Designing bio-mimetic moxifloxacin loaded hydrogel wound dressing to improve antioxidant and pharmacology properties
Baljit Singh* and
Abhishek Dhiman
Department of Chemistry, Himachal Pradesh University, Shimla - 171005, India. E-mail: baljitsinghhpu@yahoo.com; Fax: +911772830775; Tel: +911772830944
First published on 12th May 2015
Abstract
Recently, it has been found that moxifloxacin, an antibiotic drug, promotes wound healing without induction to bacterial resistance. Therefore, it has been incorporated into 2-hydroxyethylmethacrylate (HEMA) based hydrogel wound dressings, for its slow delivery and to enhance its wound curing potential, beside minimizing the adverse effects of systemic drug delivery. These polymer films were characterized by FTIR, solid state 13C NMR, SEM, AFM, XRD, TGA/DTA/DTG, DSC, and swelling measurements. Some important properties of the hydrogel wound dressings like wound fluid absorption, mechanical strength, permeability to O2, H2O vapour and microbes, blood compatibility, protein adsorption, antioxidant activity, ex-vivo mucoadhesion, in-vitro drug release and wound healing potential on an animal model have also been studied in the present work. These hydrogel dressings have taken 7.22 ± 0.26 g wound fluid per gram of gel and showed haemolytic potential of 0.95% and a water vapour transmission rate of 348.57 g per m2 per day. The release of moxifloxacin occurred through a non Fickian diffusion mechanism. The histological studies have revealed effective healing both in the case of wounds treated with drug loaded and unloaded hydrogel wound dressings, which has been proved by the absence of inflammation, matured fibrous tissues, well organised fibroblasts, and blood capillaries. Antioxidant assays showed the free radical scavenging ability of the hydrogel dressings. A correlation, between surface roughness and mucoadhesion, has been established. Overall, these dressings will not only enhance the wound curing process but also take care of the other aspects of wound health and skin regeneration.
1 Introduction
Nowadays, the prevention and treatment of infections are the major concerns in wound care. Systemic antibiotic therapy is associated with drug resistance and various adverse side effects which also include poor tissue penetration. Systemic therapy does not work, in cases where the immunity reduces and the bacterial load increases. The use of topical antimicrobial agents can help prevent the progression from colonisation to infection without any risk of drug resistance.1 Topical antimicrobial agents can be applied directly to the wound site, bypassing the need for an intact circulatory system. At the same time, antibiotic drug resistance, in wound infection, is another growing concern in wound management. Hence, all these factors, led to an increase in urgency to develop new therapeutic options for wound infections.Recently, Jacobsen and co-workers2 have studied the topical application of moxifloxacin in comparison to other commonly used antibiotics by using a porcine wound infection and a rat burn infection models. They have found that moxifloxacin promotes wound healing without induction of bacterial resistance. It has been proposed as an ideal candidate for wound therapy, due to its broad antibacterial spectrum, its high efficiency, and its potential to promote wound healing. Its better in-vitro activity against Gram-positive aerobes while retaining potent activity against Gram-negative aerobes makes it an option for the treatment of skin and skin structure infections.3 Hence, keeping in view the above facts, an attempt has been made to develop the hydrogel wound dressings loaded with moxifloxacin drug, which is efficient against resistant pathogen. The moxifloxacin is a recent addition to the fluoroquinolone class of antibiotic drugs. These hydrogel wound dressings have been prepared by using poly 2-hydroxyethylmethacrylate (poly(HEMA)), carbopol and acacia gum polysaccharide and their briefly discussion will be relevant here.
Hydrogels have all the properties which are required for a material to be use in wounds dressings. These are capable of absorbing contaminated exudates and safely retaining them within the gel structure, which provides microclimate that stimulates and regulates all cellular activities and nutritional processes during the individual phases of wound healing. Hydrogels remove from the wound without pain and risk of wound irritation.4 Hydrogels based on poly(HEMA) copolymers are of a great interest in biomedical applications, especially in wound dressing, due to their water absorption, biocompatibility, and mechanical properties.5 The poly(HEMA) copolymers have also been used to improve the biocompatibility of other materials to enhance their biomedical applications such as tissue engineering, controlled drug release and separation of bio-macromolecules.6,7 Further, the synthesis of a natural-synthetic polymer hybrid matrix induce the synergistic properties of both the polymers. Synthesis of copolymers of synthetic and natural polymers through grafting is a suitable method for the fabrication of stable, cyto-compatible natural-synthetic polymer hybrid matrices. Radhakumary et al.8 have synthesized hyaluronic acid-grafted-poly(HEMA) copolymers and have found that the copolymer films were stable in water at both acidic and neutral pH in contrast to that of virgin material and were non-cytotoxic to mammalian cells. Potorac and coworkers9 have observed that the synthesized scaffolds of collagen, modified with itaconic anhydride and poly(HEMA), have controlled the water retention and have enhanced the stability against enzymatic digestion as compared to native collagen for its applications in wound dressings.
On the other hand, gum acacia (GA) is a biocompatible, non-toxic, cost-effective natural polysaccharide which is known for its wound healing and antioxidant properties.10,11 It is used as emulsifying, stabilizing, thickening and suspending agent in food, pharmaceuticals, and cosmetics industries. It has been modified to develop the scaffold for cell culture by cross-linking with gelatine through schiff's base reaction. These scaffolds have been found to be non-cytotoxic and non-adherent nature of the scaffold.12 It has also been used to improve the fabrication of chitosan-gelatin based nanofibers meant for tissue engineering applications.13 Carbopol is a non-toxic and non-irritant hydrophilic crosslinked polymers of acrylic acid which is considered be suitable for the interface designs of materials for biomedical applications. It has been widely used in drug delivery devices owing to their mucoadhesion, swelling and biocompatibility properties.14 Das and coworkers15 have reported topical gels of cashew gum-carbopol polymers loaded with lidocaine. They have observed sustained in-vitro skin permeation of lidocaine for seven hour period through excised porcine skin. Carbopol has been used to improve mechanical properties of the hydrogels. Yoo and co-workers16 have reported that addition of carbopol has increased tensile strength of polymeric films prepared with carbopol 934P, hydroxypropyl methylcellulose and polyethylene glycol. The present study deals with the exploitation of the antioxidant property of acacia gum in designing the hydrogel dressing induced with better wound healing property. Further, these hydrogels have been applied to study the interaction of hydrogels dressings with blood and mucosal membrane, and a correlation between surface roughness and mucoadhesion has been established. The antibiotic drug moxifloxacin, which promotes wound healing without induction of bacterial resistance, has been incorporated into the dressings and its slow release profile has been studied. It reflects the exploration of the hydrogel materials for wound dressing applications and novelty of the present work. These copolymers have also been characterized by FTIR, solid state 13C NMR, SEM, AFM, XRD, TGA/DTA/DTG, DSC, and swelling measurements. Some important properties of the hydrogel wound dressings like wound fluid absorption, mechanical strength, permeability to O2, H2O vapour and microbes, blood compatibility, protein adsorption, antioxidant activity, ex-vivo mucoadhesion, in-vitro drug release and wound healing potential on animal model have also studied in the present work.
