The first catalyst and solvent-free synthesis of 2-arylimidazo[2,1-b][1,3,4]thiadiazoles: a comparative assessment of greenness†
Preeti Wadhwa,
Tanpreet Kaur and
Anuj Sharma*
Department of Chemistry, Indian Institute of Technology Roorkee, Roorkee-247667, India. E-mail: anujs77@gmail.com; Tel: +91-1332-284751
First published on 8th May 2015
Abstract
A concise and efficient one pot synthesis of bicyclic imidazo[2,1-b][1,3,4]thiadiazoles via the three component Groebke–Blackburn–Bienaymé (GBB) reaction utilizing 5-aryl-1,3,4-thiadiazol-2-amines, aromatic aldehydes and isonitriles has been developed. The reactions were carried out under microwave irradiation and ultimate green conditions excluding both catalysts and solvents. Furthermore, the “greenness” of the protocol was evaluated within the ambits of green metrics and the method exhibited an excellent score in the defined parameters such as atom economy, E-factor, reaction mass efficiency, process mass intensity and carbon efficiency. This environmentally benign GBB methodology paves easy access towards the synthesis of pharmacologically significant scaffolds.
Introduction
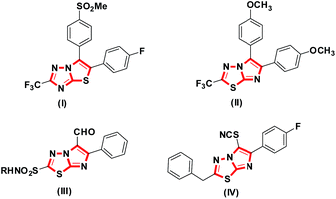
Fused heterocyclic structures over the last few decades have presented enormous opportunities in the fields of drug design and therapeutics.1,2 The bicyclic core of imidazo[2,1-b][1,3,4]thiadiazoles3 is one such scaffold widely manifested in an array of pharmacophoric activities4 such as antihyperlipidemic,5 antitubercular,6 antitumor7 and antimicrobial activities.8 Some representative members of this class are displayed in Fig. 1 e.g., 5,6-diarylsubstituted thiazolotriazole (I) acts as a COX-2 inhibitor,9 compound 2-trifluoromethyl-5,6-(4′,4′′-dimethoxyphenyl)imidazo [2,1-b][1,3,4]thiadiazole (II) as an antitubercular, 5-formyl-6-aryl-imidazo[2,1-b][1,3,4]thiadiazole sulphonamide derivatives (III) and 2-benzyl-6-(4′-fluorophenyl)-5-thiocyanato-imidazo[2,1-b][1,3,4]thiadiazole (IV) as potent anti-cancer agents.From a synthetic point of view, to date, the routes for the synthesis of imidazo[2,1-b][1,3,4]thiadiazoles are rather limited. In literature, this core was mainly synthesized by employing classical methods involving substituted 1,3,4-thiadiazol-2-amines and their reaction with a variety of reagents e.g. functionalized α-haloketones,10,11 α-haloacetic acid,12 chloroacetyl chloride,13 acetophenones14 and N,N-dimethylformamide dimethyl acetal.15 These classical methodologies possess several disadvantages, including the use of organic solvents, detrimental reagents, long reaction time, cumbersome work up procedures and moderate yields. Moreover, these methodologies are not well suited for a diversity oriented synthesis of imidazo[2,1-b][1,3,4]thiadiazole scaffolds.
In the past decade, Microwave-Assisted Organic Synthesis (MAOS) has gained prominence, particularly in developing sustainable catalyst-free and solvent-free versions of tedious reactions (CFR & SFR). Moreover and quite frequently, these approaches result in pollutant reduction, drastic reduction in reaction times, and formation of relatively pure products thereby avoiding tedious column chromatography. Hence, it represents a powerful green alternative to conventional synthesis.16
In this context, microwave-assisted multi-component Groebke–Blackburn–Bienaymé (GBB)17 can be a promising approach towards synthesis of imidazo[2,1-b][1,3,4]thiadiazoles. With this, we had hoped to harvest some of the benefits associated with MAOS as stated above.
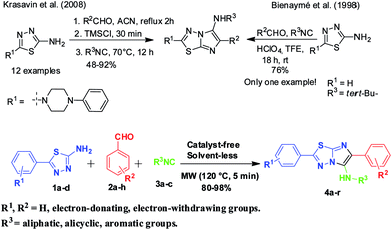
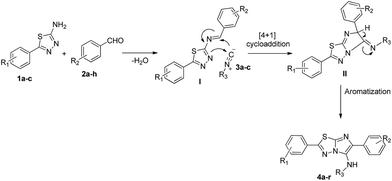
Surprisingly, there are only couple of reports for syntheses of imidazo[2,1-b][1,3,4]thiadiazoles through GBB route. First in the seminal report by Bienaymé et al.,18 where a single example of N-tert-butyl-6-phenyl-5,6-dihydroimidazo[2,1-b][1,3,4]thiadiazol-5-amine was generated using perchloric acid as catalyst in trifluoroethanol, and second by Krasavin et al.,19 where 5-piperazin-1-yl-1,3,4-thiadiazol-2-amines were synthesized using trimethylsilyl chloride as a promoter in acetonitrile (Scheme 1). Hence there is a sufficient scope for improvement of this reaction in terms of scope, reactions conditions and environment friendliness.
In continuation with our constant quest aimed at utilizing known isocyanide-based MCRs for creating libraries in a combinatorial fashion,20 we herein propose a straightforward, catalyst-free and solvent-less method that can easily access diversity-oriented imidazo[2,1-b][1,3,4]thiadiazoles via successive cyclisation of 5-aryl-1,3,4-thiadiazol-2-amines, aromatic aldehydes, and isonitriles under microwave irradiation (Scheme 1).
Moreover, most of the products were crystallized out from the crude reaction mixture in high yields and purity.
Results and discussion
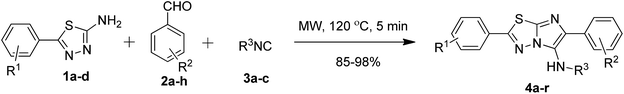
In a pursuit to develop a catalyst and solvent-free approach for the synthesis of 2-arylimidazo[2,1-b][1,3,4]thiadiazoles (Scheme 2). | ||
| Scheme 2 A microwave assisted protocol for the synthesis of 2-arylimidazo[2,1-b][1,3,4]thiadiazoles. | ||
We started our investigation with the optimization of three-component GBB reaction using 5-phenyl-1,3,4-thiadiazol-2-amines 1a (0.25 mmol), benzaldehyde 2a (0.27 mmol) and N-tert-butyl isonitrile 3a (0.30 mmol) as model substrates (Table 1) under microwave irradiation. Initially, the reaction was carried out at a mild temperature of 40 °C for 30 minutes in a sealed vial excluding solvent and catalyst (Table 1, entry 1). The reaction did not proceed at all and the starting materials remained completely unconsumed. However, by increasing the temperature to 80 °C, the desired product 4a was formed in moderate yields (61%) with still some unreacted starting material.
| Entry | Temperatureb (°C) | Time (min) | Yieldc (%) |
|---|---|---|---|
| a General condition: 5-phenyl-1,3,4-thiadiazol-2-amines 1a (0.25 mmol); benzaldehyde 2a (0.27 mmol); N-tert-butyl isonitrile 3a (0.3 mmol).b Anton Paar Monowave 300 reactor. Irradiation power: 850 W; ramp time: 1 min. 60 °C.c Isolated yield by recrystallization.d No reaction. | |||
| 1 | 40 | 10 | nrd |
| 2 | 80 | 10 | 61 |
| 3 | 100 | 10 | 73 |
| 4 | 120 | 10 | 98 |
| 5 | 140 | 10 | 85 |
| 6 | 160 | 10 | 87 |
| 7 | 120 | 5 | 98 |
| 8 | 120 | 2 | 90 |
Next, the reaction was conducted at various temperatures viz. 120 °C, 140 °C and 160 °C for 10 min, furnishing the products in 98%, 85% and 87% yields, respectively (Table 1, entries 4–6). Furthermore, to reduce the reaction time, the reaction mixture was irradiated at 5 and 2 minutes interval which furnished the product in 98% and 90% yield, respectively (Table 1, entries 7 and 8). Following this examination, the best result was obtained at 120 °C, which yielded the product 4a in 98% yield under 5 minutes of microwave irradiation (Table 1, entry 7). It is particularly advantageous; since the reaction is selective and quantitative, the product form exclusively and no column purification is required in most of the cases. The optimization of reaction yield versus the reaction temperature is mentioned in Fig. 2.
