Rational design of triazatruxene-based hole conductors for perovskite solar cells†
F. Javier Ramosa,
Kasparas Rakstysbc,
Samrana Kazima,
Michael Grätzelb,
Mohammad Khaja Nazeeruddinc and
Shahzada Ahmad*a
aAbengoa Research, Abengoa, C/ Energía Solar no 1, Campus Palmas Altas-41014, Seville, Spain. E-mail: shahzada.ahmad@abengoa.com
bLaboratory of Photonics and Interfaces, Department of Chemistry and Chemical Engineering, École Polytechnique Fédérale de Lausanne, Station 6, CH-1015, Lausanne, Switzerland
cGroup for Molecular Engineering of Functional Materials, Institute of Chemical Sciences and Engineering, École Polytechnique Fédérale de Lausanne, CH-1015-Lausanne, Switzerland
First published on 29th May 2015
Abstract
Triazatruxene core based hole transporting materials (HTMs), HMDI (5,10,15-trihexyl-3,8,13-trimethoxy-10,15-dihydro-5H-diindolo[3,2-a:3′,2′-c]carbazole) and HPDI (5,10,15-tris(4-(hexyloxy)phenyl)-10,15-dihydro-5H-diindolo[3,2-a:3′,2′-c]carbazole) were synthesized and exploited in perovskite based solar cells. The energy levels of star-shaped HMDI and HPDI were tuned by symmetrically introducing electron-rich alkoxy side groups. These soluble and easily synthesized materials exhibit optical transparency in the visible region, high thermal stability and have suitable HOMO values with respect to perovskite, making them an ideal HTM candidate for efficient perovskite solar cells. The HPDI molecule-based devices gave competitive power conversion efficiencies of ∼11% under AM 1.5G illumination. The facile synthetic approach using inexpensive precursor materials will facilitate triazatruxene-based molecules to be further exploited in thin film organic–inorganic perovskite solar cells and needs optimization to enhance power conversion efficiency.
Introduction
Hybrid organic–inorganic perovskite, a mineral that has emerged as wonder material is being used as semiconducting pigment for thin film solar cells fabrication.1,2 The recent surge of interest in perovskite-based solar cells encouraged scientist and technologist across the world to study it further3–13 and now it is clear that perovskite solar cells are more than just a lab curiosity. Perovskite is being probed as semiconducting pigment due to its excellent absorption in visible light,14,15 high charge carrier mobility (both electrons and holes) and long carrier diffusion length.14,16–18 Competitive light to energy conversion efficiencies have been obtained in labs, which are very close to established thin film solar cells and is being viewed as viable technology in photovoltaic category.19 The devices, prepared using mesoporous photoanode as an electron acceptor (hole blocking layer), perovskite as light harvester, hole transport materials (HTM) as a hole extraction layer (electron blocking layer) and gold as a contact (cathode) have gained significant interest due to the ease of fabrication, flexibility in the selection of materials and cost effective production. Moreover, reproducible results across the labs were obtained, which confirms its suitability. Currently, in most of the high performing devices Spiro-OMeTAD (2,2′,7,7′-tetrakis(N,N-di-p-methoxyphenylamine)-9,9-spirobifluorene) is being used to transport holes from the working electrode. In the recent past, sincere attempts were made to find an alternate organic HTM to rival the use of Spiro-OMeTAD. In some cases, poor matching of highest occupied molecular orbital (HOMO) level and incomplete pore filling by the perovskite hinders its successful application. The ideal HTM should have sufficient hole mobility, thermal and UV stability, and suitable HOMO energy level. A p-type polymer, poly[N-9-heptadecanyl-2,7-carbazole-alt-3,6-bis-(thiophen-5-yl)-2,5-dioctyl-2,5-dihydropyrrolo[3,4]pyrrole-1,4-dione] (PCBTDPP), was used in CH3NH3PbBr3 perovskites-based cells and gave relatively low PCE of 3%,20 while this PCE reached to 5.55% in the case of CH3NH3PbI3 and due to its better absorption.21 Other polymer based HTMs, such as P3HT [poly(3-hexylthiophene)],21,22 poly-[2,1,3-benzothiadiazole-4,7-diyl[4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b]dithiophene-2,6-diyl]] (PCPDTBT),21 poly-[[9-(1-octylnonyl)-9H-carbazole-2,7-diyl]-2,5-thiophenediyl-2,1,3-benzothiadiazole-4,7-diyl-2,5-thiophenediyl] (PCDTBT),21 and