2 Materials and methods
2.1 Materials used
Gum acacia (GA) and carbopol 940 (Loba Chemie Pvt. Ltd., Mumbai-India), N,N′-methylenebisacrylamide (NN-MBA) (Acros organics, New Jersey-USA), ammonium persulphate (APS) (Qualigens Fine Chemicals, Mumbai-India), 2-hydroxyethylmethacrylate (HEMA), nutrient broth, Folin–Ciocalteu (F–C) reagent (Merck Specialities Pvt. Ltd. Mumbai-India) nitroblue tetrazolium chloride, riboflavin, methionine, glycerol, (S.D. Fine Chemical Ltd., Mumbai, India), gallic acid and sodium dodecyl sulfate (Himedia Laboratories Pvt. Ltd., Mumbai India), bovine serum albumin (BSA) (Bio Basic Inc., Canada), 2,2-diphenyl-1-picrylhydrazyl (DPPH) (Sigma–Aldrich, Munich, Germany), and moxifloxacin hydrochloride (Lifestar Pharma Pvt. Ltd., New Delhi, India) were used as received.2.2 Synthesis and optimization of hydrogel wound dressing films
Synthesis of the polymer films was carried out by free radical polymerization method using APS as initiator and NN-MBA as cross-linker. Reaction was carried out with solution of a definite concentration of GA and carbopol, taken in beaker. After hydration, the reaction mixture was stirred to get a homogenous mixture, at constant speed for definite time and then solutions of a definite concentration of glycerol, HEMA, NN-MBA and APS were added. Then, the solution was spread in the Petri dish which was then placed in hot air oven. The cross-linked polymer formed was washed with distilled water and ethanol to remove the soluble fractions left in polymer film. These polymer films were dried in oven till constant weight was obtained and were named as [gum acacia-cl-(poly(HEMA)-co-carbopol)] (GAHCP) polymer films. In order to determine the optimum reaction parameters, [HEMA] was varied from 0.041 to 0.206 mol L−1 and carbopol content was varied from 1.5% to 3.5% (w/v) during the polymerization reaction (Table 1). The optimum HEMA and carbopol contents were obtained as 0.041 mol L−1 and 1.5% (w/v) respectively. At this optimum concentrations of carbopol and HEMA, another polymer films were synthesized without gum acacia and were named as [poly(HEMA)-co-carbopol] (HCP) polymer films. Swelling studies of hydrogels were carried out by gravimetric method.17 Swelling mechanisms of the polymer matrix was determined by the power law expression reported by Ritger and Peppas18,19 i.e. Mt/M∞ = ktn. where Mt/M∞ is the fractional swelling in time t, ‘k’ is the constant and ‘n’ is the diffusion exponent characteristic of the swelling mechanism. Mt and M∞ are the swelling at time ‘t’ and at equilibrium respectively. It is based on the relative rate of diffusion of water into polymer matrix and rate of polymer chain relaxation.| Sr. no. | HEMA (mol L−1) | Carbopol (% w/v) | Swelling after 24 h (g g−1 of gel) | Diffusion exponent ‘n’ | Gel characteristic constant ‘k’ × 102 |
|---|---|---|---|---|---|
| a GA = 5% (w/v); APS = 5.48 × 10−3 mol L−1, glycerol = 0.27 mol L−1, NN-MBA = 8.10 × 10−3 mol L−1. | |||||
| 1 | 0.000 | 2 | 8.053 ± 0.355 | 0.302 | 0.170 |
| 2 | 0.041 | 2 | 8.117 ± 0.108 | 0.266 | 0.219 |
| 3 | 0.082 | 2 | 6.805 ± 0.031 | 0.183 | 0.342 |
| 4 | 0.123 | 2 | 5.101 ± 0.148 | 0.132 | 0.454 |
| 5 | 0.164 | 2 | 4.869 ± 0.208 | 0.200 | 0.319 |
| 6 | 0.206 | 2 | 4.340 ± 0.111 | 0.215 | 0.290 |
| 7 | 0.041 | 1.5 | 8.434 ± 0.564 | 0.206 | 0.296 |
| 8 | 0.041 | 2.5 | 7.686 ± 0.254 | 0.229 | 0.260 |
| 9 | 0.041 | 3.0 | 6.659 ± 0.110 | 0.185 | 0.324 |
| 10 | 0.041 | 3.5 | 6.297 ± 0.454 | 0.210 | 0.298 |
2.3 Characterizations
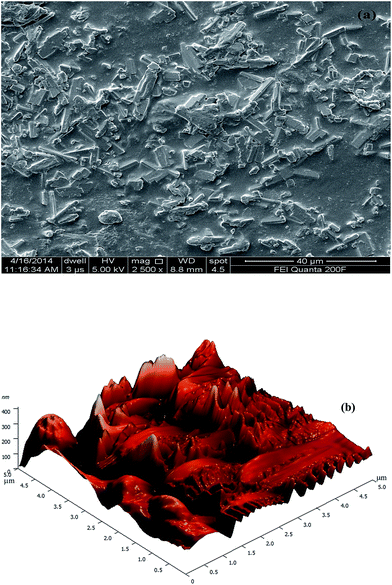
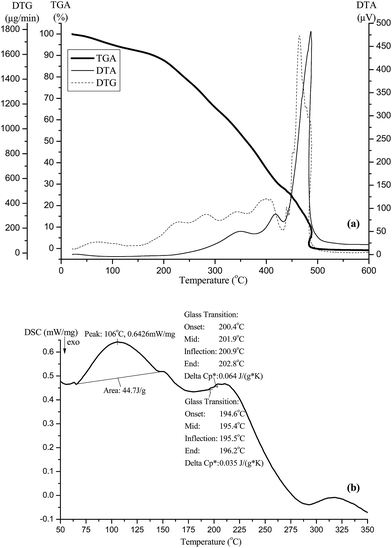
GAHCP polymers were characterized by Fourier transform infrared spectroscopy (FTIR), 13C solid state nuclear magnetic resonance (NMR) spectroscopy, scanning electron micrographs (SEMs), atomic force microscopy (AFM), X-ray diffraction study (XRD), thermogravimetric analysis (TGA), differential thermal analysis (DTA), differential thermogravimetry (DTG), and differential scanning calorimetry (DSC) studies. FTIR of dried powdered samples was recorded in KBr pellets on Nicolet 5700 FTIR THERMO (USA). The solid state 13C NMR was carried out on JEOL RESONANCE ECX 400 solid state NMR spectrometer. The spectrophotometer was operated at a magnetic field of 9.3 T and at a frequency of 100 MHz for 13C. The surface morphology of the samples was inspected using SEM (FEI Quanta 200F, The Netherlands). The SEM images of GAHCP hydrogel film was taken at high vacuum pressure using a high energy beam of electrons (5–10 kV). All SEM images were taken on carbon tape without using any staining or coating at 2500× magnification. Surface morphology was also studied by AFM (NT-MDT, Russia) in semi-contact mode. Roughness measurements were acquired from 5 μm2 areas. XRD was done using Bruker D8 advance diffractometer (USA) equipped with Cu anode and a Cu Kα source (λ = 1.54 Å). Each sample was exposed to the X-ray beam at 40 kV and 30 mA from 2θ angles varying from 10° to 80° with a step size of 0.02°. The diffraction spectrum was recorded and analyzed. Thermal degradation studies (TGA, DTA and DTG) were performed using EXSTAR TG/DTA 6300 thermal analyzer (Japan). Dried polymer samples were heated in an alumina crucible, and the degradation profile was recorded from 30 to 800 °C, at a scan rate of 10 °C min−1, under air atmosphere. DSC measurements were performed with NETZSCH DSC 204 (USA) under heating rates 10 °C min−1 in temperature range 25 °C to 500 °C.2.4 Wound fluid absorption studies of hydrogel wound dressings
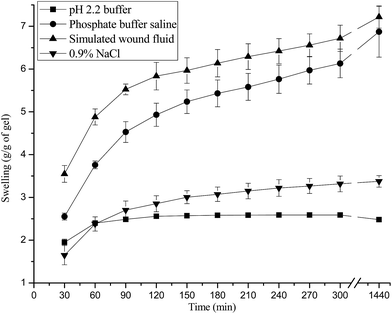
Wound fluid absorption studies of hydrogel wound dressings were carried out in simulated wound fluid (SWF) (pH = 8.0) by gravimetric method. Effect of pH (pH 2.2 buffer and phosphate buffer saline (PBS) of pH = 7.4), and [salt] on the swelling of the hydrogels was also observed.2.5 Mechanical properties of hydrogel wound dressings
A Texture Analyzer (Stable Micro Systems TA-XT2i, UK) equipped with 50 kg load cell was used to analyze the mechanical properties of hydrogel wound dressings. Tensile test of hydrogel film (50 × 20 mm) was used to evaluate breaking force, elongation at break and tensile strength. Burst strength, resilience, stress relaxation test were performed by means of texture analyzer equipped with a ball probe (P/5 s). Folding endurance test was also performed.20,21 All the tests were performed at fixed instrumental parameters.2.6 Permeability properties of hydrogel wound dressings
