Development of a novel preparation method for conductive PES ultrafine fibers with self-formed thin PES/CNTs composite layer by vapor treatment
Abstract
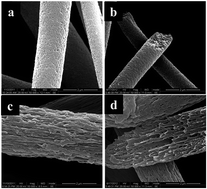
Conductive electrospun fibers have attracted widespread interest in the field of electromagnetics. However, the problem of how to effectively improve the electrical conduction of electrospun fibers has still not been adequately addressed. In this study, a new, simple and effective method was introduced to significantly improve the conductive properties of fibers. PES/PVA fibers with previous addition of 20 wt% PVA were chosen as a matrix due to the large parallel porous structure. The carbon nanotubes (CNTs) were first absorbed by the PES/PVA fibers, and then a thin polymer/CNTs composite layer was self-formed on the surface of the porous fibers by vapor treatment. Most importantly, a CNTs network structure was also formed in this vapor process, which easily gave the porous fibers a significant enhancement in conductivity with only a small amount of CNTs. Electrical conduction tests showed that the conduction of the fibers increased with increasing CNT content, and attained the maximum value when the amount of CNTs was around 7 wt%. The adsorption time and the DMSO vapor treatment time were optimized to obtain the best thin polymer/CNT composite layers. The surface microstructure of the composite layer was observed using scanning electron microscopy (SEM) and TGA. The results showed that this novel, powerful method could potentially be used to prepare novel types of conductive polymer fibers.

 Please wait while we load your content...
Please wait while we load your content...