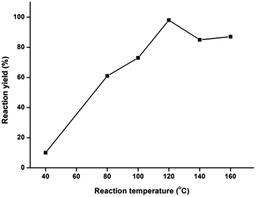 | ||
| Fig. 2 Reaction temperature vs. reaction yield (%) for the synthesis of N-tert-butyl-2,6-diphenylimidazo[2,1-b][1,3,4]thiadiazol-5-amine 4a. | ||
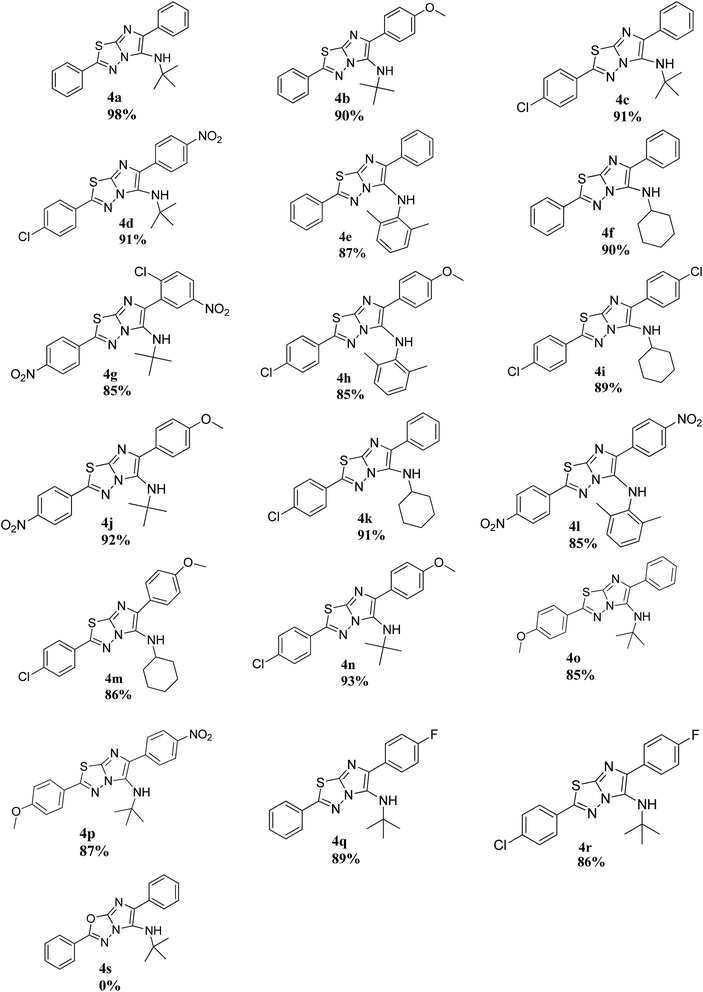
After corroborating the feasibility of the reaction, the scope and robustness of this one-pot three-component domino 2-arylimidazo[2,1-b][1,3,4]thiadiazoles synthesis was evaluated by employing a diverse range of 5-aryl-1,3,4-thiadiazol-2-amines, aldehydes and isocyanides (Table 2). To see the effect of heteroatom on the amine functionality towards the reaction efficiency, we attempted the reaction with 5-aryl-1,3,4-oxadiazol-2-amine and observed that it failed to provide the respective product 4s (0%) and only starting material was recovered.
The electronic nature of the 5-substituted-1,3,4-thiadiazol-2-amines and aldehydes had an impact on the reaction efficiency. It was found that a variety of 5-aryl-1,3,4-thiadiazol-2-amines derived from benzoic acid containing both electron-donating and electron-withdrawing groups on the phenyl ring were employed and were well tolerated under the optimal reaction conditions. 5-Aryl-1,3,4-thiadiazol-2-amines having electron-donating groups e.g., methoxy 4o (85%) & 4p (87%) and chloro 4c (91%) & 4d (91%), etc. resulted in good yields of the product. On the contrary, electron-withdrawing groups on the amine functionality e.g., nitro 4g (85%) & 4l (85%) yielded slightly lower yields.
Diverse aromatic aldehydes (electron-donating as well as electron-withdrawing) were also well accommodated. It is worth mentioning that aromatic aldehydes with an electron-withdrawing group viz. nitro 4d (91%), 4p (87%) and fluoro 4q (89%) & 4r (86%) etc. resulted in good yields of the product as expected. However, aromatic aldehydes bearing electron-donating substituents e.g. methoxy 4b (90%), 4h (85%) etc. also well accepted. Therefore, the present methodology works well with both activated and deactivated systems. It is worth mentioning that deactivating systems containing e.g. nitro & fluoro also gave excellent yields without adding any catalyst or promoters as utilized in Krasavin et al. method19 and in this context, it scores over it.
Similarly, the reaction seemed well tolerant to a range of isocyanides e.g. aliphatic (N-tert-butyl), alicyclic (cyclohexyl) and aromatic (2,6-dimethylphenyl) isocyanides. From the results, it seemed that the reaction worked efficiently and smoothly with N-tert-butyl and 2,6-dimethylphenyl isocyanides resulting in crystallization of the pure products. However, reactions employing cyclohexyl isocyanide 4f (90%), 4i (89%), 4k (91%) & 4m (86%) required column chromatography to isolate the pure products, albeit in excellent yields.
The structure of all the newly synthesized imidazo[2,1-b][1,3,4]thiadiazoles were deduced by their satisfactory spectral data (1H, 13C NMR and HRMS). By taking the entire experimental outcome into consideration, a plausible mechanistic pathway for the synthesis of 2-arylimidazo[2,1-b][1,3,4]thiadiazoles is outlined in Scheme 3. The first step is believed to be the imine (I) formation by the reaction of 5-aryl-1,3,4-thiadiazol-2-amines 1a–d and aldehyde 2a–h, followed by trapping of the imine carbon by isocyanides 3a–c through [4 + 1] cycloaddition, yields nitrilium ion intermediate (II), which upon aromatization forms 2-arylimidazo[2,1-b][1,3,4]thiadiazoles (4a–r).