The permeability properties of the hydrogels films with respect to oxygen, water vapour and microbial penetration were determined. To evaluate the O2 permeability of hydrogel dressing, equal volume of boiled distilled water was added into the flasks and hydrogel films were fixed on top of the flasks (test area: 4.22 ± 0.13 cm2) and oxygen penetration into flask after 24 hours was determined by measuring the dissolved oxygen in the distilled water (mg mL−1) using the Winkler's method.22,23Water vapour transmission rate (WVTR) of hydrogel wound dressings was determined gravimetrically using desiccant method.24 The vials containing anhydrous CaCl2, with and without polymer films on the mouth, were placed in desiccators containing saturated solution of NaCl and their weight was determined after specific interval. WVTR (g per m2 per day) was calculated using following equation;
| WVTR = [(Δw/Δt) × 24]/A. |
In this equation (Δw/Δt) is the slope of the plot ‘w’ vs. ‘t’, where ‘w’ is the weight gain (g) along the specified time period, ‘t’ (h) and ‘A’ is the effective transfer area (m2).
Microbial permeability of hydrogel wound dressings was tested by method reported elsewhere.22 The turbidity of the nutrient broth in test tubes after incubation at ambient environment was considered as microbial contamination. All the tests were carried out in triplicate.
2.7 Blood compatibility properties of hydrogel wound dressings
The blood compatibility of hydrogel wound dressings was evaluated by studying thrombogenicity and haemolytic potential of hydrogel films. The membrane disruption of red blood cells by haemolysis was measured by the optical density of the supernatant at 540 nm using a UV visible spectrophotometer (Cary 100 Bio, Varian). The evaluation of thrombus formation on polymeric surfaces was carried out using a gravimetric method.25 All studies were carried out in triplicate.2.8 Protein adsorption properties of hydrogel wound dressings
Protein adsorption of hydrogel wound dressings was determined by Lowery method.26 Hydrogel film (1 × 1 cm) was immersed in PBS containing 200 μg mL−1 bovine serum albumin (BSA) for 24 h under 100 rpm shaking at 37 °C. Afterwards, the samples were taken out, rinsed five times with PBS, and then placed in 5 mL aqueous solution of sodium dodecyl sulfate (1% w/v) and shaken under 100 rpm for 1 h at 37 °C to remove the protein adsorbed on the surface. The BSA content of each sample was measured using the Lowery method.26 The absorbance at 750 nm was measured using a UV visible spectrophotometer (Cary 100 Bio, Varian).2.9 Antioxidant properties of hydrogel wound dressings
The antioxidant activity of polymer films was evaluated by three methods i.e. 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay, superoxide radical (O2˙−) scavenging activity assay and Folin–Ciocalteu (F–C) reagent assay. In DPPH radical scavenging assay, definite amount of polymer was added in methanolic solution of DPPH radical (100 μM) and DPPH solution without polymer and it was taken as control. The reaction mixtures were kept in dark and the absorbance was measured after 12 hours at 517 nm using a UV-Vis spectrophotometer (Cary 100 Bio, Varian) to calculate percentage scavenging activity.27,28 In superoxide radical (O2˙−) scavenging activity assay method, capacity of the polymer to scavenge superoxide radicals generated in riboflavin/methionine-light system was evaluated.29 Results were expressed in percent inhibition of superoxide radicals.30 In F–C reagent assay, antioxidant capacity of polymer films was expressed as gallic acid equivalent.28 All the tests were carried out in triplicate.2.10 Ex-vivo mucoadhesion studies of hydrogel wound dressings
The adhesion of hydrogel films to the intestinal mucosa of goat was determined by Texture Analyzer (Stable Micro Systems TA-XT2i, UK equipped with a 5 kg load cell). Maximum force required to detach the film from the intestinal mucosal membrane, total work of adhesion, and distance travelled by film before detachment, were determined at a fixed instrumental parameters.312.11 In-vitro drug release studies of hydrogels
The release profile of model drug moxifloxacin hydrochloride from the drug loaded hydrogel films was determined in pH 2.2 buffer, PBS and SWF. The loading of a drug into the polymer matrix was carried out by swelling equilibrium method. In-vitro release studies of the drug were carried out by keeping the drug loaded hydrogel films in releasing medium at 37 °C. The amount of drug released was measured using a UV visible spectrophotometer (Cary 100 Bio, Varian) in pH 2.2 buffer (295 nm), PBS (288 nm), and SWF (288 nm) after every 30 min up to 300 min in each case and then after 24 h. The mechanism of drug release was determined by using mathematical equations given by Ritger and Peppas.18,192.12 Wound healing studies of hydrogel wound dressings on mouse model
Healthy, pathogen free swiss albino mice of strain Balb C weighing 22–25 g were used to assess wound healing potential of drug loaded and unloaded hydrogel films. The protocol of the present investigation was approved by Institutional Animal Ethics Committee, Himachal Pradesh University, Shimla, India. Mice were anesthetized with diethyl ether, the surgical area was shaved and a wound, approximately 1 cm2, was created on the dorsal side of the mouse, using surgical scissors. Mice were randomly divided into three groups: (i) control group in which wound was left to heal spontaneously, (ii) group in which wound were treated with GAHCP hydrogel film, (iii) group in which wound was treated with drug loaded GAHCP hydrogel films. Tissues of the wounded area were taken on 4th, 8th and 12th day, sections 5 μm thick were cut using microtome, stained with haematoxylin-eosin, and photographed with Leica photoscope, to study the changes in wounded skin. Wound healing of open wound, wound covered with drug loaded and unloaded hydrogel films were compared.3 Results and discussion
3.1 Characterization of polymers
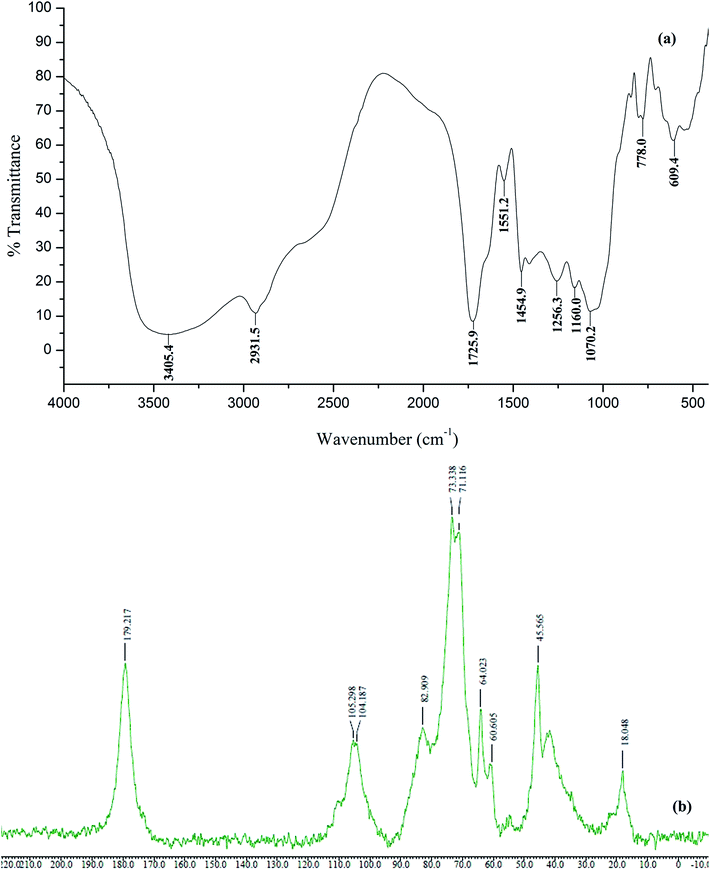