poly(triarylamine) (PTAA) were also used.19,21 Among recent reports of using different p-type polymers, only PTAA showed remarkable PV behavior. Its polymeric nature induces large chains, which will lead to defects and improper intimate contact with layers where the polymer is in between and eventually results in poor reproducibility. Additionally, polymers are known to be unstable in high vacuum conditions making it difficult for the deposition of the metallic contact on the top. Some new approaches, like p-type polymer composites with carbon nanotubes (P3HT/SWNTs)23 were able to perform well in terms of efficiency (15.3%). Contrary to the polymers, inorganic compounds like CuI24 (PCE = 6%) and small organic molecules of different nature were also reported as HTMs. Among them, promising efficiencies with Zn phthalocyanine based: TT80 (ref. 25) (6.7%), diacetylide triphenilamine based: MeODATPA26 (8.8%), carbazole based: X51 (ref. 27) (9.8%) and SGT405 (ref. 28) (14.8%), tetrathiafulvalene based: TTF-1 (ref. 29) (11.03%), pyrene based: Py-C30 (12.4%), quinolizino acridine based: Fused-F31 (12.8%), 3,4-ethylenedioxythiophene based: H101 (ref. 32) (13.8%), TIPS-pentacene (11.8%)32 and Spiro-OMeTAD derivatives33 (16.7%). These small organic molecules presented less important problems for reproducibility and stability and resulted in better contact between absorber and metal contact than their p-type polymeric counterparts.Triazatruxene-based HTM were earlier used in organic solar cells.34 Those molecules consist of a core, made of three indole units combined by one benzene ring. The presence of a benzene ring provides an extended delocalized π-system, providing excellent electron-donating potential for intramolecular charge transfer. Additionally, it permits tuning of their electronic and optical properties. However, the reported compounds were not soluble, thus limiting their deposition techniques to evaporation routes, which are not cost effective due to the requirement of huge equipment and vacuum systems. Here we report facile synthesis and characterization of two soluble, planar triazatruxene core based HTMs (5,10,15-trihexyl-3,8,13-trimethoxy-10,15-dihydro-5H-diindolo[3,2-a:3′,2′-c]carbazole) HMDI and 5,10,15-tris(4-(hexyloxy)phenyl)-10,15-dihydro-5H-diindolo[3,2-a:3′,2′-c]carbazole HPDI and their exploitation in perovskite solar cells. The energy levels of HMDI (−5.17) and HPDI (−5.41 eV) were tuned according to the perovskite energy levels by simple introduction of electron-donating methoxy- and (p-hexyloxy)phenyl-side groups on the triazatruxene core. They are easily synthesized, soluble materials, have good optical transparency in the visible region, and show high thermal stability.
Results and discussion
Fig. 1 illustrates chemical structures of HMDI and HPDI and Scheme 1 shows the synthetic routes using triazatruxene as starting molecule. The experimental details and the adopted synthesis route are explained in the experimental section. Both molecules are highly soluble in a variety of non-polar solvents and thus, enjoy the privilege to be solution processed. The high solubility and choice of solvents such as chlorobenzene or toluene, due to the presence of hexyl chains, offer their application for a variety of devices including organic solar cells, solid state dye-sensitized and perovskite solar cells. HMDI and HPDI presented solubility at room temperature >100 mg mL−1, both in chlorobenzene and toluene, thus making possible facile and cost effective solution processed deposition techniques as spin-coating, dip-coating or drop-casting.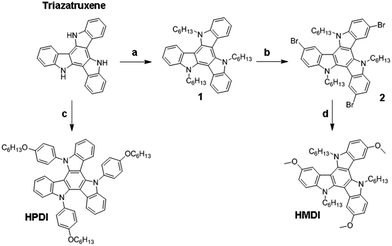 | ||
| Scheme 1 Synthetic route for the HMDI and HPDI HTMs. More details in the ESI.† | ||
The presence of alkoxy groups (methoxy for HMDI and (p-hexyloxy)phenyl in the case of HPDI) allows tuning of the HOMO level in order to adapt the molecule’s donor nature. Furthermore, alkoxy groups have been demonstrated in the literature as effective anchors for the interface perovskite/HTM.35 The UV-visible absorbance spectra of HMDI and HPDI solution in chlorobenzene are shown in Fig. 2a. Both molecules absorb in the range of 300–400 nm. The absorption spectra show broad absorption bands with a peak maximum around 317 and 325 nm, which arises due to π–π* transition.