From the standpoint of green chemistry, it would be imperative to evaluate our chemical process as environmentally benign, for which quantification of sustainable practices such as measuring the “greenness” of our method would be essential.21–24 Several green metrics such as Atom Economy (AE), E-factor, Process Mass Intensity (PMI), Reaction Mass Efficiency (RME) and Carbon Efficiency (CE) has been developed which enables us to evaluate chemical processes in terms of waste, energy usage and carbon efficiency. Table 3 provides the calculation of the illustrative metrics for all the synthesized compounds.
| S. no. | Yield (%) | %AE | %CE | E-factor | %RME | PMI |
|---|---|---|---|---|---|---|
| 4a | 98 | 95.08 | 89.65 | 0.153 | 86.05 | 1.153 |
| 4b | 90 | 95.45 | 82.85 | 0.252 | 79.74 | 1.252 |
| 4c | 91 | 95.50 | 83.83 | 0.228 | 81.39 | 1.228 |
| 4d | 91 | 95.96 | 82.91 | 0.240 | 80.73 | 1.240 |
| 4e | 87 | 95.65 | 78.61 | 0.308 | 76.43 | 1.308 |
| 4f | 90 | 90.35 | 82.15 | 0.261 | 79.32 | 1.261 |
| 4g | 85 | 96.33 | 78.34 | 0.308 | 76.40 | 1.308 |
| 4h | 85 | 96.24 | 76.99 | 0.324 | 75.51 | 1.324 |
| 4i | 89 | 96.09 | 83.98 | 0.219 | 82.02 | 1.219 |
| 4j | 92 | 95.92 | 84.70 | 0.209 | 82.66 | 1.209 |
| 4k | 91 | 95.78 | 83.07 | 0.235 | 81.01 | 1.235 |
| 4l | 85 | 96.43 | 76.80 | 0.316 | 75.76 | 1.316 |
| 4m | 86 | 96.05 | 78.49 | 0.305 | 76.05 | 1.305 |
| 4n | 93 | 95.82 | 85.47 | 0.201 | 83.26 | 1.201 |
| 4o | 85 | 95.45 | 78.63 | 0.317 | 76.31 | 1.317 |
| 4p | 87 | 95.92 | 80.48 | 0.284 | 77.85 | 1.284 |
| 4q | 89 | 95.39 | 81.91 | 0.268 | 78.84 | 1.268 |
| 4r | 86 | 95.72 | 79.26 | 0.299 | 76.98 | 1.299 |
The higher environmental compatibility factors such as smaller E-factor, higher atom economy make the present methodology an ideal green and sustainable process. Furthermore, we evaluated the greenness of our protocol with respect to existing literature reports for the synthesis of fused imidazo[2,1-b][1,3,4]thiadiazoles (Fig. 3), the results are collected and tabulated in Table 4. These results corroborates that our protocol for synthesis of imidazo[2,1-b][1,3,4]thiadiazoles has a greener profile over published traditional procedures when identical chemistries of reactions are compared. The detailed calculations are explained in the ESI.†
 | ||
| Fig. 3 E-factor and PMI profiles showing individual contributions for the synthesis of fused imidazo[2,1-b][1,3,4]thiadiazoles. | ||
In summary, we report a simple and straightforward one-pot three-component catalyst and solvent free integrated protocol for the synthesis of imidazo[2,1-b][1,3,4]thiadiazoles in good yields under microwave irradiation. This convergent and versatile method presents broad substrate scope and excellent functionality tolerance. This approach enables the rapid assembly of diverse imidazo[2,1-b][1,3,4]thiadiazoles based frameworks utilizing all the three components efficiently. More importantly, the methodology works well for both activating and deactivating starting material. This methodology therefore exemplifies the reconciliation of structural complexity and operational simplicity in an environmentally benign manner. In all the cases, but a few ones, the isolation and purification of compounds were done by mere filtration and ethanol washing which makes the process tailor made for automation in a high throughput synthetic platform. Furthermore, a variety of green metrics have been explored and our method exemplary fit in this grid.
Experimental section
General procedure for the synthesis of imidazo[2,1-b][1,3,4]thiadiazole fused heterocycles (4a–r)
5-Aryl-1,3,4-thiadiazol-2-amines 1a (0.25 mmol), aromatic aldehyde 2a (0.27 mmol) and isocyanide 3a (0.3 mmol) were taken in G-10 glass vial capped with Teflon septum. After a pre-stirring of 1 or 2 minutes at RT, the vial was subjected to microwave irradiation with the initial ramp time of 1 minute at 60 °C. The temperature was then raised to 120 °C with the holding time of 5 minutes. The products were recrystallized using EtOH. Some of the viscous products (4f, 4i, 4k, 4m) were purified by silica gel column chromatography (ethyl acetate/hexane). All compounds were fully characterized by Mp, IR, NMR, mass spectral data.N-tert-Butyl-2,6-diphenylimidazo[2,1-b][1,3,4]thiazol-5 amine (4a)
Yellow solid (98%), Mp 174–176 °C, IR (4000–600 cm−1): νmax = 3431 (stretch NH), 1642 (stretch C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1559 (bend NH), 1412, 1339 (stretch C–N). 1H NMR (CDCl3, 400 MHz): δH (ppm) 8.10–8.15 (m, 2H), 7.85–7.92 (m, 2H), 7.47–7.55 (m, 3H), 7.40 (m, 2H), 7.22–7.28 (m, 1H), 3.16 (brs, NH), 1.20 (s, 9H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 167.1, 160.5, 140.8, 138.4, 135.1, 133.6, 131.5, 130.7, 130.4, 129.3, 128.2, 127.7, 126.9, 56.3, 30.3. HR-MS (ESI) for C20H20N4S m/z calcd: 349.1481; found: 349.1516 [M + H]+.
N), 1559 (bend NH), 1412, 1339 (stretch C–N). 1H NMR (CDCl3, 400 MHz): δH (ppm) 8.10–8.15 (m, 2H), 7.85–7.92 (m, 2H), 7.47–7.55 (m, 3H), 7.40 (m, 2H), 7.22–7.28 (m, 1H), 3.16 (brs, NH), 1.20 (s, 9H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 167.1, 160.5, 140.8, 138.4, 135.1, 133.6, 131.5, 130.7, 130.4, 129.3, 128.2, 127.7, 126.9, 56.3, 30.3. HR-MS (ESI) for C20H20N4S m/z calcd: 349.1481; found: 349.1516 [M + H]+.
N-tert-Butyl-6(4-methoxyphenyl)-2-phenylimidazo[2,1-b][1,3,4]thiazol-5-amine (4b)
Yellow solid (90%), Mp 212–213 °C, IR (4000–600 cm−1): νmax = 3411 (stretch NH), 1645 (stretch C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1562 (bend NH), 1509, 1406, 1339 (stretch C–N). 1H NMR (CDCl3, 500 MHz): δH (ppm) 8.06 (d, 2H, J = 8.5 Hz), 7.83–7.91 (m, 2H), 7.46–7.54 (m, 3H), 6.94 (d, 2H, J = 8 Hz), 3.85 (s, 3H), 3.10 (brs, 1H), 2.17 (brs, enaminic proton), 1.19 (s, 9H). 13C NMR (CDCl3, 125 MHz): δC (ppm) 160.0, 158.5, 140.6, 138.3, 131.4, 130.7, 129.2, 128.1, 127.8, 126.7, 125.8, 113.6, 56.1, 55.2, 30.3. HR-MS (ESI) for C21H22N4OS m/z calcd: 379.1587; found: 379.1580 [M + H]+.