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch of GA, carbopol and HEMA), 1454.9 cm−1 (C–O stretching, and O–H in plane bending), 1256.3 cm−1 (C–O stretching), 1160.0 cm−1 (–C–O–C– symmetric stretching for acrylates) and 1070.2 cm−1 (C–O–C asymmetric stretching of β(1-6) or β(1-3) linked galactans in GA) showed the grafting of poly(HEMA) and carbopol in the polymer matrix (Fig. 1a).32,33
O stretch of GA, carbopol and HEMA), 1454.9 cm−1 (C–O stretching, and O–H in plane bending), 1256.3 cm−1 (C–O stretching), 1160.0 cm−1 (–C–O–C– symmetric stretching for acrylates) and 1070.2 cm−1 (C–O–C asymmetric stretching of β(1-6) or β(1-3) linked galactans in GA) showed the grafting of poly(HEMA) and carbopol in the polymer matrix (Fig. 1a).32,33
In case of solid state 13C NMR of polymer samples, presence of a characteristic peak at δ = 179.2 ppm (carbonyl carbons of HEMA, carbopol, glucuronic acid of GA), at 105.2 and 104.1 (C-1 of cyclic saccharides present in GA), at 82.9 (C-3 of β(1-3) linked galactans in GA), at 73.3 and at 71.1 (–C–OH of GA), 64.0 (–CH2–OH of GA), 60.6 (–CH2–OH of HEMA), at 45.5 and at 41.0 (–CH2–, –CH– of carbopol) and at 18.0 ppm (–C–CH3 of HEMA and rhamnose of GA) indicate the presence of poly(HEMA) and carbopol in the polymer matrix due to grafting and crosslinking (Fig. 1b). The absence of characteristic peak of HEMA at 137 and 127 ppm for (–CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–) indicates the conversion of sp2 to sp3 hybridized carbon during polymerization.34–36
CH–) indicates the conversion of sp2 to sp3 hybridized carbon during polymerization.34–36
| Sample | Initial decomposition temperature (°C) | Final decomposition temperature (°C) | Decomposition temperature (°C) at every 10% weight loss | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | |||
| GA | 235.60 | 515.00 | 97 | 258 | 280 | 293 | 303 | 319 | 405 | 453 | 465 | 515 |
| Carbopol | 198.99 | 462.46 | 183 | 238 | 261 | 291 | 325 | 364 | 402 | 432 | 457 | 463 |
| GAHCP | 196.73 | 495.68 | 180 | 239 | 283 | 326 | 361 | 392 | 424 | 462 | 487 | 496 |
3.2 Swelling studies
The results of effect of reaction parameters on the swelling of hydrogels are presented in Table 1. It has been found that the initially the swelling of the hydrogels increased with the addition of HEMA in the reaction system during the polymerization reaction in the synthesis of hydrogels. However, further increase in HEMA concentration; decrease the swelling of hydrogels due to increase in crosslinking and hydrophobicity of the polymer matrix, has been found. Swelling of the polymers decreased with increase in carbopol content beyond 1.5% (w/v). This may be due to increase in crosslinking in the polymer networks. The swelling of GAHCP hydrogels occurred through Fickian diffusion mechanism, wherein rate of diffusion of water in the polymer matrix is much less than the rate of relaxation of polymeric chains. Optimum parameters were determined. In general, high swelling hydrogel dressings are required which can maintain moist environment and can absorb the exudates from wound site effectively.3.3 Wound fluid absorption studies of hydrogel wound dressings
The results of wound fluid absorption studies showed that these hydrogels have taken 7.22 ± 0.26 g wound fluid per gram of polymer film. In general, it has been reported that moderate to high exuding wounds typically produce approximately 3–5 mL of exudate per 10 cm2 in 24 h. Hence, the results of present studies indicate that these dressing could absorb and retain a high volume of fluid. This could help to overcome the challenge of collection and leakage of excess exudates when applied to a moderate to highly exuding wounds.42 Whereas dressings with poor exudates absorption leads to the accumulation of wound fluid under the dressing and responsible for skin maceration, bacterial proliferation and the risk of other infections. It is also not patient friendly as it requires frequent change after their applications. Further, the effect of pH of swelling medium on swelling showed that swelling was higher in PBS and SWF as compared to pH 2.2 buffer. Decrease in the swelling has been observed in salt solution due to the screening effect of the sodium ions (Fig. 5).3.4 Mechanical properties of hydrogel wound dressings
Results of mechanical properties (like tensile strength, burst strength, resilience, stress relaxation and folding endurance) are presented in Table 3. GAHCP and HCP hydrogel films have shown tensile strength (1.35 ± 0.27) N mm−2 and (2.07 ± 0.34) N mm−2 respectively. The lower value of tensile strength of GAHCP films may be due to the less crosslinking density in GAHCP films in comparison to HCP films.43 Further, the lower value of crosslinking density of GAHCP films is substantiated by its higher swelling (8.43 g g−1) as compared to HCP films (2.18 g g−1). Similar trends have been found in case of bursting strength of these polymer films. However, GAHCP hydrogel films were more resilient than HCP films. Further, it has been found that 3.16 ± 0.60 N force was required to displace GAHCP film upto 2 mm, while it was 2.71 ± 0.49 N force for HCP films. Good resilience and considerable displacement force make these films suitable to bear various kinds of stress and strains during their application to wound healing. The results of stress relaxation experiment of both the films showed almost similar retained force (%) when a constant strain was applied for 30 s at fixed instrumental parameters. Folding endurance values for films was observed more than 300 in both the cases. High folding endurance is desirable for application at places such as joints where dressing can face folding and de-folding repeatedly. So these films can be applied successfully at such place without any risk of dressing damage. Results of mechanical properties indicate that GAHCP films can be stretched, folded, need considerable force to burst and are resilient up to certain extent, so, these dressing can provide mechanical protection to the wound. Additionally the spongy nature of the wound dressing due to its high swelling capacity can also protect the wound form external jerks.| Tensile strengtha | Breaking force (N) | Elongation at break (%) | Tensile strength (N mm−2) |
|---|---|---|---|
