Synthesis of novel Zn0.5Mg0.5Fe2O4@TiO2 nanotube arrays with enhanced photoelectrocatalytic properties†
Jingqiang Pana,
Xinyong Li*ab,
Qidong Zhaoa and
Dongke Zhang*b
aKey Laboratory of Industrial Ecology and Environmental Engineering (MOE), State Key Laboratory of Fine Chemicals, School of Environmental Science and Technology, Dalian University of Technology, Dalian 116024, China. E-mail: xyli@dlut.edu.cn
bCentre for Energy (M473), The University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia
First published on 29th May 2015
Abstract
A novel nanocomposite electrode with tight and vertically aligned Zn0.5Mg0.5Fe2O4@TiO2 nanotube arrays (NTs) has been successfully synthesized via an ultrasonically assisted electrodeposition strategy. The scanning electron microscopy (SEM), transmission electron microscopy (TEM) and X-ray diffraction (XRD) of the composite nanostructures indicated that the as-prepared electrodes were well-aligned TiO2 NTs with Zn0.5Mg0.5Fe2O4 nanoparticles. The enhanced absorption of the nanocomposite electrode under simulated sun light and visible light regions were observed. The photoelectrochemical performance of the Zn0.5Mg0.5Fe2O4@TiO2 nanotube arrays showed excellent sensitive response to visible light. The surface-interface charge separation and transfer of photo-induced electrons and holes were also demonstrated by optical characterization. Meanwhile, the photoelectrochemical investigations clearly illustrated that the Zn0.5Mg0.5Fe2O4@TiO2 composite NTs had more effective photo-conversion capability than the unloaded TiO2 NTs. The saturated photocurrent density of the doped electrode was about 6.5 fold and 4 fold as high as that of the TiO2 NTs under the illumination of visible light and simulated sun light, respectively. In addition, the enhanced photoelectrocatalytic (PEC) ability of the as-prepared electrode was demonstrated in the degradation of toxic p-nitrophenol species. The much improved PEC activity can be attributed to both the visible-light photocatalytic activity of Zn0.5Mg0.5Fe2O4 and the heterostructure between Zn0.5Mg0.5Fe2O4 and TiO2.
Introduction
Semiconductor-based photocatalysts have received considerable attention due to their potential in environmental pollution remediation and renewable energy applications.1,2 TiO2 nanostructures have in particular attracted tremendous interest due to their favourable photocatalytic (PC) activity, stability, anti-photocorrosion and non-toxicity.3–5 Among the various nanostructures, well aligned TiO2 nanotube arrays (NTs) are especially interesting and intriguing because of their large surface area and short diffusion length perpendicular to the charge collecting substrate, leading to lower recombination loss. As such, TiO2 NTs have been developed for a range of applications including photocatalysis,6–8 water splitting for hydrogen production,9 solar energy conversion,10,11 lithium ion batteries12 and gas sensors.13However, TiO2 is a semiconductor with wide band gap (3.0 and 3.18 eV for the rutile and anatase, respectively) only functioning under UV light irradiation (just about 4.5% of solar energy). Therefore, development of visible light driven photocatalysts based on TiO2 NTs becomes a key challenge but holds a key to widespread applications of this type of photocatalysts. Various strategies have been used to extend the absorption band of TiO2 to the visible light region such as metal ion doping,14–17 nonmetal ion doping18–20 and narrow-band-gap semiconductor composites.21–25 The fact that the photon-generated electrons from the conduction band of the narrow band gap semiconductor could be injected into the conduction band of TiO2 has been demonstrated, which not only can expand the spectral range of light absorption, but may also promote photon-excited electron–hole separation.17,18
Recently, spinel ferrite (MeFe2O4, where Me usually represents a metal, such as Zn, Mg, Mn, Ni etc.) crystallites are considered good candidate for photocatalysis reaction in which Me2+ cations occupy tetrahedral sites.26,27 Spinel ferrites offer the advantage of having a band gap capable of absorbing visible light, as well as the spinel crystal structure providing available extra catalytic sites by the virtue of crystal lattice.28 A great deal of work has been reported about the combination of spinel ferrites with TiO2 to enhance the PC activity. Especially, Zn0.5Mg0.5Fe2O4 with a spinel structure has been recently investigated due to its chemical stability and sensitivity to visible light.29–31 Rekha Dom has synthesized Zn0.5Mg0.5Fe2O4 via microwave irradiation,28 while S. Rahman has prepared ZnMg-ferrite nanoparticles using the co-precipitation method.32 Besides, both the conduction band and the valence band of Zn0.5Mg0.5Fe2O4 locate at higher wavelengths than those of TiO2, resulting in an excellent separation of the electron–hole pairs.27 Furthermore, the spinel ferrite properties of the dopants can enhance the separation efficiency of the photon-generated charges as well as their reactivity due to their internal electrostatic field.27,33 Consequently, coupling TiO2 NTs with Zn0.5Mg0.5Fe2O4 nanoparticles seems a promising approach to enhancing visible light absorption due to the special semiconductor structure and spatial crystal spinel structure of the Zn0.5Mg0.5Fe2O4 ferrites.
In this current work, we have synthesized visible-light-responsive Zn0.5Mg0.5Fe2O4 coupled TiO2 NTs via an ultrasonically assisted electrodeposition strategy. The properties of the heterojunction between the Zn0.5Mg0.5Fe2O4 and TiO2 NTs electrode and the photoelectrochemical properties of Zn0.5Mg0.5Fe2O4@TiO2 NTs electrode were determined and investigated using the photoelectrochemical techniques. In addition, the photoelectrocatalytic (PEC) activity of the electrodes was evaluated in detail by using the PEC degradation of toxic p-nitrophenol (PNP) species under simulated sun light and visible light irradiation conditions.