The UV-Vis absorbance and cyclic voltammetry measurements of these HTMs (Fig. 2b) were used to study the HOMO/LUMO energy levels and band gap. In the CV graphs, both HTMs show a reversible redox peak showing good electrochemical stability. The corresponding data are given in Table 1. The calculated optical band gap (Eg) from the onset of absorption band was at 3.14 eV and 3.26 eV for HMDI and HPDI, respectively. HOMO levels of HMDI (−5.17 eV) and HPDI (−5.41 eV) are well aligned with the perovskite valence band (−5.44 eV).
| Compound | λonseta (nm) | Eg optb (eV) | HOMOc (eV) | LUMOd (eV) | Tge (°C) |
|---|---|---|---|---|---|
| a Absorption spectra were measured in DCM solution.b Optical band gap (Eg) was derived from the onset of absorption.c The EHOMO was calculated by using the equation: EHOMO = −(E1/2 vs. Fc+/Fc] + 5.064) (eV), where E1/2, formal potential was calculated by averaging anodic peak potential and cathodic peak potential, E1/2 = (Epa + Epc).d ELUMO = EHOMO + Eg opt.e Tg = glass transition temperature calculated from DSC. | |||||
| HMDI | 395 | 3.14 | −5.17 | −2.03 | 1 |
| HPDI | 380 | 3.26 | −5.41 | −2.15 | 36 |
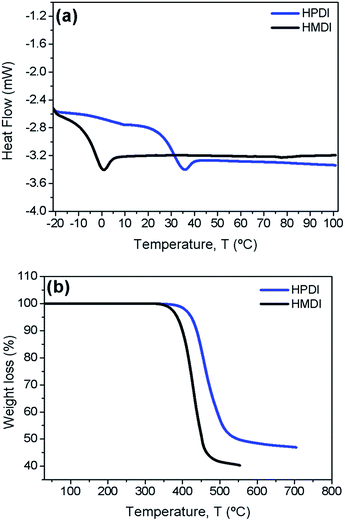
The thermal properties of novel synthesized HTMs were studied using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) measurements (Fig. 3). Both compounds show an amorphous nature, a low glass transition temperature (Tg = 1 °C for HMDI, Tg = 36 °C for HPDI) as determined by DSC (Fig. 3a). Ideally this is an essential requirement for the intimate contact between interfaces to ensure an efficient hole extraction.36 HMDI and HPDI are stable up to 350 °C and 400 °C, respectively (Fig. 3b), confirming their good thermal stability and stating practical utilization.
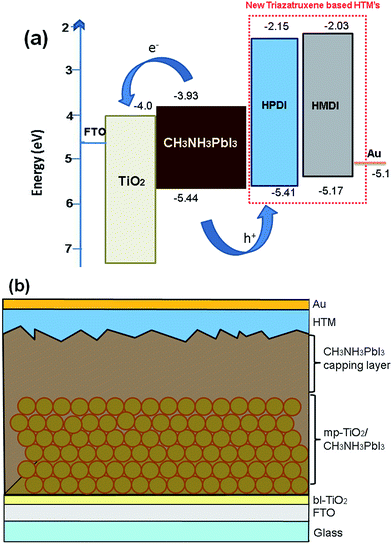
An energy level diagram of the materials is shown in Fig. 4a, while the corresponding perovskite device configuration employing triazatruxene-based HTM is presented in Fig. 4b.