| a (Trigger force = 0.029 N, test speed = 2.00 mm s−1, pre test speed = 0.20 mm s−1, post test speed = 10 mm s−1).b (Distance = 15.0 mm, trigger force = 0.049 N, test speed = 1.0 mm s−1, pre test speed = 2.0 mm s−1, post test speed = 10 mm s−1).c (Distance = 2.0 mm, trigger force = 0.049 N, test speed = 0.5 mm s−1, pre test speed = 1.0 mm s−1, post test speed = 0.5 mm s−1).d (Distance = 2.0 mm, trigger force = 0.049 N, test speed = 0.5 mm s−1, pre test speed = 1.0 mm s−1, post test speed = 10 mm s−1).e (Contact time = 60 s, return distance = 15.0 mm, applied force = 0.10 N, trigger force = 0.029 N, test speed = 0.10 mm s−1, pre test speed = 0.50 mm s−1, post test speed = 0.10 mm s−1). | |||
| GAHCP | 27.02 ± 5.33 | 23.89 ± 3.80 | 1.35 ± 0.27 |
| HCP | 41.37 ± 6.89 | 94.29 ± 32.57 | 2.07 ± 0.34 |
| Burst strengthb | Dimensions (mm2) | Bursting strength (N) | Distance at burst (mm) |
|---|---|---|---|
| GAHCP | 30 × 30 | 21.49 ± 1.10 | 8.93 ± 0.11 |
| HCP | 30 × 30 | 24.05 ± 0.95 | 18.09 ± 1.91 |
| Resiliencec | Dimensions (mm2) | Force at target distance (N) | Resilience (%) |
|---|---|---|---|
| GAHCP | 30 × 30 | 3.16 ± 0.60 | 23.82 ± 0.62 |
| HCP | 30 × 30 | 2.71 ± 0.49 | 19.69 ± 0.44 |
| Relaxationd | Dimensions (mm2) | Force at target distance (N) | Retained force (%) |
|---|---|---|---|
| GAHCP | 30 × 30 | 1.51 ± 0.18 | 21.16 ± 2.92 |
| HCP | 30 × 30 | 1.47 ± 0.18 | 21.19 ± 1.86 |
| Mucoadhesione | Wad (mN mm) | Detachment distance (mm) | Fmax (mN) |
|---|---|---|---|
| GAHCP | 25.14 ± 4.16 | 3.36 ± 0.22 | 70.20 ± 17.57 |
| HCP | 28.61 ± 3.60 | 3.50 ± 0.65 | 52.70 ± 11.21 |
3.5 Permeability properties of hydrogel wound dressings
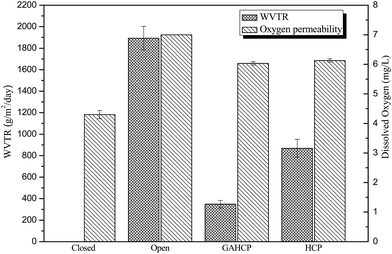
The results of oxygen permeability properties and water vapour permeability properties are presented in Fig. 6. In oxygen permeability test, the amount of dissolved oxygen in flasks sealed with hydrogel film was observed more than air tight flask and less than open flask. Similar results of oxygen permeability experiment have been observed in case of chitosan based films.22 Results indicate that both hydrogel films were permeable to oxygen. Hence, besides providing better cellular growth, oxygen availability through these wound dressings can also reduces the chances of growth of anaerobic bacteria in the wound bed.44 It is better to mention here that the oxygen is required at almost every step of wound healing process and wound hypoxia results in impaired healing, so evaluation of oxygen permeability of hydrogel film is important for its application as wound dressing. However for an efficient wound dressing, moist environment is required in order to enhance the wound healing process (winter). Water vapour transmission rate (WVTR) 348.57 and 868.57 g per m2 per day have been observed for GAHCP and HCP respectively. Addition of gum acacia decreased, the WVTR of hydrogel films.45 The WVTR of the both hydrogel films lies in the range as shown in case of normal skin (240–1920 g per m2 per day). Further, it has also been reported that the uncovered wounds can transpire upto 4800 g per m2 per day.46 The results of microbial penetration study showed that hydrogel films were impermeable to microbes in open environment. Wound bed provides a better environment for the growth of microorganisms. So prevention of microbe penetration is a very important factor for better wound healing. These results of permeability properties of hydrogel wound dressings are quit promising for its use as wound dressing.3.6 Blood compatibility properties of hydrogel wound dressings
Blood compatibility properties of hydrogel wound dressings showed that the material is biocompatible in nature. Results of hemolysis (%) values of hydrogels showed (0.95 ± 0.02)% and (4.86 ± 0.18)% of haemolysis for GAHCP and HCP respectively. The former is classified as non-haemolytic while latter is partially haemolytic. Extremely low haemolytic index of GAHCP can be attributed to higher hydrophilicity of polymer matrix which decreases in polymer-red blood corpuscles (RBC) interactions and subsequently, lower the level of RBC disruption.47 In order to evaluate the effect of hydrogel films on blood clotting, thrombogenicity test has been performed on hydrogel films. These both hydrogel films have shown higher weight of clot formation than positive control (280.33 ± 5.033 mg) which is classifying these dressings as thrombogenic in nature.48 Percent thrombogenicity of hydrogel films with and without GA were (109.28 ± 3.64)% and (140.67 ± 2.09)% respectively. These results indicate that hydrogels have increased the rate of clot formation. Thrombogenic nature of hydrogel films can be attributed to the presence of carboxylic groups on the surface of hydrogel films. Nie and coworkers49 have also reported higher thrombin generation by surface containing carboxylic groups. It has been found that negatively charged groups like –COO−, on the surface activate Factor XII and promote the formation of blood clot through various biochemical processes.3.7 Protein adsorption properties of hydrogel wound dressings
Protein adsorption property of a wound dressing is closely related to its cell adhesion behavior. Albumin is the most abundant protein in the blood. Therefore, in the present study, BSA was used as a model protein, to evaluate the protein adsorption behavior of the hydrogel films. It was observed that after 24 h GAHCP hydrogel films and HCP films showed 0.19 ± 0.02 mg cm−2 and 0.24 ± 0.02 mg cm−2 albumin adsorption respectively. Less protein adsorption on GA incorporated films can be attributed to high hydrophilic nature of GA. Less albumin adsorption is desirable property for a wound dressing.3.8 Antioxidant properties of hydrogel wound dressings
