Silicon-containing poly(esters) with halogenated bulky side groups. Synthesis, characterization and thermal studies
L. H. Tagle,
C. A. Terraza,
A. Tundidor-Camba and
D. Coll
Pontificia Universidad Católica de Chile, Faculty of Chemistry, Organic Chemistry Department, P.O. Box, 306, Santiago, Chile. E-mail: ltagle@uc.cl
First published on 22nd May 2015
Abstract
Poly(esters) (PEs) derived from diacids containing bulky side groups, which have an halogenated (Cl, Br) imide ring, an aminoacidic residue (glycine, L-alanine, L-valine) and an amide group were obtained with a silicon-containing diphenol. Also PEs without the aminoacidic residue were obtained. PEs were characterized by IR and NMR spectroscopy, and the results were in agreement with the proposed structures. PEs were obtained with good yields and moderate or high ηinh values. PEs were soluble in aprotic polar solvents and were swollen in other solvents like m-cresol and THF. The Tg values were determined and it was possible to see a tendency in the sense that when the size of the atom (Cl, Br) bonded to the imidic ring is increased, the Tg values decreased, also for those PEs obtained without the aminoacidic residue. The thermal decomposition temperatures showed that only two PEs can be considered as thermostable, considering TDT values above 400 °C at 10% of weight lost. The other PEs showed good thermal stability, showing in general a decrease of the TDT values when the volume of the side group, is increased. PEs showed UV-vis transparency at 400 nm lower than 20%, but between 500 and 600 nm, showed 80% transparency. PEs containing halogen atoms showed flame retardancy in a simple essay, with respect to PEs without halogen atoms in which the combustion was complete.
1. Introduction
Poly(esters) are condensation polymers which have been considered of interest due to their excellent mechanical properties, chemical resistance and thermal stability.1–4 However, when poly(esters) have high aromatic content, the processing is difficult due to high Tg values and insolubility in common organic solvents.5–9There are several structural changes that are possible to introduce into the monomers with the objective to improve the polymeric properties. In this sense the introduction of flexible units in the main or side chain, or cardo groups, are solutions proposed in order to increase the solubility and to decrease the Tg values, but without affecting the thermal stability.10–13
Cardo or bulky side groups have the objective of to reduce the intermolecular interactions between the polymeric chains due to the higher free volume, and consequently it is possible to improve the solubility and to decrease the Tg due to the higher internal mobility of the polymeric chains. One of the groups used with this objective is the imide one.2,5,14
On the other hand, the introduction of flexible groups in the repeating unit also has been used in order to improve the processability. Mallakpour has described poly(esters) with structural modifications, which include amino acids as flexible units with bulky groups and several functional groups, increasing the flexibility due to the central sp3 carbon of the amino acid moiety.15
Silicon-containing condensation polymers also have been developed in order to introduce in the polymeric chain heteroatoms which induce polarity in the main chain due to the difference of electronegativity between Si and C, increasing the solubility. These polymers have been studied for potential applications due to that the Si atom permits a σ–π conjugation when it is between aromatic rings and consequently participate in the electron transport in the polymeric chain.13,16,17 In this sense, Thames16,18 and Bruma19,20 have described silicon-containing polymers, with good solubility in common organic solvents and low Tg values, but maintaining the thermal stability.
The presence of halogen atoms in the polymeric chains also has been studied. In general atoms like Cl or Br can to act as flame retardant because they can remove free radicals, which diminish the burning process.21 Mallakpour has described polymers with this property, including bulky groups and halogen atoms, which led to flame-retardant materials, with good solubility, without affect the thermal properties.22
Continuing our works about the synthesis and characterization of silicon-containing condensation polymers,23–27 in this work we describe the synthesis of poly(esters) (PEs) derived from diacids containing a bulky side group, which have an imidic ring substituted with halogen atoms (Cl or Br), an aminoacidic residue (glycine, L-alanine or L-valine) and an amide group.
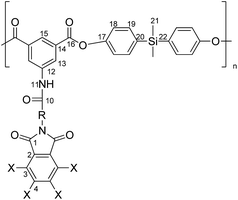
These carboxylic imide-acids were polymerized with bis(4-hydroxyphenyl)dimethylsilane and the poly(esters) were characterized by elemental analysis, IR and NMR spectroscopy. The thermal properties, Tg and TDT, were determined and the results were related with the proposed structures. Also UV-vis transparency and flame resistance were determined.
2. Experimental
2.1. Materials
Glycine, L-alanine, L-leucine and p-amino-benzoic acid, 5-amino-isophthalic acid and the anhydrides phthalic, tetrachlorophthalic and tetrabromophthalic, were obtained from Aldrich Chemical (Milwaukee, WI) and used without further purification. All other reagents and solvents were purchased commercially as analytical-grade and used without further purification.2.2. Instrumentation
The IR spectra (KBr pellets) were recorded on a Perkin-Elmer (Fremont CA) 1310 spectrophotometer over the range of 450–4000 cm−1. 1H, 13C and 29Si NMR spectra were carried out on a 400 MHz instrument (Bruker AC-200) using DMSO-d6, CDCl3 or acetone-d6 as solvents and TMS as the internal standard. Viscosimetric measurements were made in a Desreux–Bischof type dilution viscosimeter at 25 °C (c = 0.5 g dL−1). Tg values were obtained with a Mettler-Toledo (Greifensee, Switzerland) DSC 821 calorimetric system (20 °C min−1 under N2 flow) after the second heating run. Thermogravimetric analyses were carried out in a Mettler (Switzerland) TA-3000 calorimetric system equipped with a TC-10A processor, and a TG-50 thermobalance with a Mettler MT5 microbalance. Samples of 6–10 mg were placed in a platinum sample holder and the thermogravimetric measurements were carried out between 30 °C and 800 °C with a heating rate of 20 °C min−1 under N2 flow. Specific rotations were measured in an Optical Activity Automatic polarimeter, Model AA-5 at 17 °C. The UV-visible optical transmission spectra were obtained in NMP solutions (c = 5.0 g L−1) at room temperature on a UV-3101PC UV-Vis-NIR scanning spectrophotometer (Shimadzu, Japan). Elemental analyses were made on a Fisons EA 1108-CHNS-O equipment.2.3. Monomer synthesis
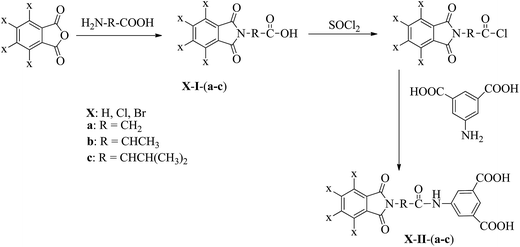
The carboxylic imide-acids: phthalimidyl-acetic acid (H-I-a),28 tetrachlorophthalimidyl-acetic acid (Cl-I-a),29 tertrabromophthalimidyl-acetic acid (Br-I-a),30 2-phthalimidyl-propanonic acid (H-I-b),31 2-tetrachlorophthalimidyl-propanoic acid (Cl-I-b),29,32 2-tetrabromophthalimidyl-propanoic acid (Br-I-b),30,32 2-phthalimidyl-3-methyl-pentanoic acid (H-I-c),33 2-tetrachlorophthalimidyl-3-methyl-pentanoic acid (Cl-I-c),29,32 and 2-tetrabromophthalimidyl-3-methyl-pentanoic acid (Br-I-c),30,32 were obtained from the respective phthalic- or tetrahalophthalic-anhydrides and the corresponding amino acid, according to a general procedure, in which the phthalic- or tetrahalophthalic-anhydride (0.05 mol) (phthalic anhydride 7.4 g, tetrachlorophthalic anhydride 14.3 g or tetrabromophthalic anhydride 23.2 g) was mixed with the amino acid (0.05 mol) (glycine 3.8 g, L-alanine 4.5 g, or L-leucine 6.5 g) in 50 mL of acetic acid and stirred during four hours. Then the mixture was refluxed by four hours and the acetic acid removed under vacuum. The residue was poured in 50 mL of a 10% HCl solution and the solid filtered, washed several times with water, dried under vacuum until constant weight (Scheme 1). The nature of all compounds was verified by IR and 1H and 13C NMR spectroscopy.The dicarboxylic imide-amide-acids: 5-(phthalimidyl-acetylamino)-isophthalic acid (H-II-a),28 5-(tetrachlorophthalimidyl-acetylamino)-isophthalic acid (Cl-II-a),34 5-(tetrabromophthalimidyl-acetylamino)-isophthalic acid (Br-II-a),34 5-(2-phthalimidyl-propionylamino)-isophthalic acid (H-II-b),31 5-(2-tetrachlorophthalimidyl-propionylamino)-isophthalic acid (Cl-II-b),34 5-(2-tetrabromophthalimidyl-propionylamino)-isophthalic acid (Br-II-b),34 5-(2-phthalimidyl-4-methyl-pentanoylamino)-isophthalic acid (H-II-c),33 5-(2-tetrachlorophthalimidyl-4-methyl-pentanoylamino)-isophthalic acid (Cl-II-c),34 and 5-(2-tetrabromophthalimidyl-4-methyl-pentanoylamino)-isophthalic acid (Br-II-c),34,35 were obtained from the respective carboxylic phthalimidyl- or tetrahalophthalimidyl-acids according to a general procedure, in which the carboxylic imide acid was mixed with thionyl chloride in order to obtain the acid chloride, which reacted with 5-amino-isophthalic acid. The mixture was poured in 10% HCl solution, and the solid filtered, washed with water and dried until constant weight (Scheme 1). The nature of all compounds was verified by IR and 1H and 13C NMR spectroscopy.