N), 1562 (bend NH), 1509, 1406, 1339 (stretch C–N). 1H NMR (CDCl3, 500 MHz): δH (ppm) 8.06 (d, 2H, J = 8.5 Hz), 7.83–7.91 (m, 2H), 7.46–7.54 (m, 3H), 6.94 (d, 2H, J = 8 Hz), 3.85 (s, 3H), 3.10 (brs, 1H), 2.17 (brs, enaminic proton), 1.19 (s, 9H). 13C NMR (CDCl3, 125 MHz): δC (ppm) 160.0, 158.5, 140.6, 138.3, 131.4, 130.7, 129.2, 128.1, 127.8, 126.7, 125.8, 113.6, 56.1, 55.2, 30.3. HR-MS (ESI) for C21H22N4OS m/z calcd: 379.1587; found: 379.1580 [M + H]+.
N-tert-Butyl-2-(4-chlorophenyl)-6-phenylimidazo[2,1-b][1,3,4]thiazol-5-amine (4c)
Yellow solid (91%), Mp 219–221 °C, IR (4000–600 cm−1): νmax = 3437 (stretch NH), 1639 (stretch C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1559 (bend NH), 1409, 1336 (stretch C–N). 1H NMR (CDCl3, 500 MHz): δH (ppm) 8.11 (d, 2H, J = 10 Hz), 7.82 (d, 2H, J = 10 Hz), 7.48 (d, 2H, J = 10 Hz), 7.40 (t, 2H, J = 10 Hz), 7.24–7.29 (m, 1H), 3.15 (brs, NH), 2.17 (brs, enaminic proton), 1.19 (s, 9H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 159.2, 138.7, 137.7, 135.0, 129.6, 129.1, 128.3, 128.0, 127.0, 126.9, 126.81, 126.80, 56.4, 30.3. HR-MS (ESI) for C20H19ClN4S m/z calcd: 383.1091; found: 383.1125 [M + H]+.
N), 1559 (bend NH), 1409, 1336 (stretch C–N). 1H NMR (CDCl3, 500 MHz): δH (ppm) 8.11 (d, 2H, J = 10 Hz), 7.82 (d, 2H, J = 10 Hz), 7.48 (d, 2H, J = 10 Hz), 7.40 (t, 2H, J = 10 Hz), 7.24–7.29 (m, 1H), 3.15 (brs, NH), 2.17 (brs, enaminic proton), 1.19 (s, 9H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 159.2, 138.7, 137.7, 135.0, 129.6, 129.1, 128.3, 128.0, 127.0, 126.9, 126.81, 126.80, 56.4, 30.3. HR-MS (ESI) for C20H19ClN4S m/z calcd: 383.1091; found: 383.1125 [M + H]+.
N-tert-Butyl-2(4-chlorophenyl)-6-(4-nitrophenyl)imidazo[2,1-b][1,3,4]thiazol-5-amine (4d)
Yellow solid (90%), Mp 221–222 °C, IR (4000–600 cm−1): νmax = 3434 (stretch NH), 2952 (sp2-CH), 2919 (sp3-CH), 1592 (stretch C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1509 (asymm. stretch NO2), 1468 (symm. stretch NO2), 1327 (stretch C–N). 1H NMR (CDCl3, 400 MHz): δH (ppm) 8.42 (d, 2H, J = 12 Hz), 8.24 (d, 2H, J = 8 Hz), 7.82 (d, 2H, J = 8 Hz), 7.51 (d, 2H, J = 12 Hz), 3.15 (brs, NH), 1.10 (s, 9H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 160.4, 160.4, 146.2, 141.6, 141.6, 138.2, 129.8, 128.7, 128.1, 127.0, 123.7, 57.0, 30.4. HR-MS (ESI) for C20H18ClN5O2S m/z calcd: 450.0761; found: 450.0750 [M + Na]+.
N), 1509 (asymm. stretch NO2), 1468 (symm. stretch NO2), 1327 (stretch C–N). 1H NMR (CDCl3, 400 MHz): δH (ppm) 8.42 (d, 2H, J = 12 Hz), 8.24 (d, 2H, J = 8 Hz), 7.82 (d, 2H, J = 8 Hz), 7.51 (d, 2H, J = 12 Hz), 3.15 (brs, NH), 1.10 (s, 9H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 160.4, 160.4, 146.2, 141.6, 141.6, 138.2, 129.8, 128.7, 128.1, 127.0, 123.7, 57.0, 30.4. HR-MS (ESI) for C20H18ClN5O2S m/z calcd: 450.0761; found: 450.0750 [M + Na]+.
N-(2,6-Dimethylphenyl)-2,6-diphenylimidazo[2,1-b][1,3,4]thiazol-5-amine (4e)
Yellow solid (87%), Mp 194–195 °C, IR (4000–600 cm−1): νmax = 3417 (stretch NH), 1642 (stretch C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1559 (bend NH), 1409 (stretch C–N). 1H NMR (CDCl3, 500 MHz): δH (ppm) 8.02 (d, 2H, J = 8 Hz), 7.69 (d, 2H, J = 7 Hz), 7.41–7.51 (m, 3H), 7.36 (t, 2H, J = 7.5 Hz), 7.22–7.27 (m, 1H), 6.99 (d, 2H, J = 7.5 Hz), 6.853 (t, 1H, J = 7.5 Hz), 5.29 (brs, NH), 2.17 (s, 6H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 161.0, 140.7, 140.5, 136.2, 133.9, 131.5, 130.3, 129.1, 129.1, 128.3, 128.1, 126.9, 126.7, 126.1, 124.7, 122.1, 18.6. HR-MS (ESI) for C24H20N4S m/z calcd: 397.1481; found: 397.1473 [M + H]+.
N), 1559 (bend NH), 1409 (stretch C–N). 1H NMR (CDCl3, 500 MHz): δH (ppm) 8.02 (d, 2H, J = 8 Hz), 7.69 (d, 2H, J = 7 Hz), 7.41–7.51 (m, 3H), 7.36 (t, 2H, J = 7.5 Hz), 7.22–7.27 (m, 1H), 6.99 (d, 2H, J = 7.5 Hz), 6.853 (t, 1H, J = 7.5 Hz), 5.29 (brs, NH), 2.17 (s, 6H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 161.0, 140.7, 140.5, 136.2, 133.9, 131.5, 130.3, 129.1, 129.1, 128.3, 128.1, 126.9, 126.7, 126.1, 124.7, 122.1, 18.6. HR-MS (ESI) for C24H20N4S m/z calcd: 397.1481; found: 397.1473 [M + H]+.
N-Cyclohexyl-2,6-diphenylimidazo[2,1-b][1,3,4]thiazol-5-amine (4f)
Yellow solid (90%), Mp 211–213 °C, IR (4000–600 cm−1): νmax = 3437 (stretch NH), 1639 (stretch C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1556 (bend NH), 1412, 1339 (stretch C–N). 1H NMR (CDCl3, 500 MHz): δH (ppm) 8.04 (d, 2H, J = 8 Hz), 7.86–7.92 (m, 2H), 7.48–7.54 (m, 3H), 7.42 (t, 2H, J = 8 Hz), 7.24–7.28 (m, 1H) (merge with CDCl3 region (7.260)), 3.24–3.40 (m, 2H), 1.92–2.05 (m, 2H), 1.68–1.78 (m, 2H), 1.58–1.62 (m, 1H), 1.15–1.36 (m, 5H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 160.9, 160.8, 134.8, 134.0, 131.6, 130.7, 129.3, 128.7, 128.6, 126.9, 126.6, 125.9, 56.8, 34.2, 25.9, 25.0. HR-MS (ESI) for C22H22N4S m/z calcd: 375.1637; found: 375.1648 [M + H]+.