The antioxidant activity of polymer films have determined by three methods i.e. 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay, superoxide radical (O2˙−) scavenging activity assay and Folin–Ciocalteu (F–C) reagent assay. DPPH free radical scavenging activity for GAHCP and HCP hydrogel films have been observed to be (79.93 ± 3.60)% and (59.09 ± 3.59)% after 12 hours. Topical wound healing formulations with DPPH scavenging ability have been reported to improve skin repair and regeneration.50,51 Moseley and coworkers52 have reported the comparative account of the antioxidant properties of wound dressing materials – carboxymethylcellulose, hyaluronan benzyl ester and hyaluronan, towards polymorphonuclear leukocyte-derived reactive oxygen species. They have observed dose-dependent antioxidant properties against O2˙− and have also found that the benzyl esterified hyaluronan showed greatest antioxidant potential.Results of SOD scavenging assay revealed that GAHCP was able to scavenge (64.21 ± 2.70)% superoxide radicals generated in riboflavin/methionine-light system, and it might be helpful for preventing delayed wound healing induced by superoxide radicals in pathological conditions. HCP hydrogels displayed 39.68 ± 1.67% scavenging. Superoxide radical scavenging may be beneficial in diminishing the formation of hydroxyl radicals, which is considered main factor for delayed healing of chronic wounds. Superoxide radical scavenging ability of GAHCP can be partially due to protein part present in GA.53
Results of F–C reagent assay also depict the antioxidant nature of GA based wound dressings, while HCP hydrogels showed no activity in this assay. 0.1 grams of GAHCP showed antioxidant activity equivalent to (13.78 ± 0.03) μg gallic acid during F–C reagent assay. Out of several factors which are involved in the enhancement of the wound healing process, the beneficial and protective effects of antioxidant molecules plays a very important role in wound healing.54 Therefore, the antioxidant property of hydrogel films may be among the most efficient contributing factors for improved wound healing in the present study. In each of three antioxidant activity tests, the GAHCP hydrogel films revealed significantly higher antioxidant activity than HCP. These findings are the result of addition of GA to the hydrogel film. The antioxidant properties of can be attributed to the AGP complex and glucoronic acid present in GA.55 The superoxide radical is a highly toxic species that can be generated by numerous biological reactions in wounds. It is a predominant cellular free radical, is involved in a large number of deleterious changes often associated with an increase in peroxidative processes. It can take part in further reactions leading to the formation of more reactive oxygen species (ROS) such as hydroxyl radical and singlet oxygen, and cause tissue damage.56 Generation of reactive oxygen species result in wound hypoxia which impairs wound healing. During the wound healing process, moderate antioxidant activity is desired because it has been shown that excessive amounts of reactive oxygen species results in impaired healing. The antioxidant properties of the wound dressing material for topical application could contribute to the healing process.50,52
3.9 Ex-vivo mucoadhesion studies of hydrogel wound dressings
Mucoadhesion of polymeric films was measured as the maximum force required detaching the film from intestinal mucosa. During mucoadhesion studies it was found GAHCP hydrogel showed more adhesion to the mucosal membrane in comparison to HCP polymers. Maximum detachment force (Fmax) required for detachment of GA incorporated hydrogel from mucosal surface was 70.20 ± 17.57 mN while in case of HCP it was 52.70 ± 11.21 mN. Relatively high Fmax can be attributed to higher hydrophilic nature of GAHCP hydrogel. Jain et al.57 have also reported that good swelling characteristics of polyvinyl alcohol and sodium carboxymethylcellulose based inserts facilitated the mucoadhesion. Absorption of water is responsible for relaxation of the polymer chains, resulting in the exposure of all sites of the polymer to occur as proper adhesion.58 On the other hand, surface roughness is another contributing factor for mucoadhesion of polymers. According to the mechanical theory of mucoadhesion, rougher surface has more bio-adhesion.59 It was further confirmed by the AFM studies of polymers in the present studies. The values of work of adhesion (Wad) and debonding distance for GAHCP and HCP polymers are presented in Table 3. The presence of the carbopol, a well-known mucoadhesive polymer, confers bio-adhesive behaviour of the hydrogels.3.10 In-vitro drug release studies of hydrogels
The in-vitro release profile of moxifloxacin hydrochloride was evaluated in three simulated biological fluids i.e. pH 2.2 buffer, PBS and SWF. Drug release did not show burst effect in release medium (Fig. 7), suggesting that the slow release profile of drug loaded hydrogel wound dressings. This may be due to the electrostatic interactions between drug and polymer films. The slow release profile has been observed in PBS (pH 7.4 buffer) due to the lower solubility of moxifloxacin hydrochloride at this pH. Langlois et al.60 have found that moxifloxacin hydrochloride is more lipophilic in pH 7.4 buffer due to the presence of neutral and zwitterions forms. Further, solubility of the drug was dominating factor over swelling during drug release studies. The release of drug followed non-Fickian diffusion mechanism (Table 4). Hence, these hydrogels can act as slow drug delivery devices to create a microbe free micro-environment for optimized wound healing.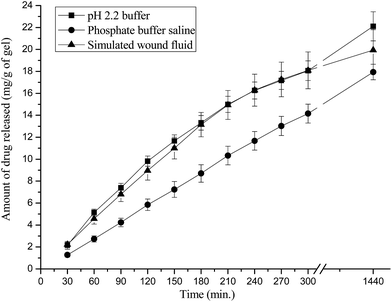 | ||
| Fig. 7 Release profile of moxifloxacin from drug loaded GAHCP polymer films in different media at 37 °C. | ||
| Drug releasing medium | Diffusion exponent ‘n’ | Gel characteristic constant ‘k’ × 103 | Diffusion coefficients (cm2 min−1) | ||
|---|---|---|---|---|---|
| Initial Di × 106 | Average DA × 106 | Late time DL × 106 | |||
| pH 2.2 buffer | 0.906 | 5.263 | 12.371 | 5.625 | 9.683 |
| Phosphate buffer saline | 1.050 | 2.061 | 13.076 | 4.491 | 9.239 |
| Simulated wound fluid | 0.924 | 5.153 | 16.946 | 6.167 | 14.432 |
3.11 Wound healing studies of hydrogel wound dressings on mouse model