To confirm the device architecture, cross sectional scanning electron microscopy (SEM) measurements were carried out (Fig. 5). Mesoporous titania can be clearly observed, with CH3NH3PbI3 absorber both infiltrated and forming large crystals on the top of the mesoporous photoanode creating a rough surface on top of the titania. This was further smoothened by the HTM layer which has a thickness of 150 nm, approximately.
 | ||
| Fig. 5 (a) and (b) Shows cross sectional scanning electron microscopy image of the photovoltaic devices using HPDI as HTM. | ||
To study the real utilization of synthesized HTM, i.e. the photovoltaic properties, i.e. current–voltage curves were recorded for the fabricated device. HMDI and HPDI were tested as hole transporting layer and the results are presented in Table 2. The devices gave very competitive PCE and the J–V curves are shown in Fig. 6a. When HMDI was used as HTM in its pristine form (as such), an average PCE of 7.13% was measured, while for the champion cell the PCE value reached to 8.62%. An unexpectedly high open circuit voltage (VOC = 907–938 mV) was obtained due to its good band alignment with the perovskite valence band.
| HTM | JSC (mA cm−2) | VOC (V) | Fill factor | PCE (%) | |
|---|---|---|---|---|---|
| a Average data and standard deviation calculated for 3 individual cells in the same batch. Mask size: 0.16 cm2 though the active area was ∼0.6 cm2.b Measured at 99.5 mW cm−2 under AM 1.5G spectra. | |||||
| HMDI | Avg ± SD | 14.43 | 0.938 | 0.637 | 8.62 |
| 14.31 ± 1.73 | 0.907 ± 0.03 | 0.551 ± 0.07 | 7.13 ± 1.14 | ||
| HPDI | Avg ± SD | 16.51 | 0.897 | 0.663 | 9.83 |
| 16.80 ± 0.23 | 0.893 ± 0.01 | 0.642 ± 0.02 | 9.62 ± 0.17 | ||
| HPDI + LiTFSIb | Avg ± SD | 19.16 | 0.976 | 0.576 | 10.82 |
| 18.69 ± 0.39 | 0.961 ± 0.02 | 0.562 ± 0.01 | 10.15 ± 0.53 | ||
| Spiro-OMeTADb | 19.62 | 944.137 | 0.694 | 12.93 | |
However, relatively low JSC (∼14 mA cm−2) and fill factor (FF = 0.55–0.64) were obtained. In order to improve the PV properties the conventional strategy of, doping was made and the additives such as LiTFSI and tert-butylpyridine (t-BP) were added for HMDI. Contrary to a report for another HTM, both VOC and JSC were reduced after doping. Doping is not very effective in this case and possibly hindering its mobility. After doping, the fill factor increased significantly, but this can be attributed to the lower JSC. The devices fabricated with pristine HPDI, showed improved value of PCE (average = 9.62%). The obtained enhanced PCE was due to higher fill factors and current (JSC = 16.80 mA cm−2, FF = 0.642), while the champion devices showed a PCE of 9.83% (JSC = 16.51 mA cm−2, VOC = 897 mV, FF = 0.663). Here it should be noted that in its pristine form both the molecules performed well and showed higher PCE compare to the standard Spiro-OMeTAD. Similarly the addition of LiTFSI and t-BP in HPDI resulted in slightly improved VOC and fill factor, but the short circuit current drops dramatically from 17 mA cm−2 to less than 10 mA cm−2. Thus the doping of synthesized HTM in both cases was found to be not effective, while in the case of Spiro-OMeTAD, other organic small molecules and PTAA, doping is crucial in order to achieve high efficiency. The drop in the current might be attributed to some undesirable molecular packing between t-BP and HTM, introduction of trap sites, which resulted in bad interface between perovskite and HTM, particularly in the case for HPDI.
Further, to improve the PV properties and the interface of HTM with perovskites, a nonpolar solvent such as toluene was used. Only, LiTFSI was added to increase the conductivity and the device PV performance was improved. Both the photocurrent and FF improved significantly, and a promising PCE of 10.82% was obtained (JSC = 19.16 mA cm−2, VOC = 976 mV, FF = 0.576). It is well known, that t-BP has a positive effect in increasing the voltage of the devices, but it also increases the recombination of electron and holes in the devices, thus making the devices less stable. Here it is notable that the devices fabricated without t-BP are working efficiently and the best results were obtained with using only 10 mg mL−1 of HPDI while in the case of Spiro-OMeTAD, much higher amount is used (72.3 mg mL−1), thus making it further cost-effective. The devices fabricated with HPDI also showed good reproducibility, having low standard deviations. In order to have real figure of merit the devices were measured from Voc → Jsc at a voltage sweep rate of 0.1 V s−1, proper mask size was also utilized to avoid any pronounced hysteresis effect. Fig. S1† shows the hysteresis curves for different HTM used for the fabrication of typical cells (not for the champion device). The incident photon to current efficiency (IPCE) spectra for the devices is shown in Fig. 6b, where the integral photocurrent obtained from the IPCE spectra are in good agreement with the experimentally obtained JSC from current–voltage performance (Fig. S2†). The doped HPDI showed improved light harvesting abilities compared to their analogous family members and in the visible range it approaches a 90% conversion rate.