The dicarboxylic imide-acids: 5-phthalimidyl-isophthalic diacid (H-III),28 5-tetrachlorophthalimidyl-isophthalic diacid (Cl-III),38,39 and 5-tetrabromophthalimidyl-isophthalic diacid (Br-III),34 were obtained by reaction of the respective phthalic- or tetrahalophthalic-anhydrides and 5-amino-isophthalic acid (Scheme 2) according to the same former procedure already described. The nature of all compounds was verified by IR and 1H and 13C NMR spectroscopy.
The diphenol bis(4-hydroxyphenyl)dimethylsilane was synthesized from 4-bromophenol and dimethyldichlorosilane, according to a describe procedure.36
2.4. Polymer synthesis and characterization
PEs were synthesized according to the following general procedure. In a typical polymerization process, 0.82 mmol of the monomeric diacid are dissolved in 1.92 mL of pyridine and are stirred by 30 minutes. After this time, a mixture of 4.1 mmol of 4-toluenesulfonyl chloride (Tos-Cl), 0.97 mL of pyridine and 0.39 mL of DMF, with a standby of 30 minutes, is added at room temperature and the reaction mixture is stirred for 30 minutes. Then, the mixture is heated at 90 °C for 10 minutes and a solution of 0.82 mmol of the silylated diphenol in 0.97 mL of pyridine is added. Now, the mixture is stirred for 3 hours at 90 °C. Then the mixture is poured into water and the solid filtered, washed, and dried under vacuum until constant weight and characterized (Scheme 3).H-PE-a.
IR (KBr) (cm−1): 3429 (NH), 3070 (CH arom.), 2947 (CH3), 1772, (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O imide), 1735 (C
O imide), 1735 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester), 1699 (C
O ester), 1699 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O amide), 1596, 1577 (C
O amide), 1596, 1577 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1454 (CH3), 1425 (Si–C arom.), 1371 (Si–C aliph.), 1102 (O–C arom.), 839 (arom. 1,3,5-subst.), 820 (arom. p-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.59 (s, 6H, CH3) (21), 4.54 (s, 2H, CH2) (5), 7.34 (s, 4H, arom.) (18), 7.64 (s, 4H, arom.) (19), 7.87–7.91 (m, 4H, arom.) (3, 4), 8.46 (s, 1H, arom.) (15), 8.56–8.60 (s, 2H, arom.) (13), 11.10 (s, 1H, NH) (11). 13C NMR (DMSO-d6) (δ) (ppm): −3.4 (21), 38.7 (5), 120.6 (18), 122.6 (3), 124.7 (15), 129.5 (13), 130.3 (2), 130.8 (4), 134.0 (14), 134.6 (20), 135.0 (12), 144.6 (19), 150.7 (17), 162.4 (16), 165.1 (10), 166.7 (1). 29Si NMR (DMSO-d6) (δ) (ppm): −7.84 (22). Elemental analysis. Calculated: (C32H24N2O7Si) (576.64 g mol−1): C: 66.65%, H: 4.20%, N: 4.86%. Found: C: 66.46%, H: 4.28%, N: 4.61%.
C arom.), 1454 (CH3), 1425 (Si–C arom.), 1371 (Si–C aliph.), 1102 (O–C arom.), 839 (arom. 1,3,5-subst.), 820 (arom. p-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.59 (s, 6H, CH3) (21), 4.54 (s, 2H, CH2) (5), 7.34 (s, 4H, arom.) (18), 7.64 (s, 4H, arom.) (19), 7.87–7.91 (m, 4H, arom.) (3, 4), 8.46 (s, 1H, arom.) (15), 8.56–8.60 (s, 2H, arom.) (13), 11.10 (s, 1H, NH) (11). 13C NMR (DMSO-d6) (δ) (ppm): −3.4 (21), 38.7 (5), 120.6 (18), 122.6 (3), 124.7 (15), 129.5 (13), 130.3 (2), 130.8 (4), 134.0 (14), 134.6 (20), 135.0 (12), 144.6 (19), 150.7 (17), 162.4 (16), 165.1 (10), 166.7 (1). 29Si NMR (DMSO-d6) (δ) (ppm): −7.84 (22). Elemental analysis. Calculated: (C32H24N2O7Si) (576.64 g mol−1): C: 66.65%, H: 4.20%, N: 4.86%. Found: C: 66.46%, H: 4.28%, N: 4.61%.
H-PE-b.
IR (KBr) (cm−1): 3448 (NH), 3095 (CH arom.), 2954 (CH3), 1775, (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O imide), 1735 (C
O imide), 1735 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester), 1637 (C
O ester), 1637 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O amide), 1588, 1559 (C
O amide), 1588, 1559 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1450 (CH3), 1425 (Si–C arom.), 1370 (Si–C aliph.), 1109 (O–C arom.), 916 (arom. 1,3,5-subst.), 824 (arom. p-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.58 (s, 6H, CH3) (21), 1.57 (s, 3H, CH3) (6), 4.99 (m, 1H, CH) (5), 7.32 (s, 4H, arom.) (18), 7.62 (s, 4H, arom.) (19), 7.84 (m, 4H, arom.) (3, 4), 8.44 (s, 1H, arom.) (15), 8.70 (s, 2H, arom.) (13), 10.41 (s, 1H, NH) (11). 13C NMR (DMSO-d6) (δ) (ppm): −2.6 (21), 14.9 (6), 48.9 (5), 121.3 (18), 123.1 (3), 125.5 (15), 129.3 (13), 130.3 (2) 131.8 (4), 134.4 (14), 135.3 (20), 135.6 (12), 140.0 (19), 151.6 (17), 163.5 (16), 167.2 (10), 168.4 (1). 29Si NMR (DMSO-d6) (δ) (ppm): −7.85 (22). Elemental analysis. Calculated: (C33H26N2O7Si) (590.66 g mol−1): C: 67.10%, H: 4.44%, N: 4.74%. Found: C: 67.15%, H: 4.39%, N: 4.64%.
C arom.), 1450 (CH3), 1425 (Si–C arom.), 1370 (Si–C aliph.), 1109 (O–C arom.), 916 (arom. 1,3,5-subst.), 824 (arom. p-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.58 (s, 6H, CH3) (21), 1.57 (s, 3H, CH3) (6), 4.99 (m, 1H, CH) (5), 7.32 (s, 4H, arom.) (18), 7.62 (s, 4H, arom.) (19), 7.84 (m, 4H, arom.) (3, 4), 8.44 (s, 1H, arom.) (15), 8.70 (s, 2H, arom.) (13), 10.41 (s, 1H, NH) (11). 13C NMR (DMSO-d6) (δ) (ppm): −2.6 (21), 14.9 (6), 48.9 (5), 121.3 (18), 123.1 (3), 125.5 (15), 129.3 (13), 130.3 (2) 131.8 (4), 134.4 (14), 135.3 (20), 135.6 (12), 140.0 (19), 151.6 (17), 163.5 (16), 167.2 (10), 168.4 (1). 29Si NMR (DMSO-d6) (δ) (ppm): −7.85 (22). Elemental analysis. Calculated: (C33H26N2O7Si) (590.66 g mol−1): C: 67.10%, H: 4.44%, N: 4.74%. Found: C: 67.15%, H: 4.39%, N: 4.64%.
H-PE-c.