N), 1556 (bend NH), 1412, 1339 (stretch C–N). 1H NMR (CDCl3, 500 MHz): δH (ppm) 8.04 (d, 2H, J = 8 Hz), 7.86–7.92 (m, 2H), 7.48–7.54 (m, 3H), 7.42 (t, 2H, J = 8 Hz), 7.24–7.28 (m, 1H) (merge with CDCl3 region (7.260)), 3.24–3.40 (m, 2H), 1.92–2.05 (m, 2H), 1.68–1.78 (m, 2H), 1.58–1.62 (m, 1H), 1.15–1.36 (m, 5H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 160.9, 160.8, 134.8, 134.0, 131.6, 130.7, 129.3, 128.7, 128.6, 126.9, 126.6, 125.9, 56.8, 34.2, 25.9, 25.0. HR-MS (ESI) for C22H22N4S m/z calcd: 375.1637; found: 375.1648 [M + H]+.
N-tert-Butyl-6-(2-chloro-5-nitrophenyl)-2-(4-nitrophenyl)imidazo[2,1-b][1,3,4]thiadiazol-5-amine (4g)
Orange solid (85%), Mp 210–211 °C, IR (4000–600 cm−1): νmax = 3428 (stretch NH), 1642 (stretch C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1559 (bend NH), 1412 (asymm. Stretch NO2), 1341 (symm. stretch NO2). 1H NMR (CDCl3, 400 MHz): δH (ppm) 8.55 (d, 1H, J = 2.4 Hz), 8.39 (dt, 2H, J = 8 Hz, J = 4 Hz), 8.17 (dd, 1H, J = 8 Hz, J = 4 Hz), 8.11 (dt, 2H, J = 8 Hz, J = 4 Hz), 7.65 (d, 1H, J = 8.8 Hz), 3.28 (brs, NH), 2.17 (brs, enaminic proton), 1.06 (s, 9H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 158.9, 149.6, 139.6, 135.9, 135.5, 135.4, 135.3, 131.0, 129.2, 127.8, 127.6, 124.7, 123.7, 56.0, 30.0. HR-MS (ESI) for C20H17ClN6O4S m/z calcd: 495.0612; found: 473.0590 [M + Na]+.
N), 1559 (bend NH), 1412 (asymm. Stretch NO2), 1341 (symm. stretch NO2). 1H NMR (CDCl3, 400 MHz): δH (ppm) 8.55 (d, 1H, J = 2.4 Hz), 8.39 (dt, 2H, J = 8 Hz, J = 4 Hz), 8.17 (dd, 1H, J = 8 Hz, J = 4 Hz), 8.11 (dt, 2H, J = 8 Hz, J = 4 Hz), 7.65 (d, 1H, J = 8.8 Hz), 3.28 (brs, NH), 2.17 (brs, enaminic proton), 1.06 (s, 9H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 158.9, 149.6, 139.6, 135.9, 135.5, 135.4, 135.3, 131.0, 129.2, 127.8, 127.6, 124.7, 123.7, 56.0, 30.0. HR-MS (ESI) for C20H17ClN6O4S m/z calcd: 495.0612; found: 473.0590 [M + Na]+.
2-(4-Chlorophenyl)-N-(2,6-dimethylphenyl)-6-(4-methoxy phenyl)imidazo[2,1-b][1,3,4]thiazol-5-amine (4h)
Yellow solid (85%), Mp 197–198 °C, IR (4000–600 cm−1): νmax = 3414 (stretch NH), 1642 (stretch C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1559 (bend NH), 1412, 1339 (stretch C–N). 1H NMR (CDCl3, 400 MHz): δH (ppm) 7.96 (d, 2H, J = 8 Hz), 7.62 (dt, 2H, J = 8 Hz, J = 4 Hz), 7.41 (dt, 2H, J = 8 Hz, J = 4 Hz), 6.99 (d, 2H, J = 8 Hz), 6.90 (d, 2H, J = 8 Hz), 6.84 (t, 1H, J = 8 Hz), 5.24 (brs, NH), 3.82 (s, 3H), 2.15 (s, 6H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 158.9, 137.7, 129.5, 129.3, 129.0, 127.9, 127.9, 127.9, 127.5, 122.1, 113.9, 55.3, 18.7. HR-MS (ESI) for C25H21ClN4OS m/z calcd: 461.1197; found: 461.1210 [M + H]+.
N), 1559 (bend NH), 1412, 1339 (stretch C–N). 1H NMR (CDCl3, 400 MHz): δH (ppm) 7.96 (d, 2H, J = 8 Hz), 7.62 (dt, 2H, J = 8 Hz, J = 4 Hz), 7.41 (dt, 2H, J = 8 Hz, J = 4 Hz), 6.99 (d, 2H, J = 8 Hz), 6.90 (d, 2H, J = 8 Hz), 6.84 (t, 1H, J = 8 Hz), 5.24 (brs, NH), 3.82 (s, 3H), 2.15 (s, 6H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 158.9, 137.7, 129.5, 129.3, 129.0, 127.9, 127.9, 127.9, 127.5, 122.1, 113.9, 55.3, 18.7. HR-MS (ESI) for C25H21ClN4OS m/z calcd: 461.1197; found: 461.1210 [M + H]+.
2,6-Bis(4-chlorophenyl)-N-cyclohexylimidazo[2,1-b][1,3,4]thiadiazol-5-amine (4i)
Yellow solid (92%), Mp 223–224 °C, IR (4000–600 cm−1): νmax = 3437 (stretch NH), 1639 (stretch C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1559 (bend NH), 1409, 1336 (stretch C–N). 1H NMR (CDCl3, 500 MHz): δH (ppm) 8.02 (d, 2H, J = 8.5 Hz), 7.82 (d, 2H, J = 8 Hz), 7.49 (d, 2H, J = 8.5 Hz), 7.37 (d, 2H, J = 8.5 Hz), 3.15–3.30 (m, 2H), 1.87–2.00 (m, 2H), 1.68–1.80 (m, 2H), 1.50–1.68 (m, 1H), 1.13–1.40 (m, 5H). 13C NMR (CDCl3, 125 MHz): δC (ppm) 159.7, 139.7, 137.7, 133.6, 133.1, 132.2, 126.6, 128.9, 128.6, 128.0, 127.1, 56.8, 33.9, 25.8, 24.8. HR-MS (ESI) for C22H20Cl2N4S m/z calcd: 465.0677; found: 465.0653 [M + Na]+.
N), 1559 (bend NH), 1409, 1336 (stretch C–N). 1H NMR (CDCl3, 500 MHz): δH (ppm) 8.02 (d, 2H, J = 8.5 Hz), 7.82 (d, 2H, J = 8 Hz), 7.49 (d, 2H, J = 8.5 Hz), 7.37 (d, 2H, J = 8.5 Hz), 3.15–3.30 (m, 2H), 1.87–2.00 (m, 2H), 1.68–1.80 (m, 2H), 1.50–1.68 (m, 1H), 1.13–1.40 (m, 5H). 13C NMR (CDCl3, 125 MHz): δC (ppm) 159.7, 139.7, 137.7, 133.6, 133.1, 132.2, 126.6, 128.9, 128.6, 128.0, 127.1, 56.8, 33.9, 25.8, 24.8. HR-MS (ESI) for C22H20Cl2N4S m/z calcd: 465.0677; found: 465.0653 [M + Na]+.