Experimental section
5,10,15-Tris(4-(hexyloxy)phenyl)-10,15-dihydro-5H-diindolo[3,2-a:3′,2′-c]carbazole (HPDI)
To a solution of triazatruxene (250 mg, 0.74 mmol, 1 eq.), 1-(hexyloxy)-4-iodobenzene37 (0.9 g, 3 mmol, 4 eq.) in 5 mL of quinoline, CuI (550 mg, 2.9 mmol, 4 eq.) and K2CO3 (400 mg, 2.9 mmol, 4 eq.) were added. After stirring at 190 °C overnight under a N2 atmosphere, the reaction mixture was allowed to cool to room temperature and subsequently diluted with DCM followed by filtering through a plug of celite. The filtrate was concentrated in a vacuum. The residue was purified by column chromatography eluting with 30% DCM in hexane to give 170 mg of pale yellow solid (55%). 1H NMR (400 MHz, CDCl3-d) δ 7.59 (d, J = 6.8 Hz, 6H), 7.30 (d, J = 8.3 Hz, 6H), 7.13–7.25 (m, 6H), 6.85 (t, J = 8.0 Hz, 3H), 6.21 (d, J = 8.1 Hz, 3H), 4.14 (t, J = 5.9 Hz, 6H), 2.13–1.83 (m, 6H), 1.44 (t, J = 57.8 Hz, 18H), 0.98 (s, 9H). 13C NMR (100 MHz, C6D6-d6) δ 159.06, 142.55, 138.43, 133.86, 130.08, 127.57, 123.18, 123.07, 120.23, 115.53, 110.28, 104.58, 68.04, 31.58, 29.15, 25.72, 22.67, 13.94. C60H63N3O3 [M+] exact mass = 873.4869, MS (MALDI-TOF) = 873.1144.5,10,15-Trihexyl-3,8,13-trimethoxy-10,15-dihydro-5H-diindolo[3,2-a:3′,2′-c]carbazole (HMDI)
In a 50 mL three-necked flask, a solution of 1.95 mL (10.5 mmol, 15 eq.) of sodium methoxide 5.4 M in methanol, dry DMF (15 mL), copper(I) iodide (810 mg, 4.3 mmol, 6 eq.), 3,8,13-tribromo-5,10,15-trihexyl-10,15-dihydro-5H-diindolo[3,2-a:3′,2′-c]carbazole (0.6 g, 0.72 mmol, 1 eq.) was heated to reflux for 3 h under a N2 atmosphere. After that, the solution was filtered while hot through the celite to remove copper(I) iodide and washed with water. The mixture was extracted with DCM and the organic phase was dried over MgSO4. The product was isolated off on a silica gel column with 50% DCM in hexane to give the product as a pale yellow solid (300 mg, 55%). 1H NMR (400 MHz, CDCl3-d) δ 8.05 (d, J = 8.0 Hz, 3H), 7.71 (s, 3H), 7.55 (d, J = 8.0 Hz, 3H), 4.95 (s, 6H), 4.03 (s, 9H), 2.02 (m, 6H), 1.41–1.28 (m, 18H), 0.84 (t, J = 7.0 Hz, 9H). 13C NMR (100 MHz, benzene-d6) δ 157.31, 143.12, 138.04, 122.55, 118.14, 107.27, 104.20, 95.97, 55.02, 46.71, 31.23, 29.28, 26.23, 22.35, 13.71. C45H57N3O3 [M+] exact mass = 687.44, MS (ESI) = 687.46.Device fabrication
FTO-coated glass (NSG10) was laser etched, cleaned with Hellmanex and rinsed with deionized water and ethanol. Then the electrodes were ultrasonicated in 2-propanol and dried with compressed air. The substrates were UV/O3 treated to eliminate organic residues before blocking layer deposition.A blocking TiO2 layer was deposited by spray pyrolysis using a titanium diisopropoxide bis(acetyl acetonate) solution (1 mL of commercial titanium diisopropoxide bis(acetyl acetonate) 75% in 2-propanol was diluted with 19 mL of absolute ethanol) using O2 as carrier gas. During this process, etched substrates were heated at 450 °C to help the anatase formation. After cooling down, substrates were immersed in TiCl4 20 mM aqueous solution and baked for 30 minutes at 70 °C. Then the samples were washed with deionized water and heated at 500 °C for 30 minutes. TiO2 mesoporous layer (mp-TiO2) was prepared by spin coating 35 μL per cell of a solution made of 1 g of 30NR-D in 3.5 g of absolute ethanol. The spin coating conditions employed were: speed = 4000 rpm, time = 30 s. Subsequently, samples were sequentially sintered: 125 °C for 5 min, 325 °C for 5 min, 375 °C for 5 min, 450 °C for 15 min and 500 °C for 15 min.