IR (KBr) (cm−1): 3432 (NH), 3088 (CH arom.), 2952, 2871, 1496 (CH3, CH2), 1775 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O imide), 1742 (C
O imide), 1742 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester), 1606 (C
O ester), 1606 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O amide), 1589, 1545 (C
O amide), 1589, 1545 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1496 (CH3), 1467 (Si–C arom.), 1368 (Si–C aliph.), 1111 (O–C arom.), 915 (arom. 1,3,5-subst.), 834 (arom. p-sust.). 1H NMR (DMSO-d6) (δ) (ppm): 0.23 (s, 3H, CH3) (21), 0.53 (s, 3H, CH3) (21′), 0.83–0.88 (d, J = 17.7 MHz, 6H, CH3) (9), 1.40 (m, 1H, CH) (8), 2.00 (m, 1H, CH2) (7), 2.13 (m, 1H, CH) (7′), 4.94 (m, 1H, CH) (5), 7.27 (s, 4H, arom.) (18), 7.58 (s, 4H, arom.) (19), 7.82–7.87 (m, 4H, arom.) (3, 4), 8.42 (s, 1H, arom.) (15), 8.67 (s, 2H, arom.) (13), 10.41 (s, 1H, NH) (11). 13C NMR (DMSO-d6) (δ) (ppm): −3.4 (21), 20.2 (9), 22.38 (9′), 23.9, (8), 36.2 (7), 51.07 (5), 120.7 (18), 122.6 (3), 124.7 (15), 127.3 (13), 129.3 (2), 133.5 (4), 133.9 (14), 134.5 (20), 134.8 (12), 139.2 (19), 150.8 (17), 162.7 (16), 166.9 (10), 167.5 (1). 29Si NMR (DMSO-d6) (δ) (ppm): −8.01 (22). Elemental analysis. Calculated: (C36H32N2O7Si) (632.74 g mol−1): C: 68.34%, H: 5.10%, N: 4.43%. Found: C: 68.25%, H: 5.07%, N: 4.39%.
C arom.), 1496 (CH3), 1467 (Si–C arom.), 1368 (Si–C aliph.), 1111 (O–C arom.), 915 (arom. 1,3,5-subst.), 834 (arom. p-sust.). 1H NMR (DMSO-d6) (δ) (ppm): 0.23 (s, 3H, CH3) (21), 0.53 (s, 3H, CH3) (21′), 0.83–0.88 (d, J = 17.7 MHz, 6H, CH3) (9), 1.40 (m, 1H, CH) (8), 2.00 (m, 1H, CH2) (7), 2.13 (m, 1H, CH) (7′), 4.94 (m, 1H, CH) (5), 7.27 (s, 4H, arom.) (18), 7.58 (s, 4H, arom.) (19), 7.82–7.87 (m, 4H, arom.) (3, 4), 8.42 (s, 1H, arom.) (15), 8.67 (s, 2H, arom.) (13), 10.41 (s, 1H, NH) (11). 13C NMR (DMSO-d6) (δ) (ppm): −3.4 (21), 20.2 (9), 22.38 (9′), 23.9, (8), 36.2 (7), 51.07 (5), 120.7 (18), 122.6 (3), 124.7 (15), 127.3 (13), 129.3 (2), 133.5 (4), 133.9 (14), 134.5 (20), 134.8 (12), 139.2 (19), 150.8 (17), 162.7 (16), 166.9 (10), 167.5 (1). 29Si NMR (DMSO-d6) (δ) (ppm): −8.01 (22). Elemental analysis. Calculated: (C36H32N2O7Si) (632.74 g mol−1): C: 68.34%, H: 5.10%, N: 4.43%. Found: C: 68.25%, H: 5.07%, N: 4.39%.
H-PE. X = H
IR (KBr) (cm−1): 3467 (NH), 3089 (CH arom.), 2952 (CH3), 1778, (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O imide), 1727 (C
O imide), 1727 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester), 1588 (C
O ester), 1588 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1453 (Si–C arom.), 1372 (Si–C aliph.), 1110 (O–C arom.), 877 (arom. 1,3,5-subst.), 834 (arom. p-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.6 (s, 6H, CH3) (21), 7.36 (s, 4H, arom.) (18), 7.64 (s, 4H, arom.) (19), 7.95 (m, J = 25.5, 4H, arom.) (3, 4), 8.6 (s, 2H, arom.) (13), 8.78 (s, 1H arom.) (15). 13C NMR (DMSO-d6) (δ) (ppm): −2.3 (21), 121.4 (18), 123.5 (3), 125.5 (15), 128.1 (13), 130.5 (2), 131.5 (4), 134.3 (14), 134.8 (20), 135.3 (12), 135.7 (19), 151.4 (17), 163.0 (16), 166.6 (1). 29Si NMR (DMSO-d6) (δ) (ppm): −7.79 (22). Elemental analysis. Calculated: (C30H21NO6Si) (591.58 g mol−1): C: 69.35%, H: 4.07%, N: 2.70%. Found: C: 69.30%, H: 4.11%, N: 2.28%.
C arom.), 1453 (Si–C arom.), 1372 (Si–C aliph.), 1110 (O–C arom.), 877 (arom. 1,3,5-subst.), 834 (arom. p-subst.). 1H NMR (DMSO-d6) (δ) (ppm): 0.6 (s, 6H, CH3) (21), 7.36 (s, 4H, arom.) (18), 7.64 (s, 4H, arom.) (19), 7.95 (m, J = 25.5, 4H, arom.) (3, 4), 8.6 (s, 2H, arom.) (13), 8.78 (s, 1H arom.) (15). 13C NMR (DMSO-d6) (δ) (ppm): −2.3 (21), 121.4 (18), 123.5 (3), 125.5 (15), 128.1 (13), 130.5 (2), 131.5 (4), 134.3 (14), 134.8 (20), 135.3 (12), 135.7 (19), 151.4 (17), 163.0 (16), 166.6 (1). 29Si NMR (DMSO-d6) (δ) (ppm): −7.79 (22). Elemental analysis. Calculated: (C30H21NO6Si) (591.58 g mol−1): C: 69.35%, H: 4.07%, N: 2.70%. Found: C: 69.30%, H: 4.11%, N: 2.28%.
Cl-PE-a.
IR (KBr) (cm−1): 3433 (NH), 3072 (CH arom.), 2954, 1450 (CH3), 1784, (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O imide), 1727 (C
O imide), 1727 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester), 1628 (C
O ester), 1628 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O amide), 1598 (C
O amide), 1598 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1450 (CH3), 1416 (Si–C arom.), 1371 (Si–C aliph.), 1110 (O–C arom.), 914 (arom. 1,3,5-subst.), 833 (arom. p-subst.), 680 (C–Cl). 1H NMR (DMSO-d6) (δ) (ppm): 0.41 (s, 6H, CH3) (21), 4.12 (m, 1H, CH2) (5), 6.32 (s, 4H arom.) (18), 6.85 (s, 4H, arom.) (19), 8.04 (s, 1H, arom.) (15), 8.91 (s, 2H, arom.) (13), 10.39 (s, 1H, NH) (11). 13C NMR (DMSO-d6) (δ) (ppm): −2.0 (21), 42.9 (5), 123.3 (18), 123.4 (15), 127.4 (13), 128.9 (2), 129.9 (3), 137.2 (14), 137.3 (4), 139.6 (20), 140.7 (12), 147.5 (19), 153.4 (17), 160.3 (1), 160.5 (16), 164.8 (10). 29Si NMR (DMSO-d6) (δ) (ppm): −7.80 (22). Elemental analysis. Calculated: (C32H20Cl4N2O7Si) (714.40 g mol−1): C: 53.80%, H: 2.82%, N: 3.92%. Found: C: 53.84%, H: 2.78%, N: 3.89%.
C arom.), 1450 (CH3), 1416 (Si–C arom.), 1371 (Si–C aliph.), 1110 (O–C arom.), 914 (arom. 1,3,5-subst.), 833 (arom. p-subst.), 680 (C–Cl). 1H NMR (DMSO-d6) (δ) (ppm): 0.41 (s, 6H, CH3) (21), 4.12 (m, 1H, CH2) (5), 6.32 (s, 4H arom.) (18), 6.85 (s, 4H, arom.) (19), 8.04 (s, 1H, arom.) (15), 8.91 (s, 2H, arom.) (13), 10.39 (s, 1H, NH) (11). 13C NMR (DMSO-d6) (δ) (ppm): −2.0 (21), 42.9 (5), 123.3 (18), 123.4 (15), 127.4 (13), 128.9 (2), 129.9 (3), 137.2 (14), 137.3 (4), 139.6 (20), 140.7 (12), 147.5 (19), 153.4 (17), 160.3 (1), 160.5 (16), 164.8 (10). 29Si NMR (DMSO-d6) (δ) (ppm): −7.80 (22). Elemental analysis. Calculated: (C32H20Cl4N2O7Si) (714.40 g mol−1): C: 53.80%, H: 2.82%, N: 3.92%. Found: C: 53.84%, H: 2.78%, N: 3.89%.
Cl-PE-b.