Experimental
Synthesis of Zn0.5Mg0.5Fe2O4@TiO2 composite electrode
Titanium foil (purity 99.6%, thickness 0.2 mm) was acquired from Beijing Academy of Steel Service, China. All the other chemicals were of analytical grade. The titanium foil was mechanically polished with sand papers of different grade (fineness) and then rinsed clean in an ultrasonic bath of deionized water for 10 min. The clean, polished titanium foil was subjected to chemically etching by immersing it in a mixture of HF and HNO3 (HF/HNO3/H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 in volume) for 40 s. The titanium foil was rinsed again in deionized water to remove the mixed acid, followed by final cleaning by immersing it in an ethanol and deionized water solution in the ultrasonic bath for 15 min. The anodization was performed in a two-electrode cell with titanium foil as the working electrode and platinum foil as the counter electrode. The anodizing voltage was set at 20 V and anodized for 30 min in 0.2 wt% HF solution. After anodization, the sample was immediately rinsed with deionized water and dried at room temperature. The sample was then annealed at 773 K in air for 2 h with heating and cooling rates of 2 °C min−1 to convert the amorphous phase to crystalline phase.
5 in volume) for 40 s. The titanium foil was rinsed again in deionized water to remove the mixed acid, followed by final cleaning by immersing it in an ethanol and deionized water solution in the ultrasonic bath for 15 min. The anodization was performed in a two-electrode cell with titanium foil as the working electrode and platinum foil as the counter electrode. The anodizing voltage was set at 20 V and anodized for 30 min in 0.2 wt% HF solution. After anodization, the sample was immediately rinsed with deionized water and dried at room temperature. The sample was then annealed at 773 K in air for 2 h with heating and cooling rates of 2 °C min−1 to convert the amorphous phase to crystalline phase.
The deposition procedure was conducted in a three-electrode setup in an uniform cell, using the TiO2 nanotube arrays electrode as the working electrode, a Pt foil as the counter electrode, and a saturated calomel electrode (SCE) as the reference electrode. The electrodeposition was carried out with a potential of −2.4 V for 20 min. Before electrodeposition, the sample was immersed in a mixture solution 50 mL containing 0.025 M (0.7425 g) Zn(NO3)2·6H2O, 0.025 M (0.641 g) Mg(NO3)2·6H2O and 0.1 M (4.04 g) Fe(NO3)3·9H2O for 20 min in an ultrasonic bath at 40 kHz frequency, which drove the salt solution to diffuse through the nanochannels of TiO2 and ensure that the inner walls of the pore channels were filled with the solution. Then the TiO2 NTs electrode was diverted into a new medium that contained the supporting electrolyte (0.1 M Na2SO4, 80 mL). The deposition in this medium resulted in the deposition of Zn, Mg and Fe ions. After repeating the ultrasonication and deposition procedure several times, a desired amount of Zn, Mg and Fe deposition in the pores was achieved. Subsequently, the electrode was electrochemically oxidized in a 1 M KOH (70 mL) solution with a constant potential of 2.5 V for 5 min. Finally, the sample was rinsed with deionized water and dried in air. Then it was annealed by heating at 773 K for 2 h at muffle furnace, with a ramp of 2 °C min−1.
Characterization
The morphology of the Zn0.5Mg0.5Fe2O4@TiO2 NTs was characterized using a field emission scanning electron microscope (SEM) (Quanta 450) with an accelerating voltage of 30.0 kV. The dopant concentration was characterized by energy-dispersive X-ray spectra (EDX) (Horiba 7593H), and EDX was performed to determine the elemental concentration distribution on the catalyst granules using Link Isis Series 300 software. Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) images were taken on a FEI Tecnai G2 F20 with an acceleration voltage of 200 and 300 kV. Chloroform solutions containing these nanocomposite samples were dropped on carbon-coated copper grids. The crystallinity of the sample was determined from X-ray diffraction patterns (XRD) using a diffractometer with Cu Kα radiation (Shimadzu Lab-X XRD-6000, source light at the wavelength (λ) of 0.15406 nm). The accelerating voltage and applied current were 30 kV and 30 mA, respectively. while the diffraction angles (2θ) was scanned from 20° to 60°. The absorption property was measured using UV-Vis diffuse reflectance spectra (DRS) (JASCO, UV-550) with a wavelength range of 200–700 nm. Photoluminescence (PL) spectra of the electrode surface was collected using a Hitachi F-4500 (excited at λ = 325 nm) fluorescence spectrophotometer in air at room temperature. X-ray photoelectron spectroscope (XPS) (PHI 5600 mode) was performed to examine the surface properties and composition of the sample. All the binding energies were calibrated by using the carbon (C 1s) 284.6 eV as a reference. The separation characteristics of photon-generated charge carriers was tested using a surface photovoltage (SPV) measurement system, which consisted of a monochromator (model Omni-λ 3005) and a lock-in amplifier (model SR830-DSP) with an optical chopper (model SR540) running at a frequency of 20 Hz.PEC activity measurement
Photoelectrochemical measurement
Photocurrent density was measured using a CHI electrochemical analyzer (CHI 760C, Shanghai Chenhua, China) in a standard three-electrode configuration with Zn0.5Mg0.5Fe2O4@TiO2 NTs as the photoanode (an effective area of 5 cm2), a platinum foil as the counter electrode, and a saturated calomel electrode (SCE) as the reference electrode. A 0.01 M Na2SO4 (80 mL) solution was used as the electrolyte. The working electrode was irradiated with visible light (λ > 420 nm) through a UV-cutoff filter (Shanghai Seagull Colored Optical Glass Co. Ltd.) from a high-pressure xenon short arc lamp (a Phillips 500 W Xe lamp). The incident light intensity was measured with a radiometer (Photoelectric Instrument Factory Beijing Normal University, model FZ-A).Photoelectrochemical experiment