N-tert-Butyl-6-(4-methoxyphenyl)-2-(4-nitrophenyl)imidazo[2,1-b][1,3,4]thiadiazol-5-amine (4j)
Red solid (92%), Mp 210–211 °C, IR (4000–600 cm−1): νmax = 3431 (stretch NH), 1645 (stretch C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1562 (asymm. stretch NO2), 1415 (symm. stretch NO2), 1338 (stretch C–N). 1H NMR (CDCl3, 400 MHz): δH (ppm) 8.34–8.38 (m, 2H), 8.03–8.08 (m, 4H), 6.92–6.97 (m, 2H), 3.85 (s, 3H), 3.11 (brs, NH), 2.17 (brs, 1H), 1.20 (s, 9H). 13C NMR (CDCl3, 125 MHz): δC (ppm) 158.9, 157.2, 149.2, 140.6, 139.4, 136.4, 128.2, 127.5, 127.3, 126.1, 124.6, 113.7, 56.3, 55.3, 30.4. HR-MS (ESI) for C21H21N5O3S m/z calcd: 424.1438; found: 424.1463 [M + H]+.
N), 1562 (asymm. stretch NO2), 1415 (symm. stretch NO2), 1338 (stretch C–N). 1H NMR (CDCl3, 400 MHz): δH (ppm) 8.34–8.38 (m, 2H), 8.03–8.08 (m, 4H), 6.92–6.97 (m, 2H), 3.85 (s, 3H), 3.11 (brs, NH), 2.17 (brs, 1H), 1.20 (s, 9H). 13C NMR (CDCl3, 125 MHz): δC (ppm) 158.9, 157.2, 149.2, 140.6, 139.4, 136.4, 128.2, 127.5, 127.3, 126.1, 124.6, 113.7, 56.3, 55.3, 30.4. HR-MS (ESI) for C21H21N5O3S m/z calcd: 424.1438; found: 424.1463 [M + H]+.
2-(4-Chlorophenyl)-N-cyclohexyl-6-phenylimidazo[2,1-b][1,3,4]thiazol-5-amine (4k)
Yellow solid (91%), Mp 201–202 °C, IR (4000–600 cm−1): νmax = 3423 (stretch NH), 1642 (stretch C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1559 (bend NH), 1415, 1336 (stretch C–N). 1H NMR (CDCl3, 500 MHz): δH (ppm) 8.03 (d, 2H, J = 7.5 Hz), 7.83 (d, 2H, J = 8.5 Hz), 7.49 (d, 2H, J = 8.5 Hz), 7.42 (t, 2H, J = 7.5 Hz), 7.24–7.28 (m, 1H), 3.23–3.36 (m, 2H), 2.170 (s, enaminic proton), 1.91–2.02 (m, 2H), 1.69–1.77 (m, 2H), 1.15–1.35 (m, 6H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 169.7, 169.6, 134.7, 129.6, 129.1, 129.1, 128.9, 128.0, 126.7, 125.9, 125.9, 125.9, 56.8, 51.0, 34.2, 25.8, 24.8. HR-MS (ESI) for C22H21ClN4S m/z calcd: 409.1248; found = 409.1225 [M + H]+.
N), 1559 (bend NH), 1415, 1336 (stretch C–N). 1H NMR (CDCl3, 500 MHz): δH (ppm) 8.03 (d, 2H, J = 7.5 Hz), 7.83 (d, 2H, J = 8.5 Hz), 7.49 (d, 2H, J = 8.5 Hz), 7.42 (t, 2H, J = 7.5 Hz), 7.24–7.28 (m, 1H), 3.23–3.36 (m, 2H), 2.170 (s, enaminic proton), 1.91–2.02 (m, 2H), 1.69–1.77 (m, 2H), 1.15–1.35 (m, 6H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 169.7, 169.6, 134.7, 129.6, 129.1, 129.1, 128.9, 128.0, 126.7, 125.9, 125.9, 125.9, 56.8, 51.0, 34.2, 25.8, 24.8. HR-MS (ESI) for C22H21ClN4S m/z calcd: 409.1248; found = 409.1225 [M + H]+.
N-(2,6-Dimethylphenyl)-2,6-bis(4-nitrophenyl)imidazo[2,1-b][1,3,4]thiazol-5-amine (4l)
Brown solid (85%), Mp 180–181 °C, IR (4000–600 cm−1): νmax = 3434 (stretch NH), 1639 (stretch CN), 1553 (asymm. stretch NO2), 1409 (symm. stretch NO2), 1341 (stretch C–N). 1H NMR (CDCl3, 500 MHz): δH (ppm) 8.32 (d, 2H, J = 8.4 Hz), 8.21 (d, 2H, J = 8.8 Hz), 8.17 (d, 2H, J = 8.6 Hz), 7.85 (d, 2H, J = 8.4 Hz), 7.04 (d, 2H, J = 7.4 Hz), 6.94 (t, 1H, J = 7.3 Hz), 5.41 (brs, NH), 2.17 (s, 6H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 149.5, 146.2, 140.7, 140.1, 139.4, 139.4, 135.6, 129.3, 129.3, 127.6, 127.6, 126.3, 124.6, 123.9, 123.7, 18.7. Anal. calcd for C24H18N6O4S; C, 59.25; H, 3.73; N, 17.27. Found: C, 59.29; H, 3.81; N, 17.31.2-(4-Chlorophenyl)-N-cyclohexyl-6-(4-methoxyphenyl)imidazo[2,1-b][1,3,4]thiazol-5-amine (4m)
Yellow solid (86%), Mp 208–209 °C, IR (4000–600 cm−1): νmax = 3428 (stretch NH), 1636 (stretch C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1565 (bend NH), 1409 (stretch C–N). 1H NMR (CDCl3, 500 MHz): δH (ppm) 7.98 (d, 2H, J = 9 Hz), 7.82 (d, 2H, J = 8.5 Hz), 7.48 (d, 2H, J = 8.5 Hz), 6.97 (d, 2H, J = 9 Hz), 3.85 (s, 3H), 3.18–3.27 (bm, 2H), 1.92–1.98 (m, 2H), 1.71–1.78 (m, 2H), 1.17–1.33 (m, 6H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 177.8, 177.8, 177.5, 161.6, 157.3, 142.0, 129.5, 127.9, 127.2, 113.9, 113.9, 113.9, 55.3, 55.3, 34.0, 25.8, 24.8. HR-MS (ESI) for C23H23ClN4OS m/z calcd: 461.1173; found: 461.1136 [M + Na]+.
N), 1565 (bend NH), 1409 (stretch C–N). 1H NMR (CDCl3, 500 MHz): δH (ppm) 7.98 (d, 2H, J = 9 Hz), 7.82 (d, 2H, J = 8.5 Hz), 7.48 (d, 2H, J = 8.5 Hz), 6.97 (d, 2H, J = 9 Hz), 3.85 (s, 3H), 3.18–3.27 (bm, 2H), 1.92–1.98 (m, 2H), 1.71–1.78 (m, 2H), 1.17–1.33 (m, 6H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 177.8, 177.8, 177.5, 161.6, 157.3, 142.0, 129.5, 127.9, 127.2, 113.9, 113.9, 113.9, 55.3, 55.3, 34.0, 25.8, 24.8. HR-MS (ESI) for C23H23ClN4OS m/z calcd: 461.1173; found: 461.1136 [M + Na]+.