A PbI2 solution 1.25 M concentrated in DMF was kept at 70 °C to avoid any precipitation. 50 μL from that solution were deposited and spun coated over the mesoporous TiO2 film (speed = 6500 rpm, time = 30 s). Immediately, the films (electrodes) were annealed at 70 °C for 30 minutes. After cooling down, the spin coating process was repeated using the same spin-coating parameters. 100 μL of CH3NH3I solution (8 mg mL−1) were spread above the PbI2 film to form perovskite, the waiting time was 20–25 s to observe the conversion of PbI2 into CH3NH3PbI3 (from yellow to black). Following this, the excess solution was removed by spin coating (speed = 4000 rpm, time = 30 s) and then the samples were annealed at 70 °C during 30 minutes.
Hole transporting materials (HTMs): 5,10,15-trihexyl-3,8,13-trimethoxy-10,15-dihydro-5H-diindolo[3,2-a:3′,2′-c]carbazole (HMDI) and 5,10,15-tris(4-(hexyloxy)phenyl)-10,15-dihydro-5H-diindolo[3,2-a:3′,2′-c]carbazole (HPDI) were both deposited by spin coating (speed = 2000 rpm, time = 30 s). HMDI and HPDI solutions were prepared by dissolving 30 mg in 1 mL of either chlorobenzene or toluene. Diluted solution were also prepared in the case of HPDI, employing LiTFSI salt as additive, for this 10 mg of product was dissolved in 1 mL of toluene and Li salt was added from a stock solution (170 mg of LiTFSI in 1 mL of acetonitrile). Finally to finish the device, a cathode of 80 nm gold was thermally evaporated.
Lead iodide, methyl ammonium iodide and Spiro-OMeTAD solutions (according to our standard procedure) were prepared inside an argon glove box with moisture and oxygen controlled conditions. PbI2 spin coating, MAI deposition and HTM spin coating depositions were developed inside a nitrogen dry box.
Conclusions
In summary, we have rationally designed solution-processable triazatruxene-based HTMs, and demonstrated their application in perovskite solar cells. The HTMs were synthesized using inexpensive precursors and state forward few synthetic steps compared to the expensive Spiro-OMeTAD. HMDI and HPDI have good thermal stability and are highly soluble in a variety of nonpolar solvents. The HOMO–LUMO energy levels of HMDI and HPDI, −5.17/−2.03 eV and −5.41/−2.15 eV, respectively, are well aligned with respect to perovskite energy level. HMDI and HPDI showed 8.6% and 9.8% PCE without the use of any additive, which is higher than reported for Spiro-OMeTAD. In the case of HPDI, the light to electricity conversion efficiency was improved to 10.82% with the addition of only LiTFSI salt, due to significantly enhancement in JSC and VOC. We believe that triazatruxene core-based HTMs have the potential to be an alternative for expensive Spiro-OMeTAD and p-type polymers in hole transporting layers due to their ease of fabrication, potentially low price, excellent solubility and remarkable thermal properties.Conflict of interest
Authors from Abengoa Research may have competing interests as Abengoa is an international company and has significant interest in the renewable energy sector. A patent disclosing the work is pending.Acknowledgements
One of us (SA) acknowledges grant from Torres Quevedo, Ministry of competitive and economics, Spain.Notes and references
- S. Kazim, M. K. Nazeeruddin, M. Grätzel and S. Ahmad, Angew. Chem., Int. Ed. Engl., 2014, 53, 2812–2824 (Angew. Chem., 2014, 126, 2854–2867) CrossRef CAS PubMed.