The PEC reactions of PNP were carried out in a single photoelectrochemical compartment. The corresponding concentration of PNP species was obtained from diluting an analytical grade powder of PNP with deionized water. The as-prepared electrode serving as the photoanode and Pt foil as the cathode were placed in parallel in a cylindrical quartz reactor, and an SCE served as the reference electrode. All electrodes were connected to a CHI 760C EC analyzer. A bias potential applied on the photoanode was 0.6 V (vs. SCE) under visible light of 33.5 mW cm−2 and simulated sun light of 36 mW cm−2, respectively. All the experiments were performed with magnetic stirring, using a 0.01 M Na2SO4 solution as the electrolyte. The initial concentration of PNP in the aqueous solution (100 mL) for the degradation tests was 20 mg L−1 under the simulated sun light and 5 mg L−1 under the visible light, respectively. The concentration of PNP was determined using a UV 1100 spectrophotometer with the detection wavelength at 318 nm.Results and discussion
Fig. 1a shows the top and cross-sectional (the inset) views of the TiO2 NTs sample. Clearly, the self-organized TiO2 nanotubes consist of vertically aligned compact nanotube arrays with a tube diameter of ∼90 nm, and length of ∼320 nm (the inset). Fig. 1b and c display the top and cross-sectional images of the Zn0.5Mg0.5Fe2O4@TiO2 NTs sample. The resulting highly ordered and vertically oriented nanotube arrays can be observed from Fig. 1b and c. It is obvious that the deposited nanoparticles not only entered the inner tubes but also surrounded the top of nanotube arrays without blocking the tube entrances. The nanotubes are still open at the top, indicating that the doping process of Zn0.5Mg0.5Fe2O4 did not destroy the structure of the nanotubes, which would induce the doped species in a much more uniform and compact manner within the inner-surface and outer-surface of the nanotube arrays. From the EDX spectrum of Zn0.5Mg0.5Fe2O4@TiO2 NTs (Fig. 1d), it can be noticed that the as-prepared composite nanotube arrays contain Zn, Mg, Fe.To further demonstrate the microstructure characteristics of Zn0.5Mg0.5Fe2O4 nanoparticles deposited on TiO2 NTAs, the low (Fig. 2a–c) and high magnification (Fig. 2d) TEM images of Zn0.5Mg0.5Fe2O4/TiO2 NTAs have been conducted. As exhibited by the low magnification TEM images in Fig. 2a and b, Zn0.5Mg0.5Fe2O4 nanoparticles are deposited on the tubes entrance while causing no damage of nanotubes. Seen from Fig. 2c, it can be found that Zn0.5Mg0.5Fe2O4 nanoparticles with a diameter of 20–30 nm are deposited into the tube. Fig. 2d depicts the HRTEM image of the selected area, where the lattice fringes of 0.253 nm and 0.351 nm correspond to the reflections from the (311) planes of spinel Zn0.5Mg0.5Fe2O4 (JCPDS no. 04-002-5442)34 and the (101) plane of anatase TiO2 (JCPDS no. 21-1272),6 respectively. The TEM testing results confirm that spinel ferrite Zn0.5Mg0.5Fe2O4 nanoparticles are successfully deposited to TiO2 NTAs.
In order to identify the crystal phases of TiO2 NTs and Zn0.5Mg0.5Fe2O4@TiO2 NTs, XRD measurements was conducted under specified conditions. Fig. 3 shows the XRD patterns of TiO2 NTs and Zn0.5Mg0.5Fe2O4@TiO2 NTs, respectively. The diffraction peaks at 35.2°, 38.4°, 40.3° and 53.1° correspond to (100), (002), (101) and (102) crystal faces of the Ti sheet (JCPDS no. 07-6265). Obviously, TiO2 NTs annealed at 773 K possesses the crystal structure of anatase (2θ = 25.3°, 48.1°. JCPDS no. 07-6175). After the deposition of Zn0.5Mg0.5Fe2O4, four additional diffraction peaks are identified at 2θ = 30.1°, 35.4°, 43.1° and 57°, which are in accordance with the (220), (331), (400) and (511) planes of spinel Zn0.5Mg0.5Fe2O4 (JCPDS no. 04-001-9289) respectively.35 The sharp peak near 35.3° in Fig. 3b is attributed to the overlap and accumulation of 35.2° from anatase and 35.4° from spinel ferrite. It should be noted that isolated MgO, Fe2O3 and ZnO phases are not found in the obtained Zn0.5Mg0.5Fe2O4@TiO2 NTs sample. The XRD results indicate that the composite nanostructures grown on electrodes prepared by the ultrasonically assisted electrodeposition approach are composed of the desired spinel Zn0.5Mg0.5Fe2O4 and anatase TiO2 crystalline structures.
 | ||
| Fig. 3 XRD patterns of Ti sheet (a), TiO2 nanotube arrays (b), and Zn0.5Mg0.5Fe2O4@TiO2 NTs (c), (ZMF in (c) represents Zn0.5Mg0.5Fe2O4). | ||
Fig. 4a shows the UV-Vis DRS of the TiO2 NTs and Zn0.5Mg0.5Fe2O4@TiO2 NTs. It can be seen that, in the visible light region, the absorption intensity of the sample Zn0.5Mg0.5Fe2O4@TiO2 is dramatically increased, which clearly reveals that Zn0.5Mg0.5Fe2O4@TiO2 NTs are more sensitive to the visible light than that of the non-doped NTs, therefore, it follows that the Zn0.5Mg0.5Fe2O4@TiO2 NTs electrode possesses a much improved photoelectrochemical capability under visible light irradiation. At the same time, an obviously red shift of the absorption threshold toward the visible light region has been successfully observed for the Zn0.5Mg0.5Fe2O4-doped sample. Two characteristic absorption peaks of TiO2 at about 410 nm and 540 nm corresponded to the absorption of the trapped hole and the trapped electron, respectively, which result from the sub-bandgap of surface defects on TiO2 nanotubes. According to literatures,36,37 the hole traps or deep electron-acceptor type defects showed a peak absorption at around λ = 410 nm. The electron traps exhibited another peak absorption around λ = 540 nm, which can be attributed to the oxygen vacancies related sub-bandgap states of the TiO2 NTs. Fig. 4b shows the (αhν)1/2 versus hν curve of TiO2 and Zn0.5Mg0.5Fe2O4 on TiO2 NTs coming from the equation (αhν)1/2 = k(hν − Eg), where α, h, ν and Eg represent the adsorption coefficient, Planck constant, light frequency and band energy, respectively.38,39 For TiO2 NTs, the absorption onset is at approximately 387 nm, which corresponds well to the band gap 3.18 eV of anatase TiO2. The absorption of TiO2 NTs in the visible range could be assigned to the sub-band gap states of the TiO2 NTs.40 After the deposition of Zn0.5Mg0.5Fe2O4 nanoparticles, the absorption threshold shifts to 570 nm. The band gap is estimated to be 2.0 eV.
