Investigation of demulsification efficiency in water-in-crude oil emulsions using dissipative particle dynamics†
Abstract
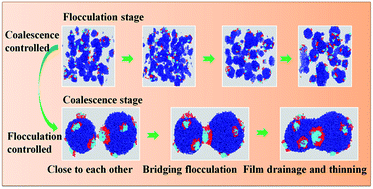
In this study, simulations of dissipative particle dynamics (DPD) were employed to investigate the efficiency of polyether demulsifiers, substituting polyoxypropylene (PPO) hydrophobic blocks for polybutylene oxide (PBO) and polytetrahydrofuran (PTF) in ultra-heavy crude oil emulsions. The simulation results showed that the demulsifiers with polybutylene oxide (PBO) hydrophobic blocks favor high levels of demulsification while the demulsifiers with polyoxypropylene (PPO) hydrophobic blocks exhibit better dehydration rates. The kinetics equation demonstrates that the demulsification process is controlled by the combination of flocculation and coalescence. As time progresses, the rate-controlling process of demulsification changes from coalescence controlled to flocculation controlled. Moreover, high performance demulsifiers which have higher rate constants for coalescence could accelerate the rate of drainage of the film much faster, thereby promoting coalescence. The root mean square end-to-end distance 〈R〉 for demulsifiers continues to grow with time, such that their configurations become more stretched. This results in interface arraying and the demulsifiers build up a continuous open network which leads to a higher possibility of droplet–droplet coalescence. The variation in radial distribution function (RDF) indicates that there is a rather strong and remarkably structured interaction between asphaltenes and demulsifiers, corresponding to the radial distribution range from 10 Å to 50 Å.

 Please wait while we load your content...
Please wait while we load your content...