- I. Chung, B. Lee, J. He, R. P. H. Chang and M. G. Kanatzidis, Nature, 2012, 485, 486–489 CrossRef CAS PubMed.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- H.-S. Kim, C.-R. Lee, J.-H. Im, K.-B. Lee, T. Moehl, A. Marchioro, S.-J. Moon, R. Humphry-Baker, J.-H. Yum, J. E. Moser, M. Grätzel and N.-G. Park, Sci. Rep., 2012, 2, 591 Search PubMed.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Science, 2012, 338, 643–647 CrossRef CAS PubMed.
- J. H. Noh, S. H. Im, J. H. Heo, T. N. Mandal and S. Il Seok, Nano Lett., 2013, 13, 1764–1769 CAS.
- J. Burschka, N. Pellet, S.-J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Grätzel, Nature, 2013, 499, 316–319 CrossRef CAS PubMed.
- J.-H. Im, I.-H. Jang, N. Pellet, M. Grätzel and N.-G. Park, Nat. Nanotechnol., 2014, 9, 927–932 CrossRef CAS PubMed.
- N. Pellet, P. Gao, G. Gregori, T.-Y. Yang, M. K. Nazeeruddin, J. Maier and M. Grätzel, Angew. Chem., Int. Ed. Engl., 2014, 53, 3151–3157 CrossRef CAS PubMed.
- A. Mei, X. Li, L. Liu, Z. Ku, T. Liu, Y. Rong, M. Xu, M. Hu, J. Chen, Y. Yang, M. Gratzel and H. Han, Science, 2014, 345, 295–298 CrossRef CAS PubMed.
- M. I. Dar, F. J. Ramos, Z. Xue, B. Liu, S. Ahmad, S. A. Shivashankar, M. K. Nazeeruddin and M. Grätzel, Chem. Mater., 2014, 26, 4675–4678 CrossRef CAS.
- F. J. Ramos, M. C. López-Santos, E. Guillén, M. K. Nazeeruddin, M. Grätzel, A. R. Gonzalez-Elipe and S. Ahmad, ChemPhysChem, 2014, 15, 1148–1153 CrossRef CAS PubMed; F. J. Ramos, M. Oliva-Ramirez, M. K. Nazeeruddin, M. Grätzel, A. R. Gonzalez-Elipe and S. Ahmad, J. Mater. Chem. A, 2015 10.1039/c5ta02238j.
- F. Hao, C. C. Stoumpos, D. H. Cao, R. P. H. Chang and M. G. Kanatzidis, Nat. Photonics, 2014, 8, 489–494 CrossRef CAS PubMed.
- K. Tanaka, T. Takahashi, T. Kondo, K. Umeda, K. Ema, T. Umebayashi, K. Asai, K. Uchida and N. Miura, Jpn. J. Appl. Phys., 2005, 44, 5923–5932 CrossRef CAS.
- C. C. Stoumpos, C. D. Malliakas and M. G. Kanatzidis, Inorg. Chem., 2013, 52, 9019–9038 CrossRef CAS PubMed.
- K. Tanaka, T. Takahashi, T. Ban, T. Kondo, K. Uchida and N. Miura, Solid State Commun., 2003, 127, 619–623 CrossRef CAS.
- V. W. Bergmann, S. A. L. Weber, F. J. Ramos, M. K. Nazeeruddin, M. Grätzel, D. Li, A. L. Domanski, I. Lieberwirth, S. Ahmad and R. Berger, Nat. Commun., 2014, 5(5001), 1–9 Search PubMed.
- E. Guillén, F. J. Ramos, J. A. Anta and S. Ahmad, J. Phys. Chem. C, 2014, 118(40), 22913–22922 Search PubMed.
- N. J. Jeon, J. H. Noh, W. S. Yang, Y. C. Kim, S. Ryu, J. Seo and S. Il Seok, Nature, 2015, 517, 476–480 CrossRef CAS PubMed.
- B. Cai, Y. Xing, Z. Yang, W.-H. Zhang and J. Qiu, Energy Environ. Sci., 2013, 6, 1480 CAS.