 | ||
| Fig. 4 (a) UV-Vis DRS of TiO2 NTs and Zn0.5Mg0.5Fe2O4@TiO2 NTs; (b) Tauc's plots to determine the band gaps for TiO2 NTs and Zn0.5Mg0.5Fe2O4@TiO2 NTs. | ||
Consequently, the DRS spectra suggest that the Zn0.5Mg0.5Fe2O4-doped nanotube arrays might exhibit a higher visible light response than the non-doped NTs. arrays might exhibit a higher visible light response than the non-doped NTs.
In order to reveal the impact of Zn0.5Mg0.5Fe2O4@TiO2 NTs photo-induced charge separation, migration and combinations,41 PL spectra of the samples are characterized. Fig. 5 presents the TiO2 NTs and Zn0.5Mg0.5Fe2O4 @TiO2 NTs PL spectra. The excitation wavelength of the samples tested was 325 nm. TiO2 NTs and doped TiO2 NTs in the vicinity of 400 nm exhibit a strong emission peak.
Two weak emission peaks also exist around 454 nm and 529 nm, respectively. The emission peak around 400 nm is attributed to the band edge emission of TiO2 NTs (intrinsic luminescence), corresponding to electron transitions between the emission band.42 The two weak peeks around 454 nm and 529 nm are ascribed to TiO2 NTs with edges of free excitons and bound excitons. These PL signals are mainly due to oxygen vacancies and defects generated on the nanoparticle surface.43,44 Compared with the PL spectra of TiO2 NTs and doped TiO2 NTs, the PL intensity of Zn0.5Mg0.5Fe2O4@TiO2 NTs is found to be significantly reduced. Since the PL spectrum results from the recombination of electrons and holes, a lower luminous intensity means less recombination of electrons and holes.
The experimental results clearly indicate that the recombination ratio of photo-generated electron–hole pairs is much lowered due to the loading of Zn0.5Mg0.5Fe2O4.45
To confirm the states of elements and composition information in the nanotubular samples, XPS was employed to reveal the valence states and surface chemical compositions of the composite nanotube arrays. Elements of Ti, Mg, Zn, Fe, O and adventitious carbon are totally proved to be presented in the composite (Fig. 6a). Carbon is ascribed to the adventitious hydrocarbon from the XPS instrument itself. In Fig. 6b, two peaks for the Ti 2p are observed at binding energy 464.8 eV and 459.2 eV, which are assigned to Ti 2p1/2 and Ti 2p3/2 respectively. These values are in good agreement with the XPS data known for Ti4+ in the pure anatase titania form46 in the corresponding literature. It seems that loading Zn0.5Mg0.5Fe2O4 species did not have any effect on the position of Ti 2p peak. As shown in Fig. 6c, XPS peaks of Zn 2p located at 1044.4 eV and 1022.8 eV can be assigned to the spectra of Zn 2p1/2 and Zn 2p3/2, suggesting that the composite electrode contains Zn2+.47 In Fig. 6d, the peaks located at 710.6 eV and 724.4 eV can be attributed to Fe 2p3/2 and Fe 2p1/2 for Fe3+, respectively, which reveal the oxidation state of Fe3+ in the nanocomposites.48 In addition, in Fig. 6e, it can be found that a sharp peak around 1303.8 eV, which matches well with Mg 1s, indicating that Mg exists in Mg2+ oxidation state in the composite electrode.33 As shown in Fig. 6f, O 1s peak is asymmetric suggesting at least two kinds of oxygen species are present in and near the surface region. Meanwhile, the XPS spectra of O 1s of the Zn0.5Mg0.5Fe2O4@TiO2 NTs are fitted using Gaussian–Lorentzian peak shapes, which can be devolved into three small peaks. The peak located at 532.4 eV is assigned to the adsorbed oxygen. The other two peaks located at 529.4 eV and 530.1 eV are attributed to the O element in TiO2 and Zn0.5Mg0.5Fe2O4, respectively.49
 | ||
| Fig. 6 The whole XPS (a), Ti 2p (b), Fe 2p (c), Zn 2p (d), Mg 1s (e) and O 1s (f) XPS of Zn0.5Mg0.5Fe2O4@TiO2 composite NTs. | ||
To further demonstrate the effect of Zn0.5Mg0.5Fe2O4 on expanding the active spectral range of doped TiO2 NTs, SPV characterization of the as-prepared electrodes was conducted (Fig. 7). TiO2 NTs electrode presents a response in the UV range from 300 to 380 nm, which is ascribed to the electron transition from valence band to conduction band of TiO2 (O2p → Ti3d).50 However, the Zn0.5Mg0.5Fe2O4@TiO2 NTs electrode displays a stronger SPV signal than that of the TiO2 NTs electrode. The modified electrode exhibits a red shift of the spectral band towards visible region, which is also confirmed by the UV-Vis DRS characterization. In view of that the strong SPV signal has direct correlation with effective separation of photo-generated charge carriers,51 the presence of Zn0.5Mg0.5Fe2O4 would favor the photo-induced charge carrier separation and surface-interface transfer, greatly enhancing the PC and PEC activity of TiO2 NTs electrode.