Wound healing studies of hydrogel film on mouse model was performed and healing of wounds covered with drug loaded and unloaded hydrogels was compared with open wound, which was evaluated by histological study of healing tissue on day 4, 8 and 12 (Fig. 8). Hydrogel dressings adhered slightly to the wound bed and could be removed from the wound surface without causing any trauma or tissue loss. Bleeding and trauma is often caused by gauze dressings during removal from wound. Wound healing process in wounds covered with hydrogel dressings seems to be proceeded quite fast. Microscopical observation at day 4 revealed that wounded tissue in all the groups were heavily in filtered with inflammatory cells, primarily polymorphonuclear leukocytes and randomly distributed fibroblasts. On day 8 less inflammation was observed, in wounds treated with drug loaded and unloaded hydrogel, when compared to untreated open wounds. Mature collagen, fibroblasts and newly formed blood vessels were observed in wounds treated with drug loaded hydrogels. Inflammation was still persistent in non-treated animals confirming a slow healing process. Day 12 observations for hydrogel treated wounds revealed effective healing, which was proved by absence of inflammation, matured fibrous tissues, well organised fibroblasts, and blood capillaries. Lack of an inflammation in the wound treated with GAHCP hydrogel wound dressings and absence of pathological abnormalities supported its histocompatibility.51,61 On the other hand, results for the open wound still showed little inflammation and lack of blood capillaries, illustrating impaired healing. On the basis of histological study in the mouse skin, it has been found that these hydrogels are effective in accelerating the wound healing process. Further, the wound healing potential of the GAHCP film can be attributed to hydrogel nature of the wound dressings, which can maintain moist environment for cellular repair and regeneration. Moreover semipermeable nature of the hydrogel wound dressings and the free radical scavenging ability may be the contributing factors for early contraction of the wound and fibrous tissue formation.50 These results indicate that these hydrogel wound dressings could act as a candidate for the repair and regeneration of wounded skin and other skin applications.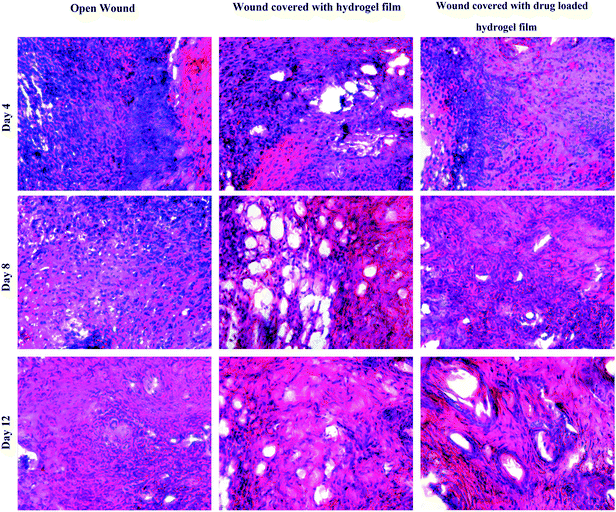 | ||
| Fig. 8 Photomicrographs of hematoxylin-eosin stained sections of open wound and wounds treated with moxifloxacin loaded and unloaded GAHCP hydrogel film. | ||
4 Conclusions
It is concluded from the forgone discussion that these hydrogel wound dressings loaded with moxifloxacin drug can act as slow drug delivery system and can overcome the various adverse effects of systemic antibiotic drug delivery. The release of moxifloxacin occurred through non Fickian diffusion mechanism in simulated wound fluid without any burst effect. In this mechanism the rate of diffusion of drug from the polymer and rate of polymer chains relaxation are comparable. Wound fluid absorption studies showed that these hydrogels can maintain moist environment and can absorb the exudates from wound site effectively. Results of mechanical properties indicate that hydrogel films can be stretched, folded, and are resilient up to certain extent, so, these dressing can provide mechanical protection to the wound. The results of permeability properties showed that these hydrogel films are permeable to O2 and H2O vapour and impermeable to microbes in open environment. Beside these permeable properties, high wound absorption, low albumin absorption, good mucoadhesion and antioxidant activity of these hydrogel films are quit promising for use as wound dressing. The histological studies have revealed effective healing both in case of wounds treated with drug loaded and unloaded hydrogels. Overall, these antibiotic containing hydrogel wound dressings will not only enhance the wound curing process but also take care about the other aspects of wound health and skin regeneration.Acknowledgements
One of the author wishes to thank University Grant commission, New Delhi, India for providing financial assistance in the work. (Letter no. F.17-40/08(SA-I), dated 27 April, 2012). Authors also wish to thank Professor Sushma Sharma, Department of Zoology, Himachal Pradesh University, Shimla, for providing necessary assistance during this work.References
- G. Gethin, J. Wound Care, 2009, 18, S4–S8 Search PubMed
.
- F. Jacobsen, C. Fisahn, M. Sorkin, I. Thiele, T. Hirsch, I. Stricker, T. Klaassen, A. Roemer, B. Fugmann and L. Steinstraesser, Antimicrob. Agents Chemother., 2011, 55, 2325–2334 CrossRef CAS PubMed
.
- D. R. P. Guay, Ther. Clin. Risk Manage., 2006, 2, 417–434 CrossRef CAS
.
- A. Jones and D. Vaughan, J. Orthop. Nurs., 2005, 9, S1–S11 CrossRef
.
- S. L. Tomic, M. M. Micic, S. N. Dobic, J. M. Filipovic and E. H. Suljovrujic, Radiat. Phys. Chem., 2010, 79, 643–649 CrossRef CAS PubMed
.
- C. He, M. Wang, X. Cai, X. Huang, L. Li, H. Zhu, J. Shen and J. Yuan, Appl. Surf. Sci., 2011, 258, 755–760 CrossRef CAS PubMed
.
- J. Huang, E. Ten, G. Liu, M. Finzen, W. Yu, J. S. Lee, E. Saiz and A. P. Tomsia, Polymer, 2013, 54, 1197–1207 CrossRef CAS PubMed
.
- C. Radhakumary, A. M. Nandkumar and P. D. Nair, Carbohydr. Polym., 2011, 85, 439–445 CrossRef CAS PubMed
.
- S. Potorac, M. Popa, L. Verestiuc and D. Le Cerf, Mater. Lett., 2012, 67, 95–98 CrossRef CAS PubMed
.
- R. G. Marwah, M. O. Fatope, R. Al-Mahrooqi, G. B. Varma, H. Al-Abadi and S. K. S. Al-Burtamani, Food Chem., 2007, 101, 465–470 CrossRef CAS PubMed
.
- I. Süntar, E. K. Akkol, L. Nahar and S. D. Sarker, Free Radicals Antioxid., 2012, 2, 1–7 CrossRef
.
- P. R. Sarika, K. Cinthya, A. Jayakrishnan, P. R. Anilkumar and N. R. James, Mater. Sci. Eng., C, 2014, 43, 272–279 CrossRef CAS PubMed
.
- R. Y. Tsai, T. Y. Kuo, S. C. Hung, C. M. Lin, T. Y. Hsien, D. M. Wang and H. J. Hsieh, Carbohydr. Polym., 2015, 115, 525–532 CrossRef CAS PubMed
.
- J. C. Cuggino, C. B. Contreras, A. Jimenez-Kairuz, B. A. Maletto and C. I. A. Igarzabal, Mol. Pharmaceutics, 2014, 11, 2239–2249 CrossRef CAS PubMed
.
- B. Das, A. K. Nayak and U. Nanda, Int. J. Biol. Macromol., 2013, 62, 514–517 CrossRef CAS PubMed
.
- J. W. Yoo, K. Dharmala and C. H. Lee, Int. J. Pharm., 2006, 309, 139–145 CrossRef CAS PubMed
.
- B. Singh and N. Sharma, Biomacromolecules, 2009, 10, 2515–2532 CrossRef CAS PubMed
.
- P. L. Ritger and N. A. Peppas, J. Controlled Release, 1987, 5, 23–36 CrossRef CAS
.
- P. L. Ritger and N. A. Peppas, J. Controlled Release, 1987, 5, 37–42 CrossRef CAS
.
- L. Varshney, Nucl. Instrum. Methods Phys. Res., Sect. B, 2007, 255, 343–349 CrossRef CAS PubMed
.
- N. V. S. Madhav and A. P. Yadav, Acta Pharm. Sin. B, 2013, 3, 408–415 CrossRef PubMed
.
- S. Wittaya-areekul and C. Prahsarn, Int. J. Pharm., 2006, 313, 123–128 CrossRef CAS PubMed
.
- L. W. Winkler, Ber. Dtsch. Chem. Ges., 1888, 21, 2843–2855 CrossRef PubMed
.