IR (KBr) (cm−1): 3432 (NH), 3071 (CH arom.), 2953, 1391 (CH3), 1782, (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O imide), 1727 (C
O imide), 1727 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester), 1627 (C
O ester), 1627 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O amide), 1588, 1513 (C
O amide), 1588, 1513 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1451 (Si–C arom.), 1368 (Si–C aliph.), 1391 (CH3), 1110 (O–C arom.), 914 (arom. 1,3,5-subst.), 825 (arom. p-subst.), 680 (C–Cl). 1H NMR (DMSO-d6) (δ) (ppm): 0.58 (s, 6H, CH3) (21), 1.59 (d, 3H, CH3) (6), 5.04 (m, 1H, CH) (5), 7.32 (s, 4H, arom.) (18), 7.62 (s, 4H, arom.) (19), 8.45 (s, 1H, arom.) (15), 8.69 (s, 2H, arom.) (13), 10.42 (s, 1H, NH) (11). 13C NMR (DMSO-d6) (δ) (ppm): −2.7 (21), 14.4 (6), 49.1 (5), 121.3 (18), 125.4 (15), 128.0 (13), 128.1 (2), 130.1 (3), 135.2 (14), 137.6 (4), 138.4 (20), 139.8 (12), 145.3 (19), 151.4 (17), 162.6 (1), 163.4 (16), 167.6 (10). 29Si NMR (DMSO-d6) (δ) (ppm): −7.82 (22). Elemental analysis. Calculated: (C33H22Cl4N2O7Si) (728.43 g mol−1): C: 54.41%, H: 3.04%, N: 3.85%. Found: C: 54.39%, H: 3.17%, N: 3.79.
C arom.), 1451 (Si–C arom.), 1368 (Si–C aliph.), 1391 (CH3), 1110 (O–C arom.), 914 (arom. 1,3,5-subst.), 825 (arom. p-subst.), 680 (C–Cl). 1H NMR (DMSO-d6) (δ) (ppm): 0.58 (s, 6H, CH3) (21), 1.59 (d, 3H, CH3) (6), 5.04 (m, 1H, CH) (5), 7.32 (s, 4H, arom.) (18), 7.62 (s, 4H, arom.) (19), 8.45 (s, 1H, arom.) (15), 8.69 (s, 2H, arom.) (13), 10.42 (s, 1H, NH) (11). 13C NMR (DMSO-d6) (δ) (ppm): −2.7 (21), 14.4 (6), 49.1 (5), 121.3 (18), 125.4 (15), 128.0 (13), 128.1 (2), 130.1 (3), 135.2 (14), 137.6 (4), 138.4 (20), 139.8 (12), 145.3 (19), 151.4 (17), 162.6 (1), 163.4 (16), 167.6 (10). 29Si NMR (DMSO-d6) (δ) (ppm): −7.82 (22). Elemental analysis. Calculated: (C33H22Cl4N2O7Si) (728.43 g mol−1): C: 54.41%, H: 3.04%, N: 3.85%. Found: C: 54.39%, H: 3.17%, N: 3.79.
Cl-PE-c.
IR (KBr) (cm−1): 3432 (NH), 3071 (CH arom.), 2954, 2852, 1452 (CH3, CH2), 1777 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O amide) 1725 (C
O amide) 1725 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester), 1626 (C
O ester), 1626 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O amide), 1587, 1570 (C
O amide), 1587, 1570 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1452 (CH3), 1419 (Si–C arom.), 1371 (Si–C aliph.), 1111 (O–C arom.), 910 (arom. 1,3,5-subst.), 834 (arom.-p-subst.), 680 (C–Cl). 1H NMR (DMSO-d6) (δ) (ppm): 0.60 (s, 6H, CH3) (21), 0.92 (d, 6H, CH3) (9), 1.57 (m, 1H, CH) (8), 2.28 (m, 2H, CH2) (7), 5.00 (m, 1H, CH) (5), 7.33 (s, 4H, arom.) (18), 7.64 (d, 4H, arom.) (19), 8.46 (s, 1H, arom.) (15), 8.70 (s, 2H, arom.) (13), 10.52 (s, 1H, NH) (11). 13C NMR (DMSO-d6) (δ) (ppm): −2.7 (21), 20.7 (9), 20.9 (9′), 23.1, (8), 36.73 (7), 52.4 (5), 121.4 (18), 123.8 (15), 125.4 (13), 128.0 (2), 128.3 (3), 135.3 (14), 137.5 (4), 138.5 (20), 145.5 (12), 149.5 (19), 151.4 (17), 163.0 (1), 163.4 (16), 167.5 (10). 29Si NMR (DMSO-d6) (δ) (ppm): −7.80 (22). Elemental analysis. Calculated: (C36H28Cl4N2O7Si) (770.51 g mol−1): C: 56.12%, H: 3.66%, N: 3.64%. Found: C: 56.23%, H: 3.54%, N: 3.71%.
C arom.), 1452 (CH3), 1419 (Si–C arom.), 1371 (Si–C aliph.), 1111 (O–C arom.), 910 (arom. 1,3,5-subst.), 834 (arom.-p-subst.), 680 (C–Cl). 1H NMR (DMSO-d6) (δ) (ppm): 0.60 (s, 6H, CH3) (21), 0.92 (d, 6H, CH3) (9), 1.57 (m, 1H, CH) (8), 2.28 (m, 2H, CH2) (7), 5.00 (m, 1H, CH) (5), 7.33 (s, 4H, arom.) (18), 7.64 (d, 4H, arom.) (19), 8.46 (s, 1H, arom.) (15), 8.70 (s, 2H, arom.) (13), 10.52 (s, 1H, NH) (11). 13C NMR (DMSO-d6) (δ) (ppm): −2.7 (21), 20.7 (9), 20.9 (9′), 23.1, (8), 36.73 (7), 52.4 (5), 121.4 (18), 123.8 (15), 125.4 (13), 128.0 (2), 128.3 (3), 135.3 (14), 137.5 (4), 138.5 (20), 145.5 (12), 149.5 (19), 151.4 (17), 163.0 (1), 163.4 (16), 167.5 (10). 29Si NMR (DMSO-d6) (δ) (ppm): −7.80 (22). Elemental analysis. Calculated: (C36H28Cl4N2O7Si) (770.51 g mol−1): C: 56.12%, H: 3.66%, N: 3.64%. Found: C: 56.23%, H: 3.54%, N: 3.71%.
Cl-PE. X = Cl
IR (KBr) (cm−1): 3434 (NH), 3071 (CH arom.), 1786, (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O imide), 1735 (C
O imide), 1735 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester), 1588, 1587 (C
O ester), 1588, 1587 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1451 (Si–C arom.), 1369 (Si–C aliph.), 1108 (O–C arom.), 918 (arom. 1,3,5-subst.), 824 (arom. p-sust.). 1H NMR (DMSO-d6) (δ) (ppm): 0.60 (s, 3H, CH3) (21), 7.35 (s, 4H, arom.) (18), 7.64 (s, 4H arom.) (19), 8.55 (s, 1H, arom.) (15), 8.83 (s, 2H, arom.) (13), 13C NMR (DMSO-d6) (δ) (ppm): −2.7 (21), 121.3 (18), 125.4 (15), 128.0 (13), 128.2 (2), 128.4 (3), 130.7 (14), 132.5 (4), 135.3 (20), 135.7 (12), 138.6 (19), 151.3 (17), 162.2 (1), 162.8 (16). 29Si NMR (DMSO-d6) (δ) (ppm): −7.8 (22). Elemental analysis. Calculated: (C30H17Cl4NO6Si) (657.35 g mol−1): C: 54.82%, H: 2.61%, N: 2.13%. Found: C: 54.79%, H: 2.57%, N: 2.22%.
C arom.), 1451 (Si–C arom.), 1369 (Si–C aliph.), 1108 (O–C arom.), 918 (arom. 1,3,5-subst.), 824 (arom. p-sust.). 1H NMR (DMSO-d6) (δ) (ppm): 0.60 (s, 3H, CH3) (21), 7.35 (s, 4H, arom.) (18), 7.64 (s, 4H arom.) (19), 8.55 (s, 1H, arom.) (15), 8.83 (s, 2H, arom.) (13), 13C NMR (DMSO-d6) (δ) (ppm): −2.7 (21), 121.3 (18), 125.4 (15), 128.0 (13), 128.2 (2), 128.4 (3), 130.7 (14), 132.5 (4), 135.3 (20), 135.7 (12), 138.6 (19), 151.3 (17), 162.2 (1), 162.8 (16). 29Si NMR (DMSO-d6) (δ) (ppm): −7.8 (22). Elemental analysis. Calculated: (C30H17Cl4NO6Si) (657.35 g mol−1): C: 54.82%, H: 2.61%, N: 2.13%. Found: C: 54.79%, H: 2.57%, N: 2.22%.
Br-PE-a.