Photoelectrochemical performance
The photoelectrochemical performance of TiO2 NTs and Zn0.5Mg0.5Fe2O4@TiO2 NTs was investigated under visible light and simulated sun light irradiation. The photocurrent densities as a function of the applied potential of the electrodes in a 0.01 M Na2SO4 solution are presented in Fig. 8a and c. The dark current densities are low and negligible under visible light and simulated sun light irradiation. However, TiO2 still displays a noticeable response while the photocurrent density of doped TiO2 NTs dramatically rises under the visible light. The increase and subsequent decrease of photocurrent density is believed to be due to the fact that the space charge region of the heterojunction promotes the separation of photo-induced carriers by the internal electrostatic field in the heterojunction region. As a consequence, more charge carriers would participate in reactions instead of recombination.52 However, this internal electrostatic field of the junction region would be weakened with increasing the applied bias potential because the thickness of the space charge layer decreased with the bias voltage. As a result, less charge carriers could be separated, and the photocurrent density decreases accordingly.53,54 The saturated photocurrent density of the Zn0.5Mg0.5Fe2O4@TiO2 NTs electrode, at 0.13 mA cm−2 is about 6.5 folds as high as that of TiO2 NTs at 0.02 mA cm−2 under visible light irradiation. In the meantime, the photocurrent density of the doped TiO2 NTs electrode is more than 4 folds as high as that of the TiO2 NTs (1.75 versus 0.4 mA cm−2) under simulated sun light irradiation. It may therefore be concluded that Zn0.5Mg0.5Fe2O4@TiO2 NTs has a much higher conversion capability of visible light than TiO2 NTs, at a 10 folds increase, and the photo-generated carrier-separation efficiency has been improved significantly after being modified with Zn0.5Mg0.5Fe2O4 nanoparticles. As shown in Fig. 8b and d, after loading Zn0.5Mg0.5Fe2O4 nanoparticles, the photo-conversion efficiency has been improved under simulated sun light and visible light irradiation. The photo-conversion efficiency (η) of light energy to chemical energy in the presence of a bias potential is calculated as follows.6where, jp means the photocurrent density (mA cm−2), jpE0rev is the total power output; jp|Eapp| represents the electrical power input; and I0 is the power density of the incident light (mA cm−2). E0rev is the standard reversible potential (which is 1.23 V for the water-splitting reaction when pH value reaches 0), and |Eapp| is the absolute value of the applied potential Eapp, which can be calculated as Eapp = Emeas − Eaoc, where Emeas is the electrode potential (vs. SCE) of the working electrode at which the photocurrent is measured under illumination and Eaoc is the electrode potential (vs. SCE) of the same working electrode under open-circuit conditions, under the same condition.
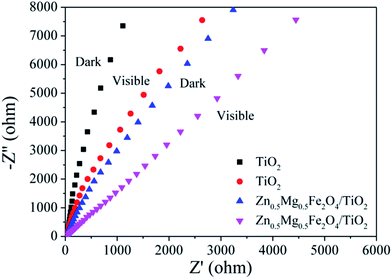
In order to study the properties of the electrodes and their interactions with the solution, the EIS measurements of the TiO2 NTs and Zn0.5Mg0.5Fe2O4@TiO2 NTs are carried out, which cover the frequencies of 105 to 10−2 Hz intervals by an amplitude of 10 mV at an open circuit potential under dark and visible light irradiation.49 By controlling the electrode current or potential, the EIS curves of the as-prepared electrode vary by slight amplitude sinusoidal curve with time, while measuring the potential or current variation with time of the electrode.55 Fig. 9 exhibits the EIS results of the TiO2 NTs and Zn0.5Mg0.5Fe2O4@TiO2 NTs electrodes under dark and visible illumination in the 0.01 M Na2SO4 electrolyte, with the bias voltage set to 0.3 V. The results in Fig. 9 suggest that without any light irradiation, the Nernst curve of TiO2 NTs becomes close to a straight line, indicating that the TiO2 NTs electrode response is poor without any light irradiation. The impedance of the radius of the TiO2 NTs electrode decreases sharply after Zn0.5Mg0.5Fe2O4 loading or with visible light irradiation, which means that the composite material has a good response to visible light region. The EIS measurement results illustrate that Zn0.5Mg0.5Fe2O4 loading effectively improves the separation of photon-generated electron–hole pairs and accelerates the photon-generated charge transfer between Zn0.5Mg0.5Fe2O4 and TiO2 NTs interface. It is concluded that the doping Zn0.5Mg0.5Fe2O4 nanoparticles effectively accelerate the transfer of photon-generated charge carriers.