N-tert-Butyl-2-(4-chlorophenyl)-6-(4-methoxyphenyl)imidazo[2,1-b][1,3,4]thiazol-5-amine (4n)
Off white solid (85%), Mp 209–210 °C, IR (4000–600 cm−1): νmax = 3428 (stretch NH), 1642 (stretch C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1562 (bend NH), 1415, 1336 (stretch C–N). 1H NMR (CDCl3, 400 MHz): δH (ppm) 8.05 (dt, 2H, J = 8 Hz, J = 4 Hz), 7.81 (dt, 2H, J = 8 Hz, J = 4 Hz), 7.48 (dt, 2H, J = 8 Hz, J = 4 Hz), 6.94 (dt, 2H, J = 8 Hz, J = 4 Hz), 3.85 (s, 3H), 3.09 (brs, NH), 2.18 (brs, enaminic proton), 1.19 (s, 9H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 158.7, 137.5, 137.5, 129.6, 129.2, 128.1, 127.9, 127.7, 127.7, 113.7, 56.2, 55.3, 30.4. Anal. calcd for C21H21ClN4OS; C, 61.08; H, 5.13; N, 13.57. Found: C, 61.13; H, 5.18; N, 13.63.
N), 1562 (bend NH), 1415, 1336 (stretch C–N). 1H NMR (CDCl3, 400 MHz): δH (ppm) 8.05 (dt, 2H, J = 8 Hz, J = 4 Hz), 7.81 (dt, 2H, J = 8 Hz, J = 4 Hz), 7.48 (dt, 2H, J = 8 Hz, J = 4 Hz), 6.94 (dt, 2H, J = 8 Hz, J = 4 Hz), 3.85 (s, 3H), 3.09 (brs, NH), 2.18 (brs, enaminic proton), 1.19 (s, 9H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 158.7, 137.5, 137.5, 129.6, 129.2, 128.1, 127.9, 127.7, 127.7, 113.7, 56.2, 55.3, 30.4. Anal. calcd for C21H21ClN4OS; C, 61.08; H, 5.13; N, 13.57. Found: C, 61.13; H, 5.18; N, 13.63.
N-tert-Butyl-2(4-methoxyphenyl)-6-phenylimidazo[2,1-b][1,3,4]thiadiazol-5-amine (4o)
Off white solid (85%), Mp 204–204.5 °C, IR (4000–600 cm−1): νmax = 3443 (stretch NH), 1640 (stretch C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1563 (bend NH), 1467 (stretch C–N), 1414 (stretch C–N). 1H NMR (CDCl3, 400 MHz): δH (ppm) 8.10 (d, 2H, J = 8 Hz), 7.80 (d, 2H, J = 8.8 Hz), 7.38 (t, 2H, J = 8 Hz), 7.22–7.26 (m, 1H), 6.99 (d, 2H, J = 8.8 Hz), 3.87 (s, 3H), 3.13 (brs, NH), 1.18 (s, 9H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 162.3, 160.4, 135.2, 135.2, 128.4, 128.2, 128.2, 126.8, 126.8, 126.8, 123.3, 114.7, 56.3, 55.6, 30.3. Anal. calcd for C21H22N4OS; C, 66.64; H, 5.86; N, 14.80. Found: C, 66.70; H, 5.80; N, 14.86.
N), 1563 (bend NH), 1467 (stretch C–N), 1414 (stretch C–N). 1H NMR (CDCl3, 400 MHz): δH (ppm) 8.10 (d, 2H, J = 8 Hz), 7.80 (d, 2H, J = 8.8 Hz), 7.38 (t, 2H, J = 8 Hz), 7.22–7.26 (m, 1H), 6.99 (d, 2H, J = 8.8 Hz), 3.87 (s, 3H), 3.13 (brs, NH), 1.18 (s, 9H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 162.3, 160.4, 135.2, 135.2, 128.4, 128.2, 128.2, 126.8, 126.8, 126.8, 123.3, 114.7, 56.3, 55.6, 30.3. Anal. calcd for C21H22N4OS; C, 66.64; H, 5.86; N, 14.80. Found: C, 66.70; H, 5.80; N, 14.86.
N-tert-Butyl-2(4-methoxyphenyl)-6-(4-nitrophenyl)imidazo[2,1-b][1,3,4]thiadiazol-5-amine (4p)
Yellow solid (87%), Mp 227–227.5 °C, IR (4000–600 cm−1): νmax = 3447 (stretch NH), 1643 (stretch C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1567 (bend NH), 1412 (stretch C–N), 1340 (stretch C–N). 1H NMR (CDCl3, 400 MHz): δH (ppm) 8.40 (d, 2H, J = 8 Hz), 8.22 (d, 2H, J = 8 Hz), 7.80 (d, 2H, J = 8 Hz), 7.00 (d, 2H, J = 8 Hz), 3.88 (s, 3H), 3.14 (brs, NH), 2.16 (s, enaminic proton), 1.20 (s, 9H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 162.6, 146.0, 141.9, 136.0, 128.6, 128.5, 126.8, 123.7, 122.8, 122.8, 114.8, 57.0, 55.7, 30.4. Anal. calcd for C21H21N5O3S; C, 59.56; H, 5.00; N, 16.54. Found: C, 59.65; H, 5.08; N, 16.61.
N), 1567 (bend NH), 1412 (stretch C–N), 1340 (stretch C–N). 1H NMR (CDCl3, 400 MHz): δH (ppm) 8.40 (d, 2H, J = 8 Hz), 8.22 (d, 2H, J = 8 Hz), 7.80 (d, 2H, J = 8 Hz), 7.00 (d, 2H, J = 8 Hz), 3.88 (s, 3H), 3.14 (brs, NH), 2.16 (s, enaminic proton), 1.20 (s, 9H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 162.6, 146.0, 141.9, 136.0, 128.6, 128.5, 126.8, 123.7, 122.8, 122.8, 114.8, 57.0, 55.7, 30.4. Anal. calcd for C21H21N5O3S; C, 59.56; H, 5.00; N, 16.54. Found: C, 59.65; H, 5.08; N, 16.61.
N-tert-Butyl-6-(4-fluorophenyl)-2-phenylimidazo[2,1-b][1,3,4]thiadiazol-5-amine (4q)
Yellow solid (89%), Mp 233.7–233.9 °C, IR (4000–600 cm−1): νmax = 3446 (stretch NH), 1639 (stretch C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1559 (bend NH), 1360 (stretch C–N). 1H NMR (CDCl3, 400 MHz): δH (ppm) 8.09–8.16 (m, 2H), 7.83–7.90 (m, 2H), 7.46–7.53 (m, 3H), 7.06 (t, 2H, J = 8 Hz), 3.07 (brs, NH), 2.16 (s, enaminic proton), 1.17 (s, 9H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 163.1, 160.7, 160.6, 131.6, 131.2, 131.2, 130.6, 130.2, 129.3, 129.1, 128.6, 128.5, 127.1, 126.8, 115.2, 115.0, 56.3, 30.3. Anal. calcd for C20H19FN4S; C, 65.55; H, 5.23; N, 15.29. Found: C, 65.62; H, 5.32; N, 15.35.
N), 1559 (bend NH), 1360 (stretch C–N). 1H NMR (CDCl3, 400 MHz): δH (ppm) 8.09–8.16 (m, 2H), 7.83–7.90 (m, 2H), 7.46–7.53 (m, 3H), 7.06 (t, 2H, J = 8 Hz), 3.07 (brs, NH), 2.16 (s, enaminic proton), 1.17 (s, 9H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 163.1, 160.7, 160.6, 131.6, 131.2, 131.2, 130.6, 130.2, 129.3, 129.1, 128.6, 128.5, 127.1, 126.8, 115.2, 115.0, 56.3, 30.3. Anal. calcd for C20H19FN4S; C, 65.55; H, 5.23; N, 15.29. Found: C, 65.62; H, 5.32; N, 15.35.