IR (KBr) (cm−1): 3435 (NH), 3070 (CH arom.), 2951, 1450 (CH3), 1776, (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O imide), 1728 (C
O imide), 1728 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester), 1626 (C
O ester), 1626 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O amide), 1597 (C
O amide), 1597 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1450 (CH3), 1414 (Si–C arom.), 1371 (Si–C aliph.), 1108 (O–C arom.), 914 (arom. 1,3,5-subst.), 832 (arom. p-sust.), 669 (C–Br). Elemental analysis. Calculated: (C32H20Br4N2O7Si) (892.22 g mol−1): C: 43.08%, H: 2.26%, N: 3.14%. Found: C: 43.19%, H: 2.21%, N: 3.03%.
C arom.), 1450 (CH3), 1414 (Si–C arom.), 1371 (Si–C aliph.), 1108 (O–C arom.), 914 (arom. 1,3,5-subst.), 832 (arom. p-sust.), 669 (C–Br). Elemental analysis. Calculated: (C32H20Br4N2O7Si) (892.22 g mol−1): C: 43.08%, H: 2.26%, N: 3.14%. Found: C: 43.19%, H: 2.21%, N: 3.03%.
Br-PE-b.
IR (KBr) (cm−1): 3432 (NH), 3072 (CH arom.), 2951 (CH3), 1777, (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O imide), 1724 (C
O imide), 1724 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester), 1627 (C
O ester), 1627 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O amide), 1587, 1560 (C
O amide), 1587, 1560 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1451 (CH3), 1408 (Si–C arom.), 1370 (Si–C aliph.), 1108 (O–C arom.), 822 (arom. p-sust.), 918 (arom. 1,3,5-sust.), 669 (C–Br). 1H NMR (DMSO-d6) (δ) (ppm): 0.58 (s, 6H, CH3) (21), 1.55 (d, 3H, CH3) (6), 5.04 (m, 1H, arom.) (5), 7.32 (s, 4H, arom.) (18), 7.62 (s, 4H, arom.) (19), 8.45 (s, 1H, arom.) (15), 8.68 (s, 2H, arom.) (13), 10.36 (s, 1H, NH) (11). 13C NMR (DMSO-d6) (δ) (ppm): −2.11 (21), 14.95 (6), 49.71 (5), 121.1 (18), 122.0 (3), 126.0 (15), 128.5 (13), 130.6 (14), 131.5 (4), 135.8 (20), 136.1 (12), 137.1 (19), 140.4 (2), 151.9 (17), 163.5 (1), 163.9 (16), 168.3 (10). 29Si NMR (DMSO-d6) (δ) (ppm): −7.80 (22). Elemental analysis. Calculated: (C33H22Br4N2O7Si) (906.25 g mol−1): C: 43.74%, H: 2.45%, N: 3.09%. Found: C: 43.78%, H: 2.39%, N: 3.10%.
C arom.), 1451 (CH3), 1408 (Si–C arom.), 1370 (Si–C aliph.), 1108 (O–C arom.), 822 (arom. p-sust.), 918 (arom. 1,3,5-sust.), 669 (C–Br). 1H NMR (DMSO-d6) (δ) (ppm): 0.58 (s, 6H, CH3) (21), 1.55 (d, 3H, CH3) (6), 5.04 (m, 1H, arom.) (5), 7.32 (s, 4H, arom.) (18), 7.62 (s, 4H, arom.) (19), 8.45 (s, 1H, arom.) (15), 8.68 (s, 2H, arom.) (13), 10.36 (s, 1H, NH) (11). 13C NMR (DMSO-d6) (δ) (ppm): −2.11 (21), 14.95 (6), 49.71 (5), 121.1 (18), 122.0 (3), 126.0 (15), 128.5 (13), 130.6 (14), 131.5 (4), 135.8 (20), 136.1 (12), 137.1 (19), 140.4 (2), 151.9 (17), 163.5 (1), 163.9 (16), 168.3 (10). 29Si NMR (DMSO-d6) (δ) (ppm): −7.80 (22). Elemental analysis. Calculated: (C33H22Br4N2O7Si) (906.25 g mol−1): C: 43.74%, H: 2.45%, N: 3.09%. Found: C: 43.78%, H: 2.39%, N: 3.10%.
Br-PE-c.
IR (KBr) (cm−1): 3433 (NH), 3066 (CH arom.), 2956, 2870, 1450 (CH3, CH2), 1778 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, imide), 1723 (O–C arom.), 1627 (C
O, imide), 1723 (O–C arom.), 1627 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O amide), 1587, 1544 (C
O amide), 1587, 1544 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1450 (CH3), 1424 (Si–C arom.), 1368 (Si–C aliph.), 1109 (C–O ester), 916 (arom. 1,3,5-subst.), 823 (arom. p-sust.), 681 (C–Br). 1H NMR (DMSO-d6) (δ) (ppm): 0.59 (s, 6H, CH3) (21), 0.90–0.91 (m, 6H, CH3) (9, 9′), 1.56 (m, 1H, CH) (8), 2.06 (m, 1H, CH2) (7), 2.28 (m, 1H, CH2) (7′), 4.99 (m, 1H, CH) (5), 7.33 (s, 4H, arom.) (18), 7.63 (d, 4H, arom.) (19), 8.46 (s, 1H, arom.) (15), 8.69 (s, 2H, arom.) (13), 10.42 (s, 1H, NH) (11). 13C NMR (DMSO-d6) (δ) (ppm): −2.8 (21), 23.2 (9), 25.0 (9′), 26.5, (8), 39.0 (7), 54.8 (5), 122.8 (18), 123.4 (3), 127.5 (15), 128.4 (13), 129.9 (14), 132.3 (4), 132.9 (20), 137.3 (12), 137.6 (19), 138.8 (2), 153.6 (17) (C arom.), 165.3 (1), 165.5 (16), 169.6 (10). 29Si NMR (DMSO-d6) (δ) (ppm): −7.10 (22). Elemental analysis. Calculated: (C36H28Br4N2O7Si) (948.33 g mol−1): C: 45.60%, H: 2.98%, N: 2.95%. Found: C: 45.63%, H: 2.92%, N: 2.89%.
C arom.), 1450 (CH3), 1424 (Si–C arom.), 1368 (Si–C aliph.), 1109 (C–O ester), 916 (arom. 1,3,5-subst.), 823 (arom. p-sust.), 681 (C–Br). 1H NMR (DMSO-d6) (δ) (ppm): 0.59 (s, 6H, CH3) (21), 0.90–0.91 (m, 6H, CH3) (9, 9′), 1.56 (m, 1H, CH) (8), 2.06 (m, 1H, CH2) (7), 2.28 (m, 1H, CH2) (7′), 4.99 (m, 1H, CH) (5), 7.33 (s, 4H, arom.) (18), 7.63 (d, 4H, arom.) (19), 8.46 (s, 1H, arom.) (15), 8.69 (s, 2H, arom.) (13), 10.42 (s, 1H, NH) (11). 13C NMR (DMSO-d6) (δ) (ppm): −2.8 (21), 23.2 (9), 25.0 (9′), 26.5, (8), 39.0 (7), 54.8 (5), 122.8 (18), 123.4 (3), 127.5 (15), 128.4 (13), 129.9 (14), 132.3 (4), 132.9 (20), 137.3 (12), 137.6 (19), 138.8 (2), 153.6 (17) (C arom.), 165.3 (1), 165.5 (16), 169.6 (10). 29Si NMR (DMSO-d6) (δ) (ppm): −7.10 (22). Elemental analysis. Calculated: (C36H28Br4N2O7Si) (948.33 g mol−1): C: 45.60%, H: 2.98%, N: 2.95%. Found: C: 45.63%, H: 2.92%, N: 2.89%.
Br-PE. R: 67%. IR (KBr) (cm−1): 3433 (NH), 3084 (H arom.), 2952, 1495 (CH3), 1770, (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O imide), 1730 (C
O imide), 1730 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester), 1587 (C
O ester), 1587 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C arom.), 1495 (CH3), 1452 (Si–C arom.), 1370 (Si–C aliph.), 1107 (O–C arom.), 917 (arom. 1,3,5-subst.), 821 (arom. p-sust.), 673 (C–Br). 1H NMR (DMSO-d6) (δ) (ppm): 0.60 (s, 6H, CH3) (21), 7.36 (s, 4H, arom.) (18), 7.65 (s, 4H, arom.) (19), 8.56 (s, 2H, arom.) (13), 8, 82 (s, 1H, arom.) (15), 13C NMR (DMSO-d6) (δ) (ppm): −2.8 (CH3) (21), 122.9 (18), 123.3 (3), 130.0 (15), 132.4 (13), 132.9 (14), 133.1 (4), 135.4 (20), 137.4 (12), 137.8 (19), 138.8 (2), 153.5 (17), 162.3, 162.7 (C
C arom.), 1495 (CH3), 1452 (Si–C arom.), 1370 (Si–C aliph.), 1107 (O–C arom.), 917 (arom. 1,3,5-subst.), 821 (arom. p-sust.), 673 (C–Br). 1H NMR (DMSO-d6) (δ) (ppm): 0.60 (s, 6H, CH3) (21), 7.36 (s, 4H, arom.) (18), 7.65 (s, 4H, arom.) (19), 8.56 (s, 2H, arom.) (13), 8, 82 (s, 1H, arom.) (15), 13C NMR (DMSO-d6) (δ) (ppm): −2.8 (CH3) (21), 122.9 (18), 123.3 (3), 130.0 (15), 132.4 (13), 132.9 (14), 133.1 (4), 135.4 (20), 137.4 (12), 137.8 (19), 138.8 (2), 153.5 (17), 162.3, 162.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 29Si NMR (δ) (ppm) (DMSO-d6): −7.83. Elemental analysis. Calculated: (C30H17Br4NO6Si) (835.17 g mol−1): C: 43.14%, H: 2.05%, N: 1.68%. Found: C: 43.16%, H: 2.00%, N: 1.53%.