- H. N. M. Chambi and C. R. F. Grosso, Cienc. Tecnol. Aliment., 2011, 31, 739–746 CrossRef PubMed
.
- K. S. C. R. dos Santos, J. F. J. Coelho, P. Ferreira, I. Pinto, S. G. Lorenzetti, E. I. Ferreira, O. Z. Higa and M. H. Gil, Int. J. Pharm., 2006, 310, 37–45 CrossRef CAS PubMed
.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, J. Biol. Chem., 1951, 193, 265–275 CAS
.
- G. Gorczyca, R. Tylingo, P. Szweda, E. Augustin, M. Sadowska and S. Milewski, Carbohydr. Polym., 2014, 102, 901–911 CrossRef CAS PubMed
.
- M. Curcio, F. Puoci, F. Iemma, O. I. Parisi, G. Cirillo, U. G. Spizzirri and N. Picci, J. Agric. Food Chem., 2009, 57, 5933–5938 CrossRef CAS PubMed
.
- C. Beauchamp and I. Fridovich, Anal. Biochem., 1971, 44, 276–287 CrossRef CAS
.
- I. Gulcin, Z. Huyut, M. Elmastas and H. Y. Aboul-Enein, Arabian J. Chem., 2010, 3, 43–53 CrossRef CAS PubMed
.
- G. M. Keegan, J. D. Smart, M. J. Ingram, L. M. Barnes, G. R. Burnett and G. D. Rees, J. Dent., 2012, 40, 229–240 CrossRef CAS PubMed
.
- L. T. Ng and S. Swami, Carbohydr. Polym., 2005, 60, 523–528 CrossRef CAS PubMed
.
- K. A. Juby, C. Dwivedi, M. Kumar, S. Kota, H. S. Misra and P. N. Bajaj, Carbohydr. Polym., 2012, 89, 906–913 CrossRef CAS PubMed
.
- H. N. Cheng and T. G. Neiss, Polym. Rev., 2012, 52, 81–114 CrossRef CAS PubMed
.
- K. Fujita and N. Nishiyama, J. Dent., 2006, 34, 123–133 CrossRef CAS PubMed
.
- S. Tanodekaew, M. Prasitsilp, S. Swasdison, B. Thavornyutikarn, T. Pothsree and R. Pateepasen, Biomaterials, 2004, 25, 1453–1460 CrossRef CAS PubMed
.
- A. Tiwari and V. Singh, Carbohydr. Polym., 2008, 74, 427–434 CrossRef CAS PubMed
.
- M. H. Kwak, J. E. Kim, J. Go, E. K. Koh, S. H. Song, H. J. Son, H. S. Kim, Y. H. Yun, Y. J. Jung and D. Y. Hwang, Carbohydr. Polym., 2015, 122, 387–398 CrossRef CAS PubMed
.
- G. G. Pereira, R. Santos-Oliveira, M. S. Albernaz, D. Canema, G. Weismüller, E. B. Barros, L. Magalhães, M. H. Lima-Ribeiro, A. R. Pohlmann and S. S. Guterres, Eur. J. Pharm. Biopharm., 2014, 86, 292–300 CrossRef CAS PubMed
.
- X. Hu, L. Feng, W. Wei, A. Xie, S. Wang, J. Zhang and W. Dong, Carbohydr. Polym., 2014, 105, 135–144 CrossRef CAS PubMed
.
- L. A. Kanis, F. C. Viel, J. S. Crespo, J. R. Bertolino, A. T. N. Pires and V. Soldi, Polymer, 2000, 41, 3303–3309 CrossRef
.
- J. S. Boateng, H. V. Pawar and J. Tetteh, Int. J. Pharm., 2013, 441, 181–191 CrossRef CAS PubMed
.
- C. Li, W. Zhu, H. Xue, Z. Chen, Y. Chen and X. Wang, Food Hydrocolloids, 2015, 43, 322–328 CrossRef CAS PubMed
.
- S. Schreml, R. M. Szeimies, L. Prantl, S. Karrer, M. Landthaler and P. Babilas, Br. J. Dermatol., 2010, 163, 257–268 CrossRef CAS PubMed
.
- C. Li, W. Zhu, H. Xue, Z. Chen, Y. Chen and X. Wang, Food Hydrocolloids, 2015, 43, 322–328 CrossRef CAS PubMed
.
- A. Nangia and C. T. Hung, Burns, 1989, 15, 385–388 CrossRef CAS
.
- J. F. Jhong, A. Venault, C. C. Hou, S. H. Chen, T. C. Wei, J. Zheng, J. Huang and Y. Chang, ACS Appl. Mater. Interfaces, 2013, 5, 6732–6742 CAS
.
- M. Bhatnagar, L. Parwani, V. Sharma, J. Ganguly and A. Bhatnagar, Carbohydr. Polym., 2014, 99, 692–699 CrossRef CAS PubMed
.
- S. Nie, H. Qin, C. Cheng, W. Zhao, S. Sun, B. Su, C. Zhao and Z. Gu, J. Mater. Chem. B, 2014, 2, 4911–4921 RSC
.
- D. Draganescu, C. Ibanescu, B. I. Tamba, C. V. Andritoiu, G. Dodi and M. I. Popa, Int. J. Biol. Macromol., 2015, 72, 614–623 CrossRef CAS PubMed
.
- H. Maalej, D. Moalla, C. Boisset, S. Bardaa, H. B. Ayed, Z. Sahnoun, T. Rebai, M. Nasri and N. Hmidet, Colloids Surf., B, 2014, 123, 814–824 CrossRef CAS PubMed
.
- R. Moseley, M. Walker, R. J. Waddington and W. Y. Chen, Biomaterials, 2003, 24, 1549–1557 CrossRef CAS
.
- Y. Chen, M. Y. Xie, S. P. Nie, C. Li and Y. X. Wang, Food Chem., 2008, 107, 231–241 CrossRef CAS PubMed
.
- V. Kant, A. Gopal, N. N. Pathak, P. Kumar, S. K. Tandan and D. Kumar, Int. Immunopharmacol., 2014, 20, 322–330 CrossRef CAS PubMed
.
- I. Vīna, R. Linde, A. Patetko and P. Semjonovs, Int. J. Res. Rev. Appl. Sci., 2013, 14, 217–230 Search PubMed
.
- J. M. Lü, P. H. Lina, Q. Yao and C. Chen, J. Cell. Mol. Med., 2010, 14, 840–860 CrossRef PubMed
.
- D. Jain, E. Carvalho and R. Banerjee, Acta Biomater., 2010, 6, 1370–1379 CrossRef CAS PubMed
.
- S. Idos, R. P. Abranches, M. T. Garcia and M. B. Pierre, J. Photochem. Photobiol., B, 2014, 140, 266–275 CrossRef PubMed
.
- A. Sosnik, J. Neves and B. Sarmento, Prog. Polym. Sci., 2014, 39, 2030–2075 CrossRef CAS PubMed
.
- M. H. Langlois, M. Montagut, J. P. Dubost, J. Grelle and M. C. Saux, J. Pharm. Biomed. Anal., 2005, 37, 389–393 CrossRef CAS PubMed
.
- X. Yang, K. Yang, S. Wu, X. Chen, F. Yu, J. Li, M. Ma and Z. Zhu, Radiat. Phys. Chem., 2010, 79, 606–611 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2015 |