef CAS.
- M. Aresta, A. Dibenedetto, C. Devita, O. A. Bourova and O. N. Chupakhin, Stud. Surf. Sci. Catal., 2004, 153, 213–220 CrossRef CAS.
- Y. Gao, W. Penga, N. Zhao, W. Wei and Y. Sun, J. Mol. Catal. A: Chem., 2011, 1, 2351–2360 Search PubMed.
- M. Wang, H. Wang, N. Zhao, W. Wei and Y. Sun, Catal. Commun., 2006, 7, 6–10 CrossRef CAS.
- M. Wang, N. Zhao, W. Wei and Y. Sun, Ind. Eng. Chem. Res., 2005, 1, 7596–7599 CrossRef.
| This journal is © The Royal Society of Chemistry 2015 |