O). 29Si NMR (δ) (ppm) (DMSO-d6): −7.83. Elemental analysis. Calculated: (C30H17Br4NO6Si) (835.17 g mol−1): C: 43.14%, H: 2.05%, N: 1.68%. Found: C: 43.16%, H: 2.00%, N: 1.53%.
3. Results and discussion
3.1. Monomer synthesis and characterization
The monoacids X-I-(a-c) were obtained from the respective phthalic anhydrides and the amino acids glycine, L-alanine and L-valine according to described procedures.28–32 (Scheme 1) The diacids X-II-(a-c) were synthesized by the reaction of acid chlorides of these monoacids with 5-amino-isophthalic acid according to previous works.28,31,33–35 (Scheme 2) The dicarboxylic imide-acids X-III were prepared by reaction of the respective phthalic anhydrides and 5-amino-isophthalic acid. All of the monomeric diacids were characterized and the results were in agreement with the proposed structures.3.2. Polymer synthesis and characterization
PEs were obtained according to the procedure described by Higashi37 showed in the Scheme 3 in which a Tos-Cl and DMF-pyridine mixture was used as polymerizing system. This method of direct polycondensation consists in the reaction of the diacid with a Vilsmeier adduct, which is prepared with Tos-Cl, pyridine in DMF. This adduct was aged previous to its use acting as activator agent of the diacids groups of the monomer and allowing this way the reaction with the silylated diphenol.PEs were obtained with almost quantitative yields (94–98%) and the particular values for each run are summarized in Table 1. Apparently, the presence of the halogen atoms in the phthalic ring moiety does not affect the final reactivity of the dicarboxylic aids.
| Yield (%) | [α]Da (°) | ηinhb (dL g−1) | Tg (°C) | TDT (°C) | Rcc (%) | LOId | |
|---|---|---|---|---|---|---|---|
| a In N,N-dimethylformamide at 25 °C.b Inherent viscosity, in NMP at 25 °C (c = 0.5 g dL−1).c Residue at 800 °C.d Limiting oxygen index.e nd: not determined. | |||||||
| H-PE-a | 94 | — | 0.27 | 219 | 402 | 44 | 35.1 |
| H-PE-b | 95 | −43 | 1.03 | 160 | 375 | 33 | 30.7 |
| H-PE-c | 96 | −31 | 0.23 | 70 | 380 | 27 | 28.3 |
| Cl-PE-a | 98 | — | 0.48 | 154 | 338 | 40 | 33.5 |
| Cl-PE-b | 98 | −51 | 0.76 | 96 | 353 | 30 | 29.5 |
| Cl-PE-c | 95 | −28 | 0.35 | 146 | 288 | 32 | 30.3 |
| Br-PE-a | 97 | — | 0.75 | 114 | 234 | 28 | 28.7 |
| Br-PE-b | 98 | −39 | 0.39 | 96 | 329 | 27 | 28.3 |
| Br-PE-c | 97 | −25 | 0.45 | nd | 341 | 22 | 26.3 |
| H-PE | 94 | — | 0.35 | 110 | 403 | 24 | 27.1 |
| Cl-PE | 97 | — | 0.27 | nd | 372 | 39 | 33.1 |
| Br-PE | 95 | — | 0.45 | 105 | 367 | 32 | 30.3 |
The structure of the repetitive unit of the polymers was characterized by means of elemental analysis and spectroscopic techniques. Thus, the C, N and H percentage contents in all samples are in agreement with the expected values.
The IR spectra showed the disappearance of the O–H stretching band associated to a carboxylic acid group and the appearance of a new band between 1740–1724 cm−1 corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the ester group. Other specific absorption bands for the polymers are listed in the Experimental Part.
O stretching of the ester group. Other specific absorption bands for the polymers are listed in the Experimental Part.
When it corresponds, the 1H NMR spectra showed the aliphatic signals of the aminoacidic residue with the expected and integration values. All PEs showed a singlet at about 0.5 ppm provided by the two CH3 groups magnetically equivalent bonded to the Si atom of the diphenol moiety. The silicon atom shifts the signal at high field due to its lower electronegativity in relation to the carbon atom. For the same polymers, the aromatic zone was very complex due to the overlapping of signals. Thus, the AB systems proposed for H18 and H19 appear like a broad singlet, however, the integration values were in agreement with the hydrogen number atoms.
The signals in 13C NMR spectra of polymers were assigned based on the spectra of monomers. The aromatic region presents a great number of C aromatic and the CH3 groups bonded to the Si atom appear between −2.1 and −3.4 ppm due to the electronic effect of the heteroatom above discussed. The spectra clearly show at low magnetic field the carbonyl groups provided by the amide, imide and ester functions that ratified the structure of the polymeric samples.
For all PEs the 29Si spectra were obtained, which showed only one signal at about −7 and −8 ppm approximately. This shift has been described in compounds in which the silicon atom is part of a –Ph–Si(CH3)2–Ph-unit.38 Fig. 1 shows as an example the 1H, 13C and 29Si NMR spectra of PE-Cl-b.
When the formation of films was attempted, the products were brittle materials and inadequate for mechanical tests.
All the PEs including L-alanine and L-valine showed optical rotation (levorotatory) and the results are summarized in Table 1.
3.3. Inherent viscosity and solubility
Table 2 shows the results of the solubility tests for the PEs. Almost all the PEs were soluble in aprotic polar organic solvents at room temperature, such as DMAc, DMF, DMSO and NMP, and the rest were partially soluble at 40 °C and/or showed swollen. In other solvents as m-cresol or THF, only PEs without an aminoacidic residue was soluble at 40 °C, and the others only showed swollen. Apparently, the aminoacidic moiety diminishes the polarity of the chain increasing its solubility. All PEs were insoluble in other solvents as CHCl3 or alcohols.| Polymer | DMSO | NMP | DMF | DMAc | m-Cresol | THF |
|---|---|---|---|---|---|---|
| a +: soluble at room temperature. ±: soluble at 40 °C. H: swollen. ±H: only a part of the sample was soluble at 40 °C, the rest was swollen. −: insoluble. | ||||||
| H-PE-a | + | + | + | + | H | H |
| Cl-PE-a | + | + | + | ±H | H | H |
| Br-PE-a | H | + | H | + | H | H |
| H-PE-b | ±H | + | ±H | ± | H | H |
| Cl-PE-b | ±H | + | ±H | ±H | H | H |
| Br-PE-b | ± | + | + | + | H | H |
| H-PE-c | + | + | + | + | H | H |
| Cl-PE-c | + | + | + | + | H | H |
| Br-PE-c | + | + | + | + | H | H |
| H-PE | + | + | + | + | + | + |
| Cl-PE | + | + | + | + | ± | ± |
| Br-PE | + | + | + | + | − | + |
Table 1 also shows the inherent viscosity values (ηinh), obtained in NMP at 25 °C and 0.5 g dL−1 as concentration. The registered values were moderate to high, obtaining the highest value for H-PE-b of 1.03 dL g−1. In general, the highest ηinh values were obtained for PEs for which the monomeric diacids were soluble in pyridine at room temperature. The higher solubility would be transferred to the polymer in the reaction media, which permits the growing of the chains. The monomer solubility can be conditioned to the volume of the halogen atoms, in the sense that when it volumes increased, the carboxylic diacids have higher separation which would permit good solvation. This effect can be observed for PEs including glycine (a) or L-valine (c), but not for those with L-alanine (b) in which the ηinh values decreases when the size of the halogen increases. In this aspect, the effect of the amino acid was not clear.