N-tert-Butyl-2-(4-chlorophenyl)-6-(4-fluorophenyl)imidazo[2,1-b][1,3,4]thiadiazol-5-amine (4r)
Yellow solid (86%), Mp 229.0–229.2 °C, IR (4000–600 cm−1): νmax = 3431 (stretch NH), 1648 (stretch C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1564 (bend NH), 1349 (stretch C–N). 1H NMR (CDCl3, 400 MHz): δH (ppm) 8.09–8.15 (m, 2H), 7.80 (d, 2H, J = 8.8 Hz), 7.47 (d, 2H, J = 8.4 Hz), 7.07 (t, 2H, J = 8.8 Hz), 3.07 (brs, NH), 2.17 (s, enaminic proton), 1.18 (s, 9H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 163.2, 160.7, 159.3, 140.7, 137.7, 131.1, 131.1, 129.6, 129.1, 128.6, 128.5, 128.0, 115.2, 115.0, 56.4, 30.3. Anal. calcd for C20H18ClFN4S; C, 59.92; H, 4.53; N, 13.98. Found: C, 59.99; H, 4.62; N, 14.06.
N), 1564 (bend NH), 1349 (stretch C–N). 1H NMR (CDCl3, 400 MHz): δH (ppm) 8.09–8.15 (m, 2H), 7.80 (d, 2H, J = 8.8 Hz), 7.47 (d, 2H, J = 8.4 Hz), 7.07 (t, 2H, J = 8.8 Hz), 3.07 (brs, NH), 2.17 (s, enaminic proton), 1.18 (s, 9H). 13C NMR (CDCl3, 100 MHz): δC (ppm) 163.2, 160.7, 159.3, 140.7, 137.7, 131.1, 131.1, 129.6, 129.1, 128.6, 128.5, 128.0, 115.2, 115.0, 56.4, 30.3. Anal. calcd for C20H18ClFN4S; C, 59.92; H, 4.53; N, 13.98. Found: C, 59.99; H, 4.62; N, 14.06.
Acknowledgements
We gratefully acknowledge the Department of Science and Technology (DST), Govt. of India for financial support (Grant no. SR/FT/CS-55/2011). The authors P. W. and T. K. thank MHRD for the award of JRF and Post-Doctoral Fellowship, respectively.References
- P. A. Jacobi, in Advances in Heterocyclic Natural Product Synthesis, ed. W. H. Pearson, Jai Press, Greenwich, CT, 1992, vol. 2, p. 251 Search PubMed.
- M. M. Mc Gee, S. Gemma, S. Butini, A. Ramunno, D. M. Zisterer, C. Fattorusso, B. Catalanotti, G. Kukreja, I. Fiorini, C. Pisano, C. Cucco, E. Novellino, V. Nacci, D. C. Williams and G. Campiani, J. Med. Chem., 2005, 48, 4367 CrossRef CAS PubMed.
- I. A. M. Khazi, A. K. Gadad, R. S. Lamani and B. A. Bhongade, Tetrahedron, 2011, 67, 3289 CrossRef CAS PubMed.
- W. S. Hamama, M. A. Gouda, M. H. A. E. Wahab and H. H. Zoorob, J. Heterocycl. Chem., 2014, 51, 1558 CrossRef CAS PubMed.
- H. M. Patel, M. N. Noolvi, A. Goyal and B. S. Thippeswamy, Eur. J. Med. Chem., 2013, 65, 119 CrossRef CAS PubMed.
- M. B. Palkar, M. N. Noolvi, V. S. Maddi, M. Ghatole and L. G. Nargund, Med. Chem. Res., 2012, 21, 1313 CrossRef CAS.
- M. Hegde, S. S. Karki, E. Thomas, S. Kumar, K. Panjamurthy, S. R. Ranganatha, K. S. Rangappa, B. Choudhary and S. C. Raghavan, PLoS One, 2012, 7, e43632 CAS.
- R. S. Lamani, N. S. Shetty, R. R. Kamble and I. A. M. Khazi, Eur. J. Med. Chem., 2009, 44, 2828 CrossRef CAS PubMed.
- A. K. Gadad, M. B. Palkar, K. Anand, M. N. Noolvi, T. S. Boreddy and J. Wagwade, Bioorg. Med. Chem., 2008, 16, 276 CrossRef CAS PubMed.
- T. Pyl, F. Wasehk and H. Beyer, Justus Liebigs Ann. Chem., 1963, 663, 113 CrossRef CAS PubMed.
- I. T. Barnish, P. E. Cross, R. P. Dickinson, B. Gadsby, M. J. Parry, M. J. Randall and I. W. Sinclair, J. Med. Chem., 1980, 23, 117 CrossRef CAS.
- A. V. Ivashchenko, S. I. Torgova, L. A. Karamysheva and A. G. Abolin, Liq. Cryst., 1990, 7, 475 CrossRef CAS PubMed.
- C. S. Andotra, T. C. Langer and A. Kotha, J. Indian Chem. Soc., 1997, 74, 125 CAS.
- F. A. Ashour, N. S. Habib, M. El Taibbi, S. El Dine and A. S. El Dine, Farmaco, 1990, 45, 1341 CAS.
- S. Fajgelj, B. Stanovnik and M. Tisler, Heterocycles, 1986, 24, 379 CrossRef CAS.
- (a) C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 2506 CrossRef PubMed; (b) R. Sarma, M. M. Sarmah and D. Prajapati, J. Org. Chem., 2012, 77, 2018 CrossRef CAS PubMed; (c) L. Shi, M. Wang, C.-A. Fan, F.-M. Zhang and Y.-Q. Tu, Org. Lett., 2003, 5, 3515 CrossRef CAS PubMed.
- (a) Multicomponent Reactions, ed. Zhu J. and Bienaymé H., Wiley-VCH, Weinheim, 2005 Search PubMed; (b) B. Maiti, K. Chanda, M. Selvaraju, C.-C. Tseng and C.-M. Sun, ACS Comb. Sci., 2013, 15, 291 CrossRef CAS PubMed; (c) A. Kadam, Z. Zhang and W. Zhang, Curr. Org. Synth., 2011, 8, 295 CrossRef CAS.
- H. Bienaymé and K. Bouzid, Angew. Chem., Int. Ed., 1998, 37, 2234 CrossRef.
- M. Krasavin, S. Tsirulnikov, M. Nikulnikov, V. Kysil and A. Ivachtchenko, Tetrahedron Lett., 2008, 49, 5241 CrossRef CAS PubMed.
- (a) M. Sharma, T. Kaur, V. K. Gupta and A. Sharma, RSC Adv., 2015, 5, 17087 RSC; (b) D. Saha, P. Wadhwa and A. Sharma, RSC Adv., 2015, 5, 33067 RSC.
- B. M. Trost, Science, 1991, 254, 1471 CAS.
- P. T. Anasta and J. C. Warner, Green Chemistry: Theory and Practical, Oxford University Press, 1998 Search PubMed.
- C. J. González, D. J. C. Constable and C. S. Ponder, Chem. Soc. Rev., 2012, 41, 1485 RSC.
- A. D. Curzons, D. J. C. Constable, D. N. Mortimer and V. L. Cunningham, Green Chem., 2001, 3, 1 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Representative 1H, 13C NMR and HRMS spectra. See DOI: 10.1039/c5ra06747b |
| This journal is © The Royal Society of Chemistry 2015 |