Degradation of PNP
To evaluate the PEC activity of the Zn0.5Mg0.5Fe2O4@TiO2 NTs electrode, a series of experiments for PNP degradation were carried out. After irradiation for 120 min under simulated sun light, the concentration of PNP was almost unchanged in the direct photocatalytic (DP) degradation without any photocatalyst, while 10.5% of PNP was degraded in the electrochemical process (EC) (Fig. 10a). The degradation efficiency of PNP by PC degradation was 36% with the Zn0.5Mg0.5Fe2O4@TiO2 composite nanotube array electrode. When a bias potential of 0.6 V (vs. SCE) was applied, the degradation efficiency increased to about 69% for the Zn0.5Mg0.5Fe2O4 loaded electrode, an enhanced of PNP than heterojunction network catalyst of Cu2O/TiO2.56 Compared with the non-doped TiO2 NTs, the composite electrodes afford a 2 folds increase in the efficiency in PNP degradation. In the meantime, trends can be observed in Fig. 10c, where the EC, DP, PC and PEC degradation efficiency of PNP by Zn0.5Mg0.5Fe2O4 are 9.3%, 10.3%, 14.8% and 59%, respectively, when illuminated by visible light while the PEC degradation of PNP is just 21% by TiO2 NTs alone. Judging from the increased degradation efficiency, there exists an obvious synergistic effect between the EC process and the PC process (under both simulated sun light irradiation and visible light irradiation, respectively). The enhanced PEC activity of the composite electrode could be attributed to the comprehensive effect between the lowered electron–hole recombination rate by the applied bias potential and the wider spectral response promoted by the Zn0.5Mg0.5Fe2O4 nanoparticles.6,56 Thus the applied bias potential effectively inhibits the recombination of photo-generated electron–hole pairs and prolongs the life of the photo-generated charge carriers, hence yielding a better photoelectrochemical performance. As shown in Fig. 10b and d, the kinetics of PNP degradation processes in EP, DP, PC, and PEC by Zn0.5Mg0.5Fe2O4 doped electrode and PEC by TiO2 NTs follows a pseudo-first-order kinetics, as described in Table 1.| Processes | Kinetic constants (min−1) | R2 |
|---|---|---|
| (a) | ||
| EC | 3 × 10−4 | 0.9441 |
| DP | 9 × 10−4 | 0.9794 |
| PC | 3.7 × 10−3 | 0.9912 |
| PEC | 8 × 10−3 | 0.9906 |
| TiO2 | 3.8 × 10−3 | 0.9872 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| (b) | ||
| EC | 4 × 10−5 | 0.9873 |
| DP | 5 × 10−5 | 0.9882 |
| PC | 8 × 10−4 | 0.9933 |
| PEC | 2.8 × 10−3 | 0.9941 |
| TiO2 | 8 × 10−4 | 0.9713 |
To further investigate the active species involved in PEC degradation, we have conducted the capture of the active species in PNP degradation to determine which species play a major role in PNP degradation. As exhibited in Fig. 11, the PEC degradation of PNP was inhibited to some extent by the addition of t-BuOH (hydroxyl radicals scavenger) under simulated sun light irradiation, indicating that the hydroxyl radicals were confirmed as active oxidative species in the PEC process. However, it was intensively suppressed when EDTA (holes scavenger) was introduced. This result indicates that photogenerated holes were the main active oxidizing species involved in the PEC process compared with other species. The direct holes transfer mainly governed the PC process. Meanwhile, ˙O2− might also serve as active species in this reaction. N2 was used to confirm the effect of O2 and the results are shown in Fig. 11. Under the anoxic condition, the degradation of PNP was not completely inhibited, indicating that ˙O2− was active species but not the main one. In summary, the photogenerated holes account for the photo activity most compared with ˙OH and ˙O2− in PEC degradation of PNP.
The stability and recyclability of the as-prepared electrode was evaluated by repeating the PNP degradation under simulated sun light irradiation several times. Before each repeating experiment, the composite electrode was ultrasonically cleaned with deionized water and each new PEC degradation cycle was carried out by introducing a fresh PNP solution and Na2SO4 electrolyte under the same conditions. As shown in Fig. 12, the efficiency of PNP degradation shows no obvious decline in the PEC activity of Zn0.5Mg0.5Fe2O4@TiO2 NTs after five recycles. Therefore, it is reasonably concluded that the Zn0.5Mg0.5Fe2O4@TiO2 NTs electrode exhibits fair stability and can be optimized as a suitable candidate for PEC purification of PNP polluted water.
 | ||
| Fig. 12 PEC degradation of PNP by the Zn0.5Mg0.5Fe2O4@TiO2 NTs electrode over five consecutive cycles under simulated sun light illumination. | ||
Mechanism of enhanced PEC activity
To gain a further insight into the enhanced PEC activity, the charge transfer behavior during PNP degradation is illustrated in Fig. 13. The involved photoelectrocatalytic reactions of PNP degradation over Zn0.5Mg0.5Fe2O4@TiO2 nanotube arrays have been described as follows (herein, ZMF represents Zn0.5Mg0.5Fe2O4). To calculate the band structures of Zn0.5Mg0.5Fe2O4, periodic density functional calculations (DFT) computations were performed using a plane-wave method implemented in the Cambridge Sequential Total Energy Package (CASTEP) code.57,58 It can be obtained that the band gap of Zn0.5Mg0.5Fe2O4 is approximately 1.9 eV, in good agreement with DRS experimental values 2.0 eV (the conduction band and valence band structure of Zn0.5Mg0.5Fe2O4 can be seen in ESI†). As shown in Fig. 13, under visible light irradiation, Zn0.5Mg0.5Fe2O4 with a narrow band gap can be easily excited to generate electron–hole pairs while TiO2 with a band gap of 3.2 eV can not be excited. The photo-generated electrons can be forced to transfer from the conduction band of Zn0.5Mg0.5Fe2O4 to TiO2 under an applied bias potential of 0.6 V, leaving more holes in the valence band of Zn0.5Mg0.5Fe2O4, significantly reducing the charge recombination. However, the holes spontaneously transfer from TiO2 NTs to the valence band of Zn0.5Mg0.5Fe2O4 nanoparticle. This separation effectively prevent the Zn0.5Mg0.5Fe2O4 react with H2O or hydroxyl ions (OH−) to form hydroxyl radicals (˙OH). The accumulated electrons in the conduction band of TiO2 are transferred to the adsorbed O2 on the TiO2 surface to form superoxide radical anions (˙O2−), which are then reduced to ˙OH. Eventually, the photo-generated active oxidizing species such as ˙OH, ˙O2− and h+ are involved in the self-sensitized photoelectrocatalysis degradation of PNP.| ZMF + visible light → ZMF(h+) + ZMF(e−) |
| ZMF(e−) + TiO2 → ZMF + TiO2(e−) |
| TiO2(e−) + O2 → TiO2 + ˙O2− |
| ˙O2− + H+ → ˙OOH → H2O2 |
| H2O2 + ˙O2− → ˙OH + OH− |
| TiO2(e−) + external electrostatic field → external circuit → counter electrode |
| ZMF(h+) + OH− → ZMF + ˙OH |
| PNP + ˙OH, ˙O2−, h+ → intermediate + H2O + CO2 |
Conclusions
A novel Zn0.5Mg0.5Fe2O4@TiO2 composite-nanotube-array photo-electrode was successfully synthesized using an ultrasonically assisted electrodeposition technique. The electrodeposition strategy effectively promoted the deposition of the Zn0.5Mg0.5Fe2O4 nanoparticles within self-organized and vertically oriented TiO2 nanotube arrays while minimizing the clogging of the nanotube entrances. The PEC properties demonstrated that the composite nanotube arrays showed typical heterojunction characteristics. The loading of Zn0.5Mg0.5Fe2O4 nanoparticles significantly extended the response of the TiO2 nanotube arrays to the visible light region. The composite nanotube arrays showed a significantly higher photocurrent density and improved photo-conversion efficiency due to better charge separation and collection efficiency under simulated sun light and visible light illumination. In addition, the composite electrode was found to possess excellent PEC activity for the degradation of PNP as compared with the non-doped TiO2 electrode under both simulated sun light and visible light illumination. Consequently, the Zn0.5Mg0.5Fe2O4@TiO2 heterostructure photoelectrode described herein may be used in a wide range of applications including photocatalysis.Acknowledgements
This work received partial financial support from the National Nature Science Foundation of China (no. NSFC21377015, 21207015), the Major State Basic Research Development Program of China (973 Program) (no. 2011CB936002) and the Key Laboratory of Industrial Ecology and Environmental Engineering, China Ministry of Education.Notes and references
- X. Chen, S. Shen, L. Guo and S. S. Mao, Chem. Rev., 2010, 110, 6503–6570 CrossRef CAS PubMed.
- H. Kisch, Angew. Chem., Int. Ed., 2013, 52, 812–847 CrossRef CAS PubMed.
- A. L. Linsebigler, G. Lu and J. T. Yates Jr, Chem. Rev., 1995, 95, 735–758 CrossRef CAS.
- S. G. Kumar and L. G. Devi, J. Phys. Chem. A, 2011, 115, 13211–13241 CrossRef CAS PubMed.
- K. Lee, D. Kim, P. Roy, I. Paramasivam, B. I. Birajdar, E. Spiecker and P. Schmuki, J. Am. Chem. Soc., 2010, 132, 1478–1479 CrossRef CAS PubMed.
- A. Zhu, Q. Zhao, X. Li and Y. Shi, ACS Appl. Mater. Interfaces, 2014, 6, 671–679 CAS.
- T. Li, X. Li, Q. Zhao, Y. Shi and W. Teng, Appl. Catal., B, 2014, 156–157, 362–370 CrossRef CAS PubMed.
- A. E. R. Mohamed and S. Rohani, Energy Environ. Sci., 2011, 4, 1065–1086 CAS.
- R. M. Navarro, M. C. Alvarez-Galván, J. A. V. de la Mano, S. M. Al-Zahrani and J. L. G. Fierro, Energy Environ. Sci., 2010, 3, 1865–1882 CAS.
- C. X. Kronawitter, L. Vayssieres, S. Shen, L. Guo, D. A. Wheeler, J. Z. Zhang, B. R. Antoun and S. S. Mao, Energy Environ. Sci., 2011, 4, 3889–3899 CAS.
- A. Lamberti, A. Sacco, S. Bianco, D. Manfredi, F. Cappelluti, S. Hernandez, M. Quaglio and C. F. Pirri, Phys. Chem. Chem. Phys., 2013, 15, 2596–2602 RSC.
- L. Xue, Z. Wei, R. Li, J. Liu, T. Huang and A. Yu, J. Mater. Chem., 2011, 21, 3216–3220 RSC.
- L. Sun, S. Zhang, X. W. Sun, X. Wang and Y. Cai, Langmuir, 2010, 26, 18424–18429 CrossRef CAS PubMed.
- W. Teng, X. Y. Li, Q. D. Zhao and G. H. Chen, J. Mater. Chem. A, 2013, 1, 9060–9068 CAS.
- W. Wang, M. Tian, A. Abdulagatov, S. M. George, Y. C. Lee and R. Yang, Nano Lett., 2012, 12, 655–660 CrossRef CAS PubMed.
- J. Choi, H. Park and M. R. Hoffmann, J. Phys. Chem. C, 2009, 114, 783–792 Search PubMed.
- M. A. Hashemian, E. Palacios, I. I. Nedrygailov, D. Diesing and E. Karpov, ACS Appl. Mater. Interfaces, 2013, 5, 12375–12379 CAS.
- W. Tu, Y. Zhou, Q. Liu, S. Yan, S. Bao, X. Wang, M. Xiao and Z. Zou, Adv. Funct. Mater., 2013, 23, 1743–1749 CrossRef CAS PubMed.
- H. U. Lee, S. C. Lee, S. H. Choi, B. Son, S. J. Lee, H. J. Kim and J. Lee, Appl. Catal., B, 2013, 129, 106–113 CrossRef CAS PubMed.
- A. Ghicov, J. M. Macak, H. Tsuchiya, J. Kunze, V. Haeublein, L. Frey and P. Schmuki, Nano Lett., 2006, 6, 1080–1082 CrossRef CAS.
- M. Wang, L. Sun, Z. Lin, J. Cai, K. Xie and C. Lin, Energy Environ. Sci., 2013, 6, 1211 CAS.
- G. Li, L. Wu, F. Li, P. Xu, D. Zhang and H. Li, Nanoscale, 2013, 5, 2118–2125 RSC.
- A. Kongkanand, K. Tvrdy, K. Takechi, M. Kuno and P. V. Kamat, J. Am. Chem. Soc., 2008, 130, 4007–4015 CrossRef CAS PubMed.
- Q. Kang, S. Liu, L. Yang, Q. Cai and C. A. Grimes, ACS Appl. Mater. Interfaces, 2011, 3, 746–749 CAS.
- W.-T. Sun, Y. Yu, H.-Y. Pan, X.-F. Gao, Q. Chen and L.-M. Peng, J. Am. Chem. Soc., 2008, 130, 1124–1125 CrossRef CAS PubMed.
- K. Thummer, M. Chhantbar, K. Modi, G. Baldha and H. Joshi, J. Magn. Magn. Mater., 2004, 280, 23–30 CrossRef CAS PubMed.
- E. Casbeer, V. K. Sharma and X.-Z. Li, Sep. Purif. Technol., 2012, 87, 1–14 CrossRef CAS PubMed.
- R. Dom, R. Subasri, K. Radha and P. H. Borse, Solid State Commun., 2011, 151, 470–473 CrossRef CAS PubMed.
- M. Gaudon, N. Pailhé, A. Wattiaux and A. Demourgues, Mater. Res. Bull., 2009, 44, 479–484 CrossRef CAS PubMed.
- K. A. Mohammed, A. D. Al-Rawas, A. M. Gismelseed, A. Sellai, H. M. Widatallah, A. Yousif, M. E. Elzain and M. Shongwe, Phys. B, 2012, 407, 795–804 CrossRef CAS PubMed.
- A. Xia, S. Liu, C. Jin, L. Chen and Y. Lv, Mater. Lett., 2013, 105, 199–201 CrossRef CAS PubMed.
- S. Rahman, K. Nadeem, M. Anis-ur-Rehman, M. Mumtaz, S. Naeem and I. Letofsky-Papst, Ceram. Int., 2013, 39, 5235–5239 CrossRef CAS PubMed.
- Y. Hou, F. Zuo, A. Dagg and P. Feng, Angew. Chem., 2013, 52, 1248–1252 CrossRef CAS PubMed.
- K. Mukherjee and S. Majumder, Nanotechnology, 2010, 21, 255504 CrossRef CAS PubMed.
- K. Mukherjee and S. Majumder, Sens. Actuators, B, 2013, 177, 55–63 CrossRef CAS PubMed.
- Z. Wu, Y. Wang, L. Sun, Y. Mao, M. Wang and C. Lin, J. Mater. Chem. A, 2014, 2, 8223–8229 CAS.
- S. Kurian, H. Seo and H. Jeon, J. Phys. Chem. C, 2013, 117, 16811–16819 CAS.
- M. Paulose, K. Shankar, S. Yoriya, H. E. Prakasam, O. K. Varghese, G. K. Mor, T. A. Latempa, A. Fitzgerald and C. A. Grimes, J. Phys. Chem. B, 2006, 110, 16179–16184 CrossRef CAS PubMed.
- K. Shankar, G. K. Mor, A. Fitzgerald and C. A. Grimes, J. Phys. Chem. C, 2007, 111, 21–26 CAS.
- H. E. Prakasam, K. Shankar, M. Paulose, O. K. Varghese and C. A. Grimes, J. Phys. Chem. C, 2007, 111, 7235–7241 CAS.
- N. Serpone, D. Lawless and R. Khairutdinov, J. Phys. Chem., 1995, 99, 16646–16654 CrossRef CAS.
- V. Salas, E. Olias, A. Barrado and A. Lazaro, Sol. Energy Mater. Sol. Cells, 2006, 90, 1555–1578 CrossRef CAS PubMed.
- Y. Lei, L.-D. Zhang, G.-W. Meng, G.-H. Li, X. Zhang, C. Liang, W. Chen and S. Wang, Appl. Phys. Lett., 2001, 78, 1125–1127 CrossRef CAS PubMed.
- T. Tachikawa and T. Majima, Langmuir, 2009, 25, 7791–7802 CrossRef CAS PubMed.
- T. Tachikawa, N. Wang, S. Yamashita, S. C. Cui and T. Majima, Angew. Chem., Int. Ed., 2010, 49, 8593–8597 CrossRef CAS PubMed.
- Y. Hou, X.-Y. Li, Q.-D. Zhao, X. Quan and G.-H. Chen, Adv. Funct. Mater., 2010, 20, 2165–2174 CrossRef CAS PubMed.
- Y.-Q. Chu, Z.-W. Fu and Q.-Z. Qin, Electrochim. Acta, 2004, 49, 4915–4921 CrossRef CAS PubMed.
- Y. Sharma, N. Sharma, G. Rao and B. Chowdari, Electrochim. Acta, 2008, 53, 2380–2385 CrossRef CAS PubMed.
- M. Wang, L. Sun, J. Cai, P. Huang, Y. Su and C. Lin, J. Mater. Chem. A, 2013, 1, 12082 CAS.
- Y. Mizukoshi, N. Ohtsu, S. Semboshi and N. Masahashi, Appl. Catal., B, 2009, 91, 152–156 CrossRef CAS PubMed.
- Q. Li and J. K. Shang, Environ. Sci. Technol., 2009, 43, 8923–8929 CrossRef CAS PubMed.
- Y. Chen, J. C. Crittenden, S. Hackney, L. Sutter and D. W. Hand, Environ. Sci. Technol., 2005, 39, 1201–1208 CrossRef CAS.
- Y. Hou, F. Zuo, A. Dagg and P. Feng, Nano Lett., 2012, 12, 6464–6473 CrossRef CAS PubMed.
- H. Yu, X. Quan, S. Chen and H. Zhao, J. Phys. Chem. C, 2007, 111, 12987–12991 CAS.
- X. Zhao, T. Xu, W. Yao, C. Zhang and Y. Zhu, Appl. Catal., B, 2007, 72, 92–97 CrossRef CAS PubMed.
- L. Yang, S. Luo, Y. Li, Y. Xiao, Q. Kang and Q. Cai, Environ. Sci. Technol., 2010, 44, 7641–7646 CrossRef CAS PubMed.
- L. Sun, L. Xiang, X. Zhao, C.-J. Jia, J. Yang, Z. Jin, X. Cheng and W. Fan, ACS Catal., 2015, 5, 3540–3551 CrossRef CAS.
- M. Nolan, ACS Appl. Mater. Interfaces, 2012, 4, 5863–5871 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra06443k |
| This journal is © The Royal Society of Chemistry 2015 |