3.4. Thermal properties
Table 1 also shows the Tg values obtained for the PEs. If the influence of the atoms bonded to the imide ring (H, Cl, Br) is analyzed, it is possible to see that when the size of this atom is increased, the Tg values decrease, due probably to higher separation between the polymeric chains which confers them more mobility. The exception to this rule was the PEs series with L-valine as aminoacidic residue, which showed a high value for Cl-PE-c and a low value for H-PE-c. For the series without aminoacidic residue, also it was possible to see a slight decrease of the Tg when the atom of the imidic ring increased in size, with the exception of Cl-PE whose Tg was not detected. Fig. 2 shows the Tg values of PEs which include glycine in the structure.On the other hand, if the nature of the amino acid is considered, also it is possible to see a tendency. When the volume of the side group is increased, the Tg values decreases, due probably to a less symmetry and higher chain flexibility. This fact would imply less packing forces inter-chains which permit the segmental motions and consequently lower Tg values. The exception was Cl-PE-c, including L-valine as aminoacidic residue.
In general the same behavior was observed for poly(amides) derived from the same dicarboxylic acid with an aromatic diamine containing Si as central atom.28,34
The thermal degradation temperatures (TDT) of the PEs were determined, for which was considered the temperature at which the polymer lost 10% of weight. In general, a polymer is considered as thermostable when the weight loss is less than 10% at 400 °C.
According to the above definition, only two PEs can be considered as thermostable, H-PE-a and H-PE. The other PEs showed lower values, but can be considered with good thermal stability, due principally to the great aromatic content of the repeating unit and the micropolarity contributed by the Si–C bond. If the influence of the amino acid is considered, there is not a clear tendency of the TDT values. However, when the influence of atoms bonded to the imidic ring is analyzed, it is possible to see that when the size of these atoms is increased (H, Cl, Br), the TDT values decreased. Thus, the polymeric series with Br shows the lowest TDT values with Cl-PE-c as the only exception to this rule which showed a very low TDT value.
It is possible that when the size of the halogen atom is increased from H to Br, the distance between the polymeric chains also increased due to the higher volume of the imidic side group. According to this, the interactions between the chains are lower and the degradation process occurs at lower temperatures. The same situation was possible to see in the PEs without the aminoacidic moiety. In general, the exception was the same to that observed for the Tg values. Fig. 3 shows the results obtained for the degradation process for PEs which include L-alanine in the structure. Also there are included the respective derivative curves where is possible to evidence the main degradation process of the chains at lower temperature for the halogenated samples PE-H-b.
 | ||
| Fig. 3 Thermal degradation process for poly(esters) PE-H-b, PE-Cl-b and Br-PE-b (20 °C min−1 under N2 flow). | ||
Table 1 also shows the residue (%) of the thermogravimetric analysis at 800 °C, which probably corresponds principally to silicon oxide, but without to discard other inorganic compounds due to the analysis was in N2 atmosphere.
3.5. Optical properties
The optical transparency of the PEs was determined from the transmittance, which depends on the wavelength of the incident light. A sample is considered as transparent when the transmittance at 400 nm is higher than 80%. In the case of organic polymers with a high aromatic content like these PEs, there are interactions between the π electrons of the aromatic rings which absorb energy in the UV-vis region, and as a consequence the materials can be colorless with lower transparency. In this work the transparence was measured in solution of NMP at 15 °C with a concentration of 0.5 g dL−1.For these PEs, the transparence at 400 nm was lower than 20%, but between 510 and 640 nm all polymers showed 80% of transmittance. On the other hand, the cut-off wavelength of the samples is very similar and the obtained values are between 325 and 388 nm with the exception of Br-PE-b which presented a lower value. These results are summarized in Table 3, and the transparence curves are shown in the Fig. 4.
| PEs | T400 (%) | λT = 80% (nm) | λcutoff (nm) |
|---|---|---|---|
| H-PE-a | 15.7 | 574 | 336 |
| H-PE-b | 20.8 | 564 | 325 |
| H-PE-c | 3.7 | 555 | 359 |
| H-PE | 2.7 | 561 | 362 |
| Cl-PE-a | 3.7 | 556 | 330 |
| Cl-PE-b | 1.9 | 561 | 331 |
| Cl-PE-c | 0.2 | 634 | 388 |
| Cl-PE | 0.6 | 577 | 335 |
| Br-PE-a | 0.6 | 577 | 342 |
| Br-PE-b | 16.8 | 517 | 285 |
| Br-PE-c | 1.7 | 580 | 338 |
| Br-PE | 8.2 | 506 | 335 |
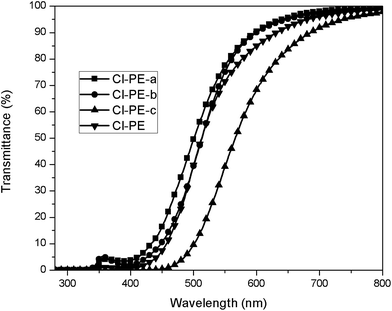 | ||
| Fig. 4 UV-vis transparency curves for poly(esters) Cl-PE-a, Cl-PE-b, Cl-PE-c, Cl-PE (NMP solution, 0.5 g dL−1 and 15 °C). | ||
3.6. Flame retardancy
Halogen-containing materials are known as flame retardance, due to that the free radicals responsible of the flame propagation are inhibited by the halogen atoms, which act in the following order: I > Br > Cl > F.39 In Table 1 it is possible to see the limiting oxygen index (LOI) calculated according to the Van Krevelen and Hoftyzer equation, in which R is the residue chart at 800 °C.40| LOI = 17.5 + 0.4R |
Also in this work a very simple essay was made, consisting in to put the PE for 10 seconds in the flame. For all the PEs containing Cl or Br when the flame was eliminated, the combustion was stopped immediately. For those PEs without halogen atoms in the repeating unit, the combustion continued until the complete disappearance of the material.
4. Conclusions
New poly(esters) containing an amide and/or imide group in the side chain and halogen atoms (Cl or Br) bonded to the imide ring, were synthesized from the respective dicarboxylic acids and bis(4-hydroxyphenyl)dimethylsilane. Polymers were characterized by elemental analysis, IR and 1H, 13C and 29Si NMR spectroscopy and the results were in agreement with the proposed structures.PEs were obtained with good results yields and moderated ηinh values. The solubility was good in polar aprotic solvents and some of them in THF. Almost of them showed swollen in THF and m-cresol. These results were analyzed respect to the presence of the halogen atom and when it corresponded, to the nature of the aminoacidic moiety. In all cases, the different interactions between the chains and therefore their solubility, was related to the size of the halogen atom and side group of the aminoacidic moiety.
The Tg and TDT values showed a tendency in the sense that when the size of the halogen atom is increased, the values decrease, due to the higher distance between the polymeric chains, which increases their flexibility and diminishes the interactions between them.
PEs showed UV-vis transparence between 500 and 600 nm approximately and those containing halogen atoms autoextinction when were put at the flame.
Acknowledgements
Authors acknowledge “Fondo Nacional de Investigación Científica y Tecnológica”, FONDECYT, through Project 1100015. D.C. acknowledges CONICYT for a Ph.D, fellowship.References
- P. Zhang, W. Linbo and L. Bo-Geng, Thermal stability of aromatic polyesters prepared from diphenolic acid and its esters, Polym. Degrad. Stab., 2009, 94, 1261 CrossRef CAS.
- M. Arroyo, in Handbook of thermoplastics, ed. O. Olabisi, New York, Marcel Dekker, Inc., 1997, p. 599 Search PubMed.
- L. M. Maresca and L. M. Robeson, in Engineering thermoplastics: properties and applications, ed. J. M. Margolis, New York, Marcel Dekker Inc., 1985, p. 255 Search PubMed.
- P. W. Morgan, Aromatic Polyesters with Large Cross-Planar Substituents, Macromolecules, 1970, 3, 536 CrossRef CAS.
- S. Mallakpour and Z. Rafiee, Synthesis and Characterization of Novel Organosoluble, Thermal Stable and Optically Active Polyesters Derived from 5-(2-Phthalimidiylpropanoylamino)isophthalic Acid, Polym. J., 2007, 39, 1185 CrossRef CAS.
- G. S. Liou, S. H. Hsiao, H. M. Huang, C. W. Chang and H. J. Yen, Synthesis and photophysical properties of novelorgano-soluble polyarylates bearing triphenylamine moieties, J. Polym. Res., 2007, 14, 191 CrossRef CAS.
- S. H. Hsiao and H. W. Chiang, Synthesis and Structure-Property Study of Polyarylates Derived from Bisphenols with Different Connector Groups, J. Polym. Res., 2005, 12, 211 CrossRef CAS.
- G. S. Liou, S. H. Hsiao, H. M. Huang and C. W. Chang, Synthesis and Photoluminescence of Novel Organo-Soluble Polyarylates Bearing (N-Carbazolyl)triphenylamine Moieties, Polym. J., 2007, 39, 448 CrossRef CAS.