- J. H. Heo, S. H. Im, J. H. Noh, T. N. Mandal, C.-S. Lim, J. A. Chang, Y. H. Lee, H. Kim, A. Sarkar, M. K. Nazeeruddin, M. Graetzel and S. Il Seok, Nat. Photonics, 2013, 7, 486–491 CrossRef CAS PubMed.
- D. Bi, L. Yang, G. Boschloo, A. Hagfeldt and E. M. J. Johansson, J. Phys. Chem. Lett., 2013, 4, 1532–1536 CrossRef CAS.
- S. N. Habisreutinger, T. Leijtens, G. E. Eperon, S. D. Stranks, R. J. Nicholas and H. J. Snaith, Nano Lett., 2014, 14, 5561–5568 CrossRef CAS PubMed.
- J. A. Christians, R. C. M. Fung and P. V. Kamat, J. Am. Chem. Soc., 2013, 136, 758–764 CrossRef PubMed.
- F. J. Ramos, M. Ince, M. Urbani, A. Abate, M. Grätzel, S. Ahmad, T. Torres and M. K. Nazeeruddin, Dalton Trans., 2015, 44, 10847–10851 RSC.
- A. Abate, M. Planells, D. J. Hollman, V. Barthi, S. Chand, H. J. Snaith and N. Robertson, Phys. Chem. Chem. Phys., 2015, 17, 2335–2338 RSC.
- B. Xu, E. Sheibani, P. Liu, J. Zhang, H. Tian, N. Vlachopoulos, G. Boschloo, L. Kloo, A. Hagfeldt and L. Sun, Adv. Mater., 2014, 26, 6629–6634 CrossRef CAS PubMed.
- S. Do Sung, M. S. Kang, I. T. Choi, H. M. Kim, H. Kim, M. Hong, H. K. Kim and W. I. Lee, Chem. Commun., 2014, 50, 14161–14163 RSC.
- J. Liu, Y. Wu, C. Qin, X. Yang, T. Yasuda, A. Islam, K. Zhang, W. Peng, W. Chen and L. Han, Energy Environ. Sci., 2014, 7, 2963–2967 CAS.
- N. J. Jeon, J. Lee, J. H. Noh, M. K. Nazeeruddin, M. Grätzel and S. Il Seok, J. Am. Chem. Soc., 2013, 135, 19087–19090 CrossRef CAS PubMed.
- P. Qin, S. Paek, M. I. Dar, N. Pellet, J. Ko, M. Grätzel and M. K. Nazeeruddin, J. Am. Chem. Soc., 2014, 136, 8516–8519 CrossRef CAS PubMed.
- H. Li, K. Fu, A. Hagfeldt, M. Grätzel, S. G. Mhaisalkar and A. C. Grimsdale, Angew. Chem., Int. Ed. Engl., 2014, 53, 4085–4088 CrossRef CAS PubMed; S. Kazim, F. J. Ramos, P. Gao, M. K. Nazeeruddin, M. Grätzel and S. Ahmad, Energy Environ. Sci., 2015, 8, 1816–1823 Search PubMed.
- N. J. Jeon, H. G. Lee, Y. C. Kim, J. Seo, J. H. Noh, J. Lee and S. Il Seok, J. Am. Chem. Soc., 2014, 136, 7837–7840 CrossRef CAS PubMed.
- C. Kulshreshtha, G. W. Kim, R. Lampande, D. H. Huh, M. Chae and J. H. Kwon, J. Mater. Chem. A, 2013, 1, 4077 CAS.
- A. Torres and L. G. C. Rego, J. Phys. Chem. C, 2014, 118, 26947–26954 CAS.
- T. Leijtens, I. Ding, T. Giovenzana, J. T. Bloking, M. D. Mcgehee and A. Sellinger, ACS Nano, 2012, 6, 1455–1462 CrossRef CAS PubMed.
- Y. Arakawa, S. Nakajima, R. Ishige, M. Uchimura, S. Kang, G. Konishi and J. Watanabe, J. Mater. Chem., 2012, 22, 8394 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, synthesis, and characterization. IPCE with integrated photocurrent is also included. See DOI: 10.1039/c5ra06876b |
| This journal is © The Royal Society of Chemistry 2015 |