- G. S. Liou and Y. T. Chern, Synthesis and Properties of New Polyarylates from 1,4-Bis(4-carboxyphenoxy)naphthyl or 2,6-Bis(4-carboxyphenoxy)naphthyl and Various Bisphenols, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 645 CrossRef CAS.
- D. J. Liaw, B. Y. Liaw, J. J. Hsu and Y. C. Cheng, Synthesis and Characterization of New SolublePolyesters Derived from Various Cardo Bisphenols by Solution Polycondensation, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 4451 CrossRef CAS.
- M. V. Rao, A. J. Rojivadiya, P. H. Parsania and H. H. Parekh, Synthesis and Characterization of the Polyester Made from 1,1′-Bis(3-Methyl-4-Hydroxyphenyl)Cyclohexane with Isophthalic/Terephthalic Acids, J. Macromol. Sci., Part A: Pure Appl.Chem., 1990, 27, 53 Search PubMed.
- S. V. Vinogradova and Y. S. Vygodski, Cardopolymers, Russ. Chem. Rev., 1973, 42, 551 CrossRef.
- M. D. Joshi, A. Sarkar, O. S. Yemul, P. P. Wadgaonkar and S. V. Lonikar, Synthesis and characterization of silicon-containing cardo polyesters, J. Appl. Polym. Sci., 1997, 64, 1329 CrossRef CAS.
- N. P. Honkhambe, N. S. Bhairamadgi, M. V. Biyani, P. P. Wadgaonkar and M. M. Salunkhe, Synthesis and characterization of new aromatic polyesters containing cardo decahydronaphthalene groups, Eur. Polym. J., 2010, 46, 709 CrossRef.
- S. Mallakpour and E. Hashemi, Synthesis and characterization of novel optically active and photoactive aromatic polyesters containing 1,8-naphthalimidyl pendant group by step-growth polymerization, Polym. Bull., 2010, 65, 551 CrossRef CAS.
- S. F. Thames and K. G. Malone, A study of polyesters synthesized from an arylsilane and its carbon analog, J. Polym. Sci., Part A: Polym. Chem., 1993, 31, 521 CrossRef CAS.
- J. Zhang, Q. Sun and X. Hou, Gas Permeability in an Aromatic Polyester, Macromolecules, 1993, 26, 7176 CrossRef CAS.
- S. D. Pace, K. G. Malone and S. F. Thames, Arylsilane polyarylates: novel high temperature thermoplastic solvent cast coatings, J. Coat. Technol. Res., 1990, 62, 101 CAS.
- M. Bruma, I. Sava, F. Mercer, V. N. Reddy, T. Köpnick, B. Stller and B. Schulz, Silicon-containing Poly(amide-ether)s, Polym. Adv. Technol., 1998, 9, 752 CrossRef CAS.
- M. Bruma, B. Schulz, T. Köpnick and J. Robinson, Silicon-containing polyesterimides, High Perform. Polym., 2000, 12, 429 CrossRef CAS.
- C. C. Grant, Halogen Design Calculations, The SFPE Handbook of Fire Protection Engineering, 3rd edn, 2002, section 4, cap. 6 Search PubMed.
- S. Mallakpour and M. Taghavi, Direct polyamidation in green media: Studies on thermal degradation of novel organosoluble and optically active flame retardant polyamides, React. Funct. Polym., 2009, 69, 206 CrossRef CAS.
- C. M. González-Henríquez, C. A. Terraza, L. H. Tagle, A. Barriga-González, U. G. Volkmann, A. L. Cabrera, E. Ramos-Moore and M. Pavez-Moreno, Inclusion and electrical percolation effect of gold and cooper in a poly(amide) matrix that contains a thiphene moiety and Si or Ge atoms in the main chain, J. Mater. Chem., 2012, 22, 6782 RSC.
- C. M. González-Henríquez, L. H. Tagle, C. A. Terraza, A. Barriga-González, U. G. Volkmann, A. L. Cabrera, E. Ramos and M. Pavez, Structural symmetry breaking of silicon-containing poly(amide-imide) oligomers and its relation to electrical conductivity and Raman-active vibrations, Polym. Int., 2012, 61, 197 CrossRef.
- C. A. Terraza, L. H. Tagle, A. Tundidor-Camba, C. M. González-Henríquez, P. Ortiz and D. Coll, Poly(amides) obtained from 4-(4-(4-(4-aminophenoxy)phenyl) diphenylsilyl)phenoxybenzeneamine and diphenylsilarylene containing-dicarboxilic acids. Synthesis and characterization, Eur. Polym. J., 2012, 48, 649 CrossRef CAS.
- C. M. González-Henríquez, L. H. Tagle, C. A. Terraza, A. Cañete, A. Leiva, A. Barriga-González, U. G. Volkmann and A. L. Cabrera, Thiophene and silarylene-containing polyesters. Resonance effect on conductivity after polarization in an external electric field, Polym. Int., 2012, 61, 810 CrossRef.
- L. H. Tagle, C. A. Terraza, A. Tundidor-Camba and P. A. Ortiz, Silicon-containing oligomeric poly(imido-amides) with amino moieties. Synthesis, characterization and thermal studies, RSC Adv., 2014, 4, 30197 RSC.
- L. H. Tagle, C. A. Terraza, A. Leiva and D. Coll, Silicon-Containing Poly(amides) with Phthalamidyl-Amide Side Groups. Synthesis, Characterization and thermal studies, High Perform. Polym., 2012, 24, 77 CrossRef CAS.
- E. Cros, M. Plana, G. Barandy and E. Bardaji, N-tetrachlorophthaloyl (TPC) protection for solid phase peptide synthesis, Eur. J. Org. Chem., 2004, 17, 3633 CrossRef.
- N. S. Khalaf, H. A. Eyada, R. A. El-Sayed and H. M. Hassan, Synthesis of some new halogenated phthaloylamino acids containing sulfonamide and carbamate moieties with expected antimicrobial activities, J. Pharmaceut. Sci., 1994, 14, 70 CAS.
- S. Mallakpour and M. Kolahdoozan, Synthesis and Properties of Thermally Stable and Optically Active Novel Wholly Aromatic Polyesters Containing a Chiral Pendent Group, Eur. Polym. J., 2007, 43, 3344 CrossRef CAS.
- A. G. Golub, O. Y. Yakovenko, O. A. Prykhod'ko, S. S. Lukashov, V. G. Bdzhola and S. M. Yarmoluk, Evaluation of 4,5,6,7-tetrahalogeno-1H,isoindole-1,3(2H)-dienes as inhibitors of human protein kinase CK2, Biochim. Biophys. Acta, Proteins Proteomics, 2008, 1748, 143 CrossRef PubMed.
- S. Mallakpour and M. Dinari, Microwave Step-growth Polymerization of 5-(4-methyl-2-phthalimidylpentanoylamino)isophthalic Acid with Different Diisocyanates, Polym. Adv. Technol., 2008, 19, 1334 CrossRef CAS.
- (a) L. H. Tagle, C. A. Terraza, A. Tundidor-Camba and D. Coll, Silicon-containing halogenated poly(amides) derived from diacids with aminoacidic and imidic moieties in the side chain, Synthesis, characterization, optical studies and thermal properties, submitted Search PubMed; (b) D. Coll, Ph.D. dissertation, Pontificia Universidad Católica de Chile, 2014.
- J. B. Alvarado and H. W. Goecke, New iodine-containing X-ray opaque materials, Rev. R. Acad. Cienc. Exactas, Fis. Nat., 1959, 53, 737 CAS.
- W. Davidsohn, B. R. Laliberte, C. M. Goddard and C. M. Henry, Organometallic bis(p-hydroxyphenyl) derivatives of group IV elements and related compounds, J. Organomet. Chem., 1972, 36, 283 CrossRef CAS.
- F. Higashi, N. Akiyama, I. Takahashi and T. Koyama, Further Study of the Direct Polycondensation Reaction of Hydroxybenzoic Acids with Tosyl Chloride and N,N-Dimethylformamide, J. Polym. Sci., Polym. Chem. Ed., 1984, 22, 3607 CrossRef CAS.
- C. A. Terraza, L. H. Tagle, F. Concha and L. Poblete, Synthesis and characterization of new bifunctional monomers based on germarylene or silarylene units: 4,4′-(R1R2-silylene)bis(phenyl chloroformates) and 4,4′-(R1R2-germylene)bis(phenyl chloroformates), Des. Monomers Polym., 2007, 10, 253 CrossRef CAS.
- C. A. Wilkie and A. B. Morgan, Fire retardancy of polymetic materials, CRC Press, 2010 Search PubMed.
- D. W. Van Krevelen and P. J. Hoftyzer, Properties of Polymers, Elsevier, Amsterdam, 2nd edn